Lactiplantibacillus argentoratensis and Candida tropicalis Isolated from the Gastrointestinal Tract of Fish Exhibited Inhibitory Effects against Pathogenic Bacteria of Nile Tilapia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Probiotic Microorganisms
2.3. Pathogenic Bacterial Strains Used
2.4. Pathogenic Bacteria Inhibiting Test
2.4.1. Probiotic Bacterial Isolate Preparation
2.4.2. Probiotic Yeast Isolates Preparation
2.4.3. Inhibitory Capacity against Fish Pathogenic Bacteria
2.5. Probiotic Properties Tests
2.5.1. Bile Salt Tolerance Test
2.5.2. Acid Tolerance Test
2.5.3. Adhesion Property Test
2.5.4. Biofilm Formation Test
2.6. Detection of Antibacterial Factor Secretion of Selected Probiotic Isolates
2.7. Simulated GI Tract Tolerance Test
2.8. Safety Evaluation of Selected Bacterial and Yeasts Isolates
2.8.1. Hemolytic Activity Test
2.8.2. Antibiotics Susceptibility
2.9. Probiotic Bacteria Identification
2.10. Probiotic Yeast Identification
2.11. Nucleotide Sequencing and Analysis
2.12. Statistical Analysis
3. Results
3.1. Isolation of Probiotic Microbes
3.2. Pathogenic Bacteria Inhibiting Test
3.3. Probiotic Properties Tests
3.3.1. Acid Tolerance Test
3.3.2. Bile Salt Tolerance Test
3.3.3. Adhesion Property Test
3.3.4. Biofilm Formation Test
3.4. Detection of Antibacterial Factor
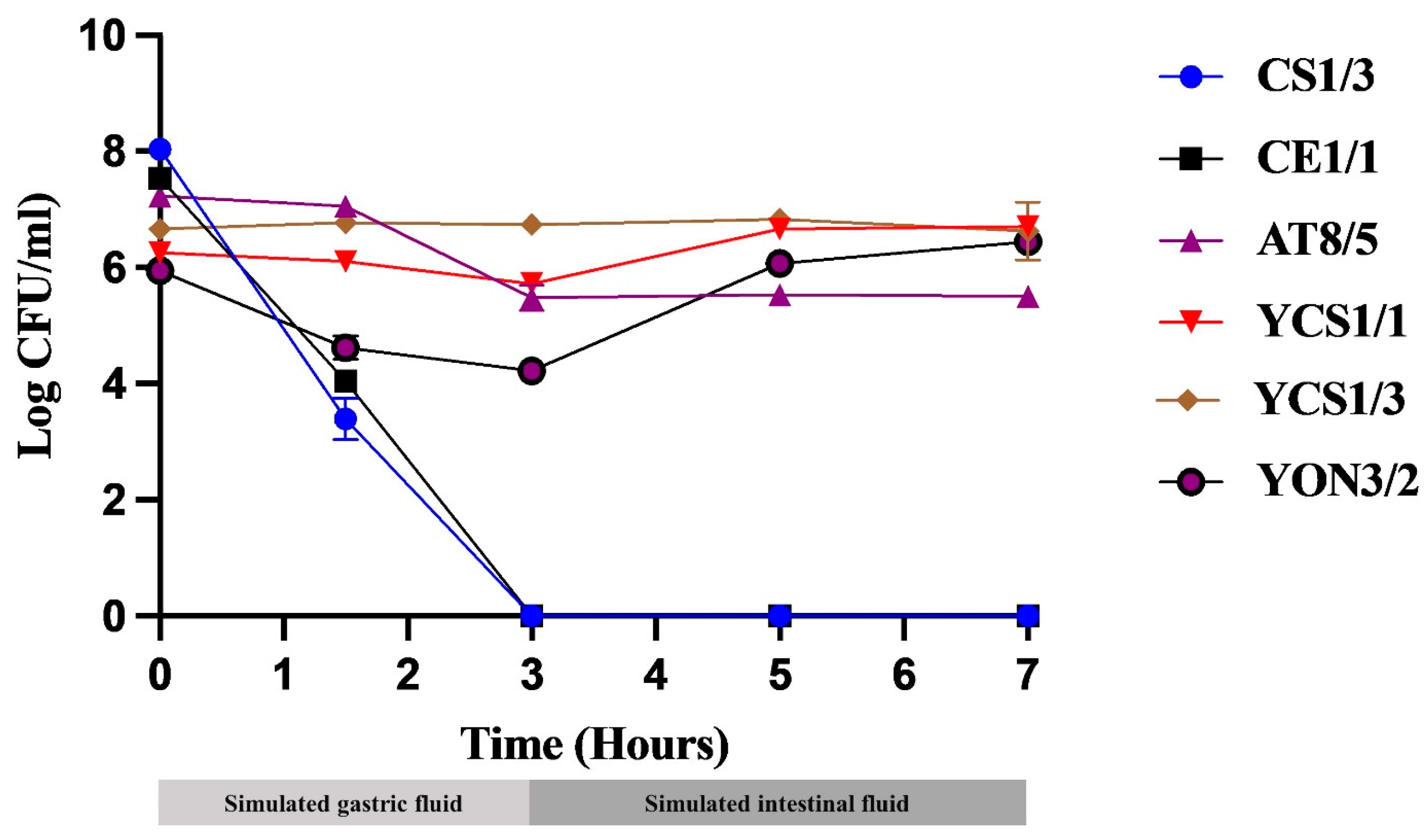
3.5. Simulated GI Tract Tolerance Test
3.6. Safety of Selected Probiotic Isolates
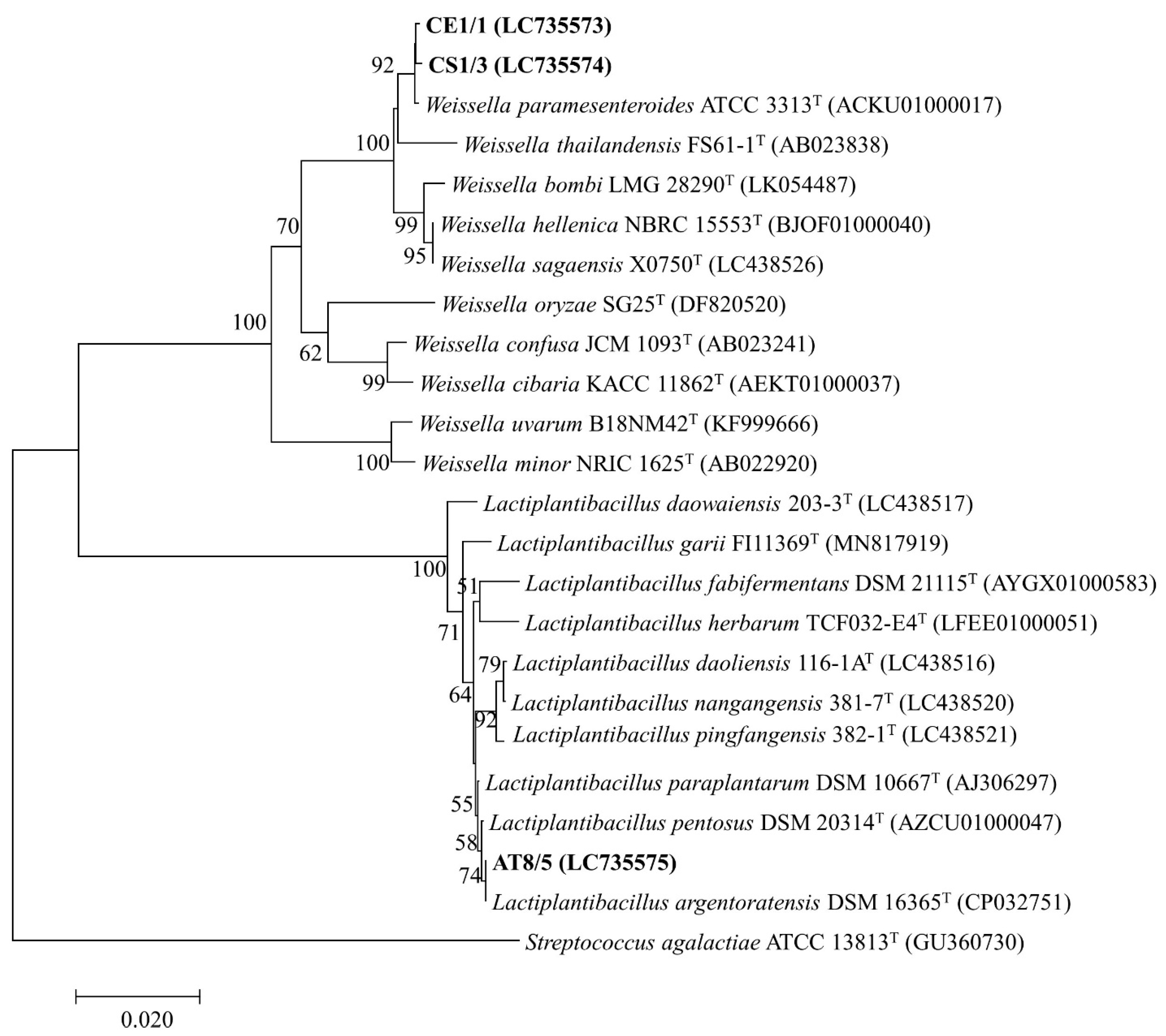
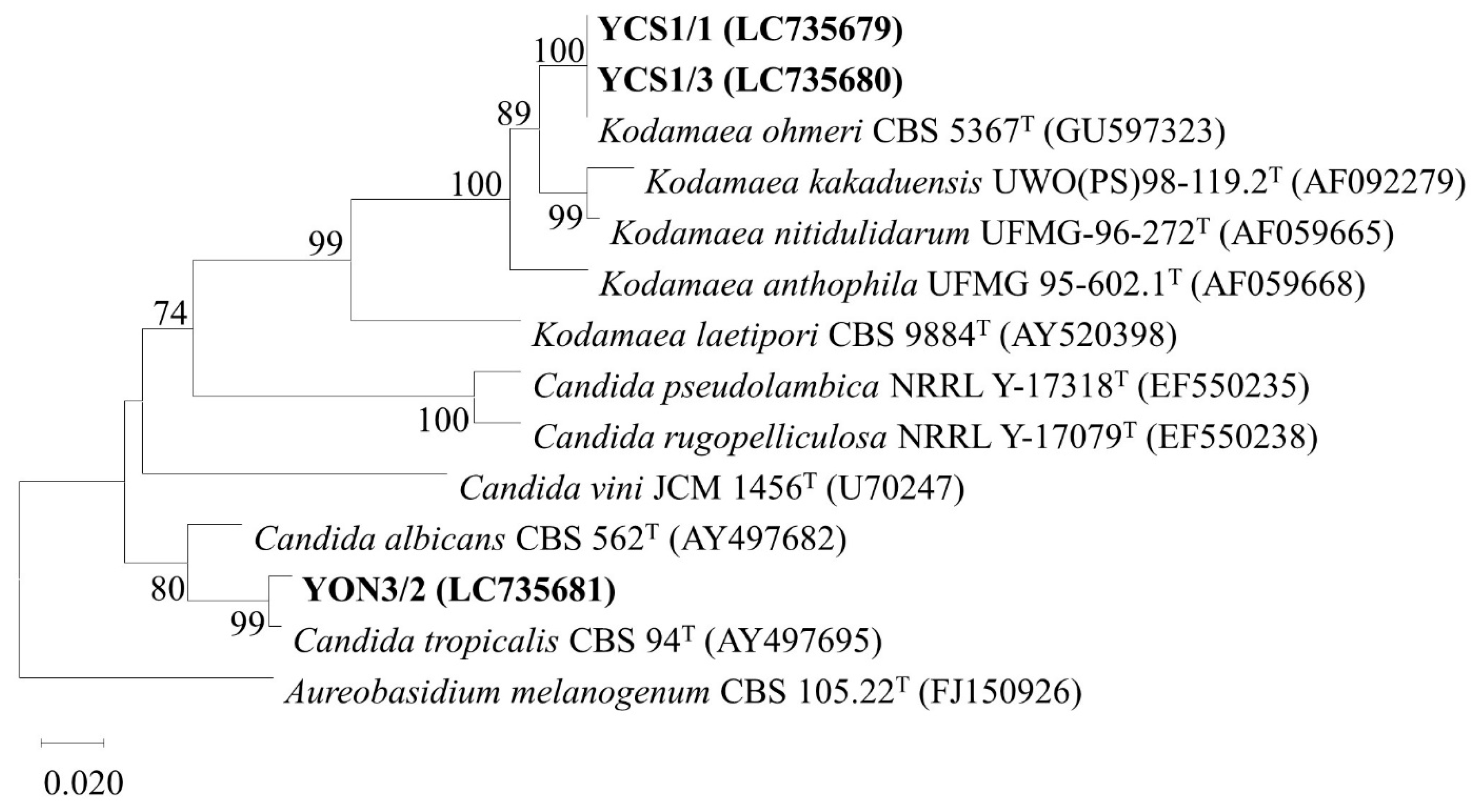
3.7. Genotypic Identification of Selected Probiotics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delphino, M.; Joshi, R.; Alvarez, A.T. Economic appraisal of using genetics to control Streptococcus agalactiae in Nile tilapia under cage and pond farming system in Malaysia. Sci. Rep. 2022, 12, 8754. [Google Scholar] [CrossRef] [PubMed]
- Suanyuk, N.; Kong, F.; Ko, D.; Gilbert, G.L.; Supamattaya, K. Occurrence of rare genotypes of Streptococcus agalactiae in cultured red tilapia Oreochromis sp. and Nile tilapia O. niloticus in Thailand-Relationship to human isolates? Aquaculture 2008, 284, 35–40. [Google Scholar] [CrossRef]
