Osteopontin Concentration in Prostates Fractions: A Novel Marker of Sperm Quality in Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Semen Collection
2.2. Semen Processing and Evaluation
2.3. Determination of OPN Levels in Seminal Plasma
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Exploring OPN’s Role in Sperm Motility and Morphology
4.2. Comparative İnsights of OPN on Fertility in Other Species
4.3. Potential Implications and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Essawe, E.M.; Wallgren, M.; Wulf, M.; Aurich, C.; Macías-Garcia, B.; Sjunnesson, Y. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 2018, 115, 99–107. [Google Scholar] [CrossRef]
- Ohaneje, U.L.; Osuagwuh, U.I.; Alvarez-Rodríguez, M.; Yánez-Ortiz, I.; Tabarez, A.; Palomo, M.J. The Re-Addition of Seminal Plasma after Thawing Does Not Improve Buck Sperm Quality Parameters. Animals 2021, 11, 3452. [Google Scholar] [CrossRef]
- Souza, F.F.D.; Chirinea, V.H.; Martins, M.I.M.; Lopes, M.D. Osteopontin in seminal plasma and sperm membrane of dogs. Reprod. Domest. Anim. 2009, 44, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Brito-Nery, L.T.; van Tilburg, M.F.; Menezes, E.B.; Machado, V.P.; Moura, A.A.A.; Oliveira, E.C.S. Protein patterns of canine seminal plasma. Rev. Bras. Reprod. Anim. 2014, 38, 110–115. [Google Scholar]
- Aquino-Cortez, A.; Pinheiro, B.Q.; Lima, D.B.C.; Silva, H.V.R.; Mota-Filho, A.C.; Martins, J.A.M. Proteomic characterization of canine seminal plasma. Theriogenology 2017, 95, 178–186. [Google Scholar] [CrossRef] [PubMed]
- D’Occhio, M.J.; Campanile, G.; Zicarelli, L.; Visintin, J.A.; Baruselli, P.S. Adhesion molecules in gamete transport, fertilization, early embryonic development, and implantation-role in establishing a pregnancy in cattle: A review. Mol. Reprod. Dev. 2020, 87, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919159-1–e919159-9. [Google Scholar] [CrossRef] [PubMed]
- Abedin, S.N.; Leela, V.; Devendran, P.; Suganya, G.; Rangasamy, S.; Loganathasamy, K. Seminal Plasma Osteopontin: A Marker for Potential Fertility in Dogs. Indian J. Anim. Res. 2021, 55, 758–762. [Google Scholar] [CrossRef]
- Erikson, D.W.; Way, A.L.; Chapman, D.A.; Killian, G.J. Detection of osteopontin on Holstein bull spermatozoa, in cauda epididymal fluid and testis homogenates, and its potential role in bovine fertilization. Reproduction 2007, 133, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.M.; Day, J.R.; Killian, G.J. Osteopontin gene expression in the Holstein bull reproductive tract. J. Androl. 2000, 21, 414–420. [Google Scholar] [CrossRef]