- Figueiredo, H.C.P.; Nobrega Netto, L.; Leal, C.A.G.; Pereira, U.P.; Mian, G.F. Streptococcus iniae outbreaks in Brazilian Nile tilapia (Oreochromis niloticus L.) farms. Braz. J. Microbiol. 2012, 43, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Kesarcodi-Watson, A.; Kaspar, H.; Lategan, M.J.; Gibson, L. Probiotics in aquaculture: The need, principles and mechanisms of action and screening processes. Aquaculture 2008, 274, 1–14. [Google Scholar] [CrossRef]
- Gastalho, S.; Silva, G.J.; Ramos, F. Antibiotics in aquaculture and bacterial resistance: Health care impact. Acta Farm. Port. 2014, 3, 28–44. [Google Scholar]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations/World Health Organization: London, ON, Canada, 2002. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R. Probiotics. Indian J. Med. Microbiol. 2009, 27, 202–209. [Google Scholar] [CrossRef]
- Yamashita, M.M.; Pereira, S.A.; Cardoso, L.; De Araujo, A.P.; Oda, C.E.; Schmidt, É.C.; Bouzon, Z.L.; Martins, M.L.; Mouriño, J.L.P. Probiotic dietary supplementation in Nile tilapia as prophylaxis against streptococcosis. Aquac. Nutr. 2017, 23, 1235–1243. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M.; El-Sayed, H.M.; El-Hady, M.A. In vitro selection and identification of potential probiotics isolated from the gastrointestinal tract of Nile tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 2018, 10, 692–703. [Google Scholar] [CrossRef]
- Midhun, S.J.; Neethu, S.; Vysakh, A.; Sunil, M.A.; Radhakrishnan, E.K.; Jyothis, M. Antibacterial activity of autochthonous bacteria isolated from Anabas testudineus (Bloch, 1792) and it’s in vitro probiotic characterization. Microb. Pathog. 2017, 113, 312–320. [Google Scholar] [CrossRef]
- Caruffo, M.; Navarrete, N.; Salgado, O.; Díaz, A.; López, P.; García, K.; Feijóo, C.G.; Navarrete, P. Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front. Microbiol. 2015, 6, 1093. [Google Scholar] [CrossRef]
- Laconi, S.; Pompei, R. Study and characterization of intestinal yeasts of mullet (Mugil spp.) for potential probiotic use. J. Food Agric. Environ. 2007, 5, 475–480. [Google Scholar]
- Gatesoupe, F.J. Live yeasts in the gut: Natural occurrence, dietary introduction, and their effects on fish health and development. Aquaculture 2007, 267, 20–30. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and potentially probiotic yeasts-characteristics and food application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Khagwal, N.; Sharma, P.; Sharma, D.C. Screening and evaluation of Lactobacillus spp. for the development of potential probiotics. Afr. J. Microbiol. Res. 2014, 8, 1573–1579. [Google Scholar]
- Ngov, S.; Sukboonyasatit, D.; Paseephol, T. Enhancement of probiotic survival in low pH and bile salt condition using alginate-hi-maize starch encapsulation. Asia-Pac. J. Sci. Technol. 2014, 19, 141–147. [Google Scholar]
- Yegorenkova, I.V.; Tregubova, K.V.; Matora, L.Y.; Burygin, G.L.; Ignatov, V.V. Biofilm formation by Paenibacillus polymyxa strains differing in the production and rheological properties of their exopolysaccharides. Curr. Microbiol. 2011, 62, 1554–1559. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Hmila, Z.; Behi, A.; Bakhrouf, A. Preliminary characterization of the probiotic properties of Candida famata and Geobacillus thermoleovorans. Iran. J. Microbiol. 2011, 3, 129–134. [Google Scholar]
- Schillinger, U. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Pennacchia, C.; Blaiotta, G.; Pepe, O.; Villani, F. Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J. Appl. Microbiol. 2008, 105, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Adimpong, D.B.; Nielsen, D.S.; Sørensen, K.I.; Derkx, P.M.F.; Jespersen, L. Genotypic characterization and safety assessment of lactic acid bacteria from indigenous African fermented food products. BMC Microbiol. 2012, 12, 75. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Saito, H.; Miura, K.I. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim. Biophys. Acta 1963, 72, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Tanasupawat, S.; Takehana, T.; Yoshida, S.; Hiraga, K.; Oda, K. Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades poly (ethylene terephthalate). Int. J. Syst. Evol. Microbiol. 2016, 66, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Eden, P.A.; Schmidt, T.M.; Blakemore, R.P.; Pace, N.R. Phylogenetic analysis of Aquaspirillum magnetotacticum using polymerase chain reaction-amplified 16S rRNA-specific DNA. Int. J. Syst. Evol. Microbiol. 1991, 41, 324–325. [Google Scholar] [CrossRef]
- Jutakanoke, R.; Endoh, R.; Takashima, M.; Ohkuma, M.; Tanasupawat, S.; Akaracharanya, A. Allodekkera sacchari gen. nov., sp. nov., a yeast species in the Saccharomycetales isolated from a sugar factory. Int. J. Syst. Evol. Microbiol. 2017, 67, 250–255. [Google Scholar] [CrossRef]