- Zhang, G.M.; Lan, S.; Jia, R.X.; Yan, G.Y.; Wang, L.Z.; Nie, H.T.; Wang, F. Age-associated and tissue-specific expression of osteopontin in male Hu sheep reproductive tract. Tissue Cell 2016, 48, 496–502. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Li, S.; Meng, P. Salinomycin triggers prostate cancer cell apoptosis by inducing oxidative and endoplasmic reticulum stress via suppressing Nrf2 signaling. Exp. Ther. Med. 2021, 22, 946. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Gezmen-Karadag, M. Determination of the Effect of Nutritional Status on PRAL Level in Patients with Nephrolithiasis. Gümüşhane Univ. J. Health Sci. 2018, 7, 1–9. [Google Scholar]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.A. Seminal plasma proteins and fertility indexes in the bull. The case for osteopontin. Anim. Reprod. 2005, 2, 3–10. [Google Scholar]
- Luedtke, C.C.; McKee, M.D.; Cyr, D.G.; Gregory, M.; Kaartinen, M.T.; Mui, J.; Hermo, L. Osteopontin expression and regulation in the testis, efferent ducts, and epididymis of rats during postnatal development through to adulthood. Biol. Reprod. 2002, 66, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, C.; Cortes-Araya, Y.; Donadeu, F.X.; Ashworth, C.J. Associations between testicular development and fetal size in the pig. J. Anim. Sci. Biotechnol. 2022, 13, 24. [Google Scholar] [CrossRef]
- Brandon, C.I.; Heusner, G.L.; Caudle, A.B.; Fayrer-Hosken, R.A. Two-dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their correlation with fertility. Theriogenology 1999, 52, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Amedeo, S.; Cerruti, F.; Capucchio, M.T.; Palmerini, D.; Apicella, M.; Giberti, M.; Mioletti, S. Osteopontin expression in normal and criptorchid boar testes: Preliminary results. In Proceedings of the Atti della Società Italiana di Patologia ed Allevamento dei Suini, XXXV Meeting Annuale, Modena, Italy, 12–13 March 2009; Società Italiana di Patologia ed Allevamento dei Suini (SIPAS): Modena, Italy, 2009; pp. 308–313. [Google Scholar]
- Rowell, J.L.; McCarthy, D.O.; Alvarez, C.E. Dog models of naturally occurring cancer. Trends Mol. Med. 2011, 17, 380–388. [Google Scholar] [CrossRef]
- Surmacz, P.; Niwinska, A.; Kautz, E.; Gizinski, S.; Faundez, R. Comparison of two staining techniques on the manual and automated canine sperm morphology analysis. Reprod. Domest. Anim. 2022, 57, 678–684. [Google Scholar] [CrossRef]
- Fontbonne, A. Infertility in male dogs: Recent advances. Rev. Bras. Reprod. Anim. 2011, 35, 266–273. [Google Scholar]
- Lee, J.; Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Miki, H.; Toyokuni, S. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development 2007, 134, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Cunha Bustamante-Filho, I.; Renato Menegassi, S.; Ribas Pereira, G.; Dias Salton, G.; Mosena, M.F.; Schneider, R.M. Bovine seminal plasma osteopontin: Structural modelling, recombinant expression and its relationship with semen quality. Andrologia 2021, 53, e13905. [Google Scholar] [CrossRef] [PubMed]