- Manitis, T.; Fritsch, E.F.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1982. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jutakanoke, R.; Tanasupawat, S.; Akaracharanya, A. Characterization and ethanol fermentation of Pichia and Torulaspora strains. J. Appl. Pharm. 2014, 4, 052–056. [Google Scholar]
- Raman, J.; Kim, J.S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.J.; Kim, S.J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
- Ammor, S.; Tauveron, G.; Dufour, E.; Chevallier, I. Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility: 1-Screening and characterization of the antibacterial compounds. Food Control 2006, 17, 454–461. [Google Scholar] [CrossRef]
- Karaoglu, Ş.A.; Aydin, F.; Kilic, S.S.; Kilic, A.O. Antimicrobial activity and characteristics of bacteriocins produced by vaginal lactobacilli. Turk. J. Med. Sci. 2003, 33, 7–13. [Google Scholar]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and probiotic properties of yeasts: From fundamental to novel applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef]
- Aasen, I.M.; Møretrø, T.; Katla, T.; Axelsson, L.; Storrø, I. Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl. Microbiol. Biotechnol. 2000, 53, 159–166. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Kostoń, D. Comparison of different methods for detection of antimicrobial activity of probiotic strains of Lactobacillus rhamnosus against some food spoilage microorganisms. Ann. UMCS 2009, 64, 15–24. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Muzquiz, J.L.; Girones, O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 2008, 278, 188–191. [Google Scholar] [CrossRef]
- Sahoo, T.K.; Jena, P.K.; Nagar, N.; Patel, A.K.; Seshadri, S. In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catla catla. Probiotics Antimicrob. Proteins 2015, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W.; Nannen, N.L. pH homeostasis in lactic acid bacteria. J. Dairy Sci. 1993, 76, 2354–2365. [Google Scholar] [CrossRef]
- Carmelo, V.; Santos, H.; Sá-Correia, I. Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim. Biophys. Acta Biomembr. 1997, 1325, 63–70. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Geraylou, Z.; Vanhove, M.P.; Souffreau, C.; Rurangwa, E.; Buyse, J.; Ollevier, F. In vitro selection and characterization of putative probiotics isolated from the gut of Acipenser baerii (Brandt, 1869). Aquac. Res. 2014, 45, 341–352. [Google Scholar] [CrossRef]
- Hernández-Gómez, J.G.; López-Bonilla, A.; Trejo-Tapia, G.; Ávila-Reyes, S.V.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. In vitro bile salt hydrolase (BSH) activity screening of different probiotic microorganisms. Foods 2021, 10, 674. [Google Scholar] [CrossRef]
- Kazuń, B.; Kazuń, K.; Żylińska, J.; Siwicki, A.K. In vitro study of Lactobacillus plantarum properties as a potential probiotic strain and an alternative method to antibiotic treatment of fish. Fish. Aquat. Life 2018, 26, 47–55. [Google Scholar] [CrossRef]
- Mello, T.P.; Oliveira, S.S.; Frasés, S.; Branquinha, M.H.; Santos, A.L. Surface properties, adhesion and biofilm formation on different surfaces by Scedosporium spp. and Lomentospora prolificans. Biofouling 2018, 34, 800–814. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; Amato, M.D.; Bonfiglio, F.; McDonald, D.; Gonzales, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Uscanga, A.; Moyano, F.J.; Alvarez, C.A. Assessment of enzymatic efficiency on protein digestion in the tilapia Oreochromis niloticus. Fish Physiol. Biochem. 2010, 36, 1079–1085. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Lin, X.; Qi, Y.; Yan, D.; Liu, H.; Chen, X.; Liu, L. CgMED3 changes membrane sterol composition to help Candida glabrata tolerate low-pH stress. Appl. Environ. Microbiol. 2017, 83, e00972-17. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ramírez, M.C.; Jaimez-Ordaz, J.; Escorza-Iglesias, V.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Ramírez-Godínez, J.; Castañeda-Ovando, A.; Morales-Estrada, A.I.; Felix-Reyes, N.; González-Olivares, L.G. Lactobacillus pentosus ABHEAU-05: An in vitro digestion resistant lactic acid bacterium isolated from a traditional fermented Mexican beverage. Rev. Argent. Microbiol. 2020, 52, 305–314. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Casquete, R.; Serradilla, M.J.; de Guía Córdoba, M. Safety and functional aspects of pre-selected lactobacilli for probiotic use in Iberian dry-fermented sausages. Meat Sci. 2009, 83, 460–467. [Google Scholar] [CrossRef]
- Shin, H.J.; Choi, H.J.; Kim, D.W.; Ahn, C.S.; Lee, Y.G.; Jeong, Y.K. Probiotic potential of Pediococcus pentosaceus BCNU 9070. J. Life Sci. 2012, 22, 1194–1200. [Google Scholar] [CrossRef]
- Seker, E. Identification of Candida species isolated from bovine mastitic milk and their in vitro hemolytic activity in Western Turkey. Mycopathologia 2010, 169, 303–308. [Google Scholar] [CrossRef]