- Michos, I.; Tsantarliotou, M.; Boscos, C.M.; Tsousis, G.; Basioura, A.; Tzika, E.D. Effect of boar sperm proteins and quality changes on field fertility. Animals 2021, 11, 1813. [Google Scholar] [CrossRef]
- Viswam, V.; Loganathasamy, K.; Gomathy, V.S.; Reena, D. Ameliorative effects of osteopontin on sperm morphology of frozen thawed buffalo semen treated with sodium nitroprusside. J. Entomol. Zool. Stud. 2020, 8, 891–894. [Google Scholar] [CrossRef]
- Novak, S.; Ruiz-Sánchez, A.; Dixon, W.T.; Foxcroft, G.R.; Dyck, M.K. Seminal plasma proteins as potential markers of relative fertility in boars. J. Androl. 2010, 31, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.M.; Ghoneim, I.M.; Alhaider, A.K. Seminal plasma and serum fertility biomarkers in dromedary camels (Camelus dromedarius). Theriogenology 2015, 83, 650–654. [Google Scholar] [CrossRef]
- Baruah, K.K.; Dhall, A.; Bora, B.; Mech, A.; Mondal, M. Detection of osteopontin transcript in seminal plasma and its association with post-freeze-thaw quality of cryopreserved spermatozoa in mithun (Bos frontalis). Indian J. Anim. Res. 2017, 51, 648–653. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signalling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Young Group | Older Group | ||
|---|---|---|---|---|
| Age (year) | 1.5 ± 0.3 (n = 19) | 4.7 ± 1.3 (n = 20) | ||
| Fractions | 1st | 3rd | 1st | 3rd |
| Volume (mL) | 3 ± 2 | 22 ± 9.8 | 2.5 ± 1.02 | 18.5 ± 9 |
| OPN concentration (ng/mL) | 17.06 ± 7.16 | 8.27 ± 4.54 | 11.58 ± 5.23 | 6.10 ± 4.05 |
| OPN average (ng/mL) | 1st 10.4 ± 5.3 | 3rd 7.4 ± 5 | ||
| Motility % | 79.8 ± 12.5 | 66.5 ± 20.10 | ||
| Concentration × 106 | 311.8 ± 68.3 | 279 ± 69.41 | ||
| Dead spermatozoa % | 14.68 ± 7.7 | 18.5 ± 8.9 | ||
| Abnormal Spermatozoa % | 15.02 ± 9.2 | 30.9 ± 17.45 | ||
| Head Abnormality % | 2.8 ± 2.1 | 4.2 ± 3.4 | ||
| Acrosome defects % | 2.2 ± 3.5 | 2.8 ± 3.2 | ||
| Mid-piece defects % | 2.09 ± 2.26 | 7.8 ± 6.2 | ||
| Tail % | 5.9 ± 4 | 11.5 ± 8.1 | ||
| Cytoplasmic Droplet % | 2.8 ± 2.2 | 7.5 ± 7.9 | ||
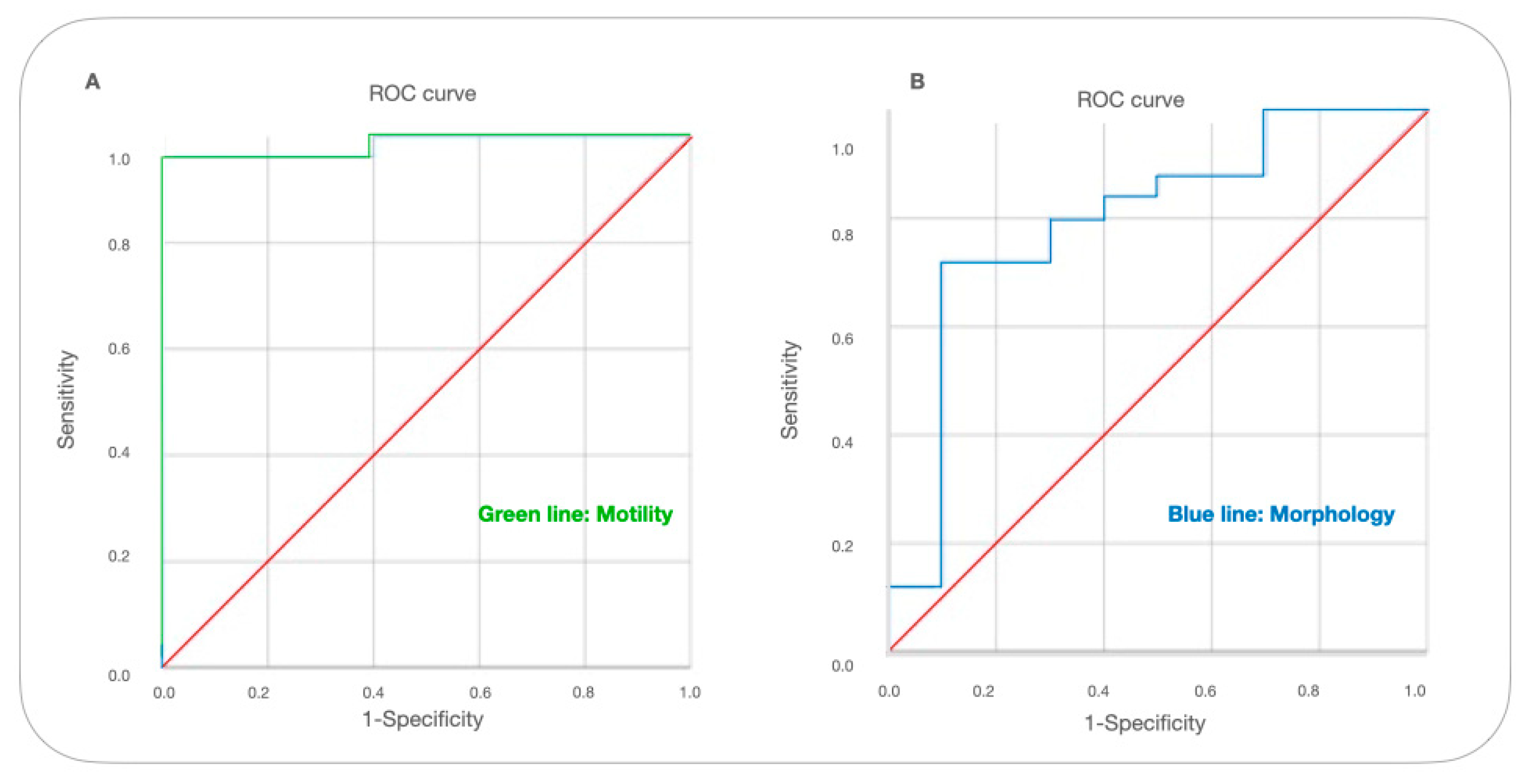
| AUC | SE | SP | P | 95%Cl | |
|---|---|---|---|---|---|
| Motility | 0.985 | 0.962 | 0.95 | 0.001 | (0.951–1) |
| Morphology | 0.796 | 0.72 | 0.9 | 0.05 | (0.619–0.973) |
| OPN | FR-1 | FR-3 | CON | Dead | Norm | ACR | Head | Mid-P | Tail | C-Drop | ABN | MOT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPN | 1 | −0.170 | −0.092 | 0.292 | −0.161 | 0.418 | −0.493 * | −0.359 | −0.711 ** | −0.304 | −0.303 | −0.417 | 0.763 ** |
| Volume−1 | 1 | −0.205 | 0.247 | −0.225 | 0.054 | −0.070 | 0.020 | 0.067 | −0.137 | 0.136 | −0.052 | 0.070 | |
| Volume−3 | 1 | −0.139 | 0.400 | 0.159 | 0.049 | −0.107 | 0.038 | −0.203 | −0.131 | −0.157 | −0.066 | ||