- Diguță, C.F.; Mihai, C.; Toma, R.C.; Cîmpeanu, C.; Matei, F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods. 2023, 12, 124. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.A.G.; Tsakalidou, E.; Nychas, G.J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a probiotic strain with a potent anti-Helicobacter pylori activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Curragh, H.J.; Collins, M.A. High levels of spontaneous drug resistance in Lactobacillus. J. Appl. Bacteriol. 1992, 73, 31–36. [Google Scholar] [CrossRef]
- Ammor, M.S.; Flórez, A.B.; Mayo, B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 2007, 24, 559–570. [Google Scholar] [CrossRef]
- Danielsen, M.; Wind, A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003, 82, 1–11. [Google Scholar] [CrossRef]
- Nguyen, T.D.T.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef]
- Syal, P.; Vohra, A. Probiotic attributes of a yeast-like fungus, Geotrichum klebahnii. Afr. J. Microbiol. Res. 2014, 8, 2037–2043. [Google Scholar]
- do Vale Pereira, G.; Da Cunha, D.G.; Pedreira Mourino, J.L.; Rodiles, A.; Jaramillo-Torres, A.; Merrifield, D.L. Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. J. Appl. Microbiol. 2017, 123, 1298–1311. [Google Scholar] [CrossRef]
- Liu, D.D.; Gu, C.T. Proposal to reclassify Lactobacillus zhaodongensis, Lactobacillus zeae, Lactobacillus argentoratensis and Lactobacillus buchneri subsp. silagei as Lacticaseibacillus zhaodongensis comb. nov., Lacticaseibacillus zeae comb. nov., Lactiplantibacillus argentoratensis comb. nov. and Lentilactobacillus buchneri subsp. silagei comb. nov., respectively and Apilactobacillus kosoi as a later heterotypic synonym of Apilactobacillus micheneri. Int. J. Syst. Evol. Microbiol. 2020, 70, 6414–6417. [Google Scholar]
- Saraniya, A.; Jeevaratnam, K. In vitro probiotic evaluation of phytase producing Lactobacillus species isolated from Uttapam batter and their application in soy milk fermentation. J. Food Sci. Technol. 2015, 52, 5631–5640. [Google Scholar] [CrossRef]
- Azhar, M.A.; Munaim, M.S.A. Identification and evaluation of probiotic potential in yeast strains found in kefir drink samples from Malaysia. Int. J. Food Eng. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Karasu-Yalcin, S.; Senses-Ergul, S.; Ozbas, Z.Y. Yeast strains with technological and probiotic traits isolated from Mihalic cheese. Int. Food Res. J. 2019, 26, 1359–1370. [Google Scholar]
- Ogunremi, O.; Sanni, A.; Agrawal, R. Probiotic potentials of yeasts isolated from some cereal-based Nigerian traditional fermented food products. J. Appl. Microbiol. 2015, 119, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.J., Jr.; Ahearn, D.G.; Fell, J.W.; Meyers, S.P.; Meyer, S.A. Ecology and taxonomy of yeasts isolated from various marine substrates. Limnol. Oceanogr. 1962, 7, 178–185. [Google Scholar] [CrossRef]
- Hirimuthugoda, N.Y.; Chi, Z.; Li, X.; Wang, L.; Wu, L. Diversity of phytase-producing marine yeasts. Cienc. Mar. 2006, 32, 673–682. [Google Scholar] [CrossRef]

| Isolates | OD640 | |||
|---|---|---|---|---|
| Control Group | Tested Group | |||
| 6 h | 24 h | 6 h | 24 h | |
| CS1/3 | 1.86 A | 3.23 a | 0.39 B | 1.42 b |
| CE1/1 | 1.51 A | 2.99 a | 0.45 B | 1.39 b |
| AT8/5 | 1.19 A | 1.90 a | 0.39 B | 0.67 b |
| Isolates | OD640 | |||
|---|---|---|---|---|
| Control Group | Tested Group | |||
| 6 h | 24 h | 6 h | 24 h | |
| YCS1/1 | 0.39 A | 2.89 a | 0.21 B | 5.55 b |
| YCS1/2 | 0.43 A | 3.15 a | 0.20 B | 4.67 b |
| YCS1/3 | 0.39 A | 2.74 a | 0.36 A | 6.20 b |
| YON3/4 | 1.24 A | 4.45 a | 0.89 B | 5.07 b |
| YAT1/6 | 0.71 A | 6.25 a | 0.35 B | 6.64 b |
| YAT8/2 | 0.95 A | 2.75 a | 0.44 B | 4.58 b |
| YAT10/8 | 0.93 A | 2.52 a | 0.93 A | 4.43 b |
| YAT10/9 | 1.09 A | 2.83 a | 0.99 A | 4.34 b |
| Isolates | Inhibition Zone (mm) of Pathogenic Bacteria | |||||
|---|---|---|---|---|---|---|
| AH | AS | AV | EI | ET | SA | |
| Positive control | 26.00 ± 0.00 | 28.5 ± 0.50 | 26.17 ± 0.29 | 30.17 ± 0.29 | 33.00 ± 1.00 | 36.00 ± 1.41 |
| Negative control | - | - | - | - | - | - |
| CS1/3 | 9.25 ± 0.25 | 9.42 ± 0.14 | - | - | 12.17 ± 0.29 | 9.42 ± 0.14 |
| CE1/1 | 8.67 ± 0.29 | 8.75 ± 0.35 | - | - | 10.33 ± 0.29 | 8.33 ± 0.29 |
| AT8/5 | 13.50 ± 0.71 | 22.83 ± 1.15 | 7.17 ± 0.29 | 16.33 ± 0.58 | 13.17 ± 0.29 | 9.83 ± 0.76 |
| YCS1/1 | - | - | - | - | - | 7.00 ± 0.00 |
| YCS1/3 | - | - | - | - | - | 7.08 ± 0.14 |
| YON3/2 | - | - | - | 7.17 ± 0.29 | - | 7.25 ± 0.43 |
| Probiotic Properties | Probiotic Bacteria | Probiotic Yeast | ||||
|---|---|---|---|---|---|---|
| CS1/3 | CE1/1 | AT8/5 | YCS1/1 | YCS1/3 | YON3/2 | |
| Acid tolerance at 24 h | - | + | - | +++ | +++ | +++ |
| Bile salt tolerance at 24 h | ++ | ++ | + | +++ | +++ | +++ |