| CON | 1 | −0.262 | 0.241 | −0.141 | −0.284 | −0.305 | −0.082 | 0.029 | −0.233 | 0.178 | |||
| Dead | 1 | −0.357 | 0.477 * | 0.243 | 0.575 * | 0.147 | 0.208 | 0.353 | −0.403 | ||||
| Normal | 1 | −0.553 * | −0.351 | −0.510 * | −0.874 ** | −0.872 ** | −1.000 ** | 0.293 | |||||
| ACR | 1 | 0.583 * | 0.560 * | 0.240 | 0.268 | 0.546 * | −0.574 * | ||||||
| Head | 1 | 0.259 | −0.026 | 0.010 | 0.348 | −0.306 | |||||||
| Mid-P | 1 | 0.343 | 0.364 | 0.508 * | −0.758 ** | ||||||||
| Tail | 1 | 0.878 ** | 0.879 ** | −0.172 | |||||||||
| Cyto-Drop | 1 | 0.876 ** | −0.125 | ||||||||||
| Abnormal | 1 | −0.288 | |||||||||||
| Motility | 1 |
| OPN | FR-1 | FR-3 | CON | Dead | Normal | ACR | Head | Mid-P | Tail | Cyto-Drop | Abnormal | Motility | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPN | 1 | 0.713 | 0.144 | 0.497 | −0.282 | 0.173 | 0.031 | 0.133 | −0.523 | 0.316 | −0.283 | −0.307 | 0.355 |
| Volume−1 | 1 | −0.064 | 0.743 | −0.656 | 0.846 | 0.635 | −0.089 | −0.963 * | −0.364 | −0.733 | −0.896 | 0.898 | |
| Volume−3 | 1 | −0.843 | 0.728 | −0.446 | 0.716 | −0.248 | 0.488 | 0.317 | 0.571 | 0.505 | −0.769 | ||
| CON | 1 | −0.899 * | 0.770 | −0.304 | 0.089 | −0.873 | −0.455 | −0.827 | −0.841 | 0.961 ** | |||
| Dead | 1 | −0.874 | 0.044 | −0.297 | 0.870 | 0.749 | 0.978 ** | 0.908 * | −0.865 | ||||
| Normal | 1 | 0.252 | −0.128 | −0.929 * | −0.836 | −0.919 * | −0.989 ** | 0.860 | |||||
| ACR | 1 | −0.089 | −0.187 | −0.300 | −0.165 | −0.200 | −0.225 | ||||||
| Head | 1 | 0.065 | −0.083 | −0.264 | 0.070 | −0.149 | |||||||
| Mid-P | 1 | 0.599 | 0.901 * | 0.971 ** | −0.897 * | ||||||||
| Tail | 1 | 0.804 | 0.767 | −0.555 | |||||||||
| Cyto-Drop | 1 | 0.942 * | −0.812 | ||||||||||
| Abnormal | 1 | −0.898 * | |||||||||||
| Motility | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin, K.; Kurtdede, E.; Salmanoğlu, B.; Uysal, O.; Stelletta, C. Osteopontin Concentration in Prostates Fractions: A Novel Marker of Sperm Quality in Dogs. Vet. Sci. 2023, 10, 646. https://doi.org/10.3390/vetsci10110646
Tekin K, Kurtdede E, Salmanoğlu B, Uysal O, Stelletta C. Osteopontin Concentration in Prostates Fractions: A Novel Marker of Sperm Quality in Dogs. Veterinary Sciences. 2023; 10(11):646. https://doi.org/10.3390/vetsci10110646
Chicago/Turabian StyleTekin, Koray, Efe Kurtdede, Berrin Salmanoğlu, Ongun Uysal, and Calogero Stelletta. 2023. "Osteopontin Concentration in Prostates Fractions: A Novel Marker of Sperm Quality in Dogs" Veterinary Sciences 10, no. 11: 646. https://doi.org/10.3390/vetsci10110646
APA StyleTekin, K., Kurtdede, E., Salmanoğlu, B., Uysal, O., & Stelletta, C. (2023). Osteopontin Concentration in Prostates Fractions: A Novel Marker of Sperm Quality in Dogs. Veterinary Sciences, 10(11), 646. https://doi.org/10.3390/vetsci10110646