| Adhesion property | * | ** | * | *** | *** | * |
| Biofilm formation | - | - | ✓ | - | - | - |
| Isolates | Inhibition Zone (mm) for Fish Pathogenic Bacteria | ||||||
|---|---|---|---|---|---|---|---|
| AH | AS | AV | EI | ET | SA | ||
| CS1/3 | C | 7.17 ± 0.29 | 15.00 ± 1.00 | X | X | 7.17 ± 0.29 | 6.83 ± 0.29 |
| N | - | - | X | X | - | - | |
| CE1/1 | C | 8.67 ± 0.29 | 13.83 ± 0.76 | X | X | 10.33 ± 0.29 | 9.00 ± 0.50 |
| N | - | - | X | X | - | - | |
| AT8/5 | C | 10.33 ± 0.58 | 22.83 ± 1.15 | 7.00 ± 0.00 | 16.00 ± 0.00 | 11.67 ± 0.29 | 9.83 ± 0.29 |
| N | - | - | - | - | - | - | |
| YCS1/1 | C | X | X | X | X | X | - |
| N | X | X | X | X | X | - | |
| YCS1/3 | C | X | X | X | X | X | - |
| N | X | X | X | X | X | - | |
| YON3/2 | C | X | X | X | - | X | - |
| N | X | X | X | - | X | - | |
| Antibiotics | Isolates | |||||
|---|---|---|---|---|---|---|
| CS1/3 | CE1/1 | AT8/5 | YCS1/1 | YCS1/3 | YON3/2 | |
| Bacitracin | S | S | S | S | R | R |
| Cefpirome | R | R | R | R | R | R |
| Chloramphenicol | S | S | S | S | R | R |
| Clarithromycin | S | S | S | S | R | R |
| Penicillin | R | R | S | S | R | R |
| STX | R | R | R | S | R | R |
| Tetracycline | S | S | S | S | R | R |
| Vancomycin | R | R | R | S | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siangpro, N.; Chuakrut, S.; Sirimanapong, W.; Tanasupawat, S.; Phongsopitanun, W.; Meksiriporn, B.; Boonnorat, J.; Sarin, S.; Kucharoenphaibul, S.; Jutakanoke, R. Lactiplantibacillus argentoratensis and Candida tropicalis Isolated from the Gastrointestinal Tract of Fish Exhibited Inhibitory Effects against Pathogenic Bacteria of Nile Tilapia. Vet. Sci. 2023, 10, 129. https://doi.org/10.3390/vetsci10020129
Siangpro N, Chuakrut S, Sirimanapong W, Tanasupawat S, Phongsopitanun W, Meksiriporn B, Boonnorat J, Sarin S, Kucharoenphaibul S, Jutakanoke R. Lactiplantibacillus argentoratensis and Candida tropicalis Isolated from the Gastrointestinal Tract of Fish Exhibited Inhibitory Effects against Pathogenic Bacteria of Nile Tilapia. Veterinary Sciences. 2023; 10(2):129. https://doi.org/10.3390/vetsci10020129
Chicago/Turabian StyleSiangpro, Noppadon, Songkran Chuakrut, Wanna Sirimanapong, Somboon Tanasupawat, Wongsakorn Phongsopitanun, Bunyarit Meksiriporn, Jarungwit Boonnorat, Siripun Sarin, Siriwat Kucharoenphaibul, and Rumpa Jutakanoke. 2023. "Lactiplantibacillus argentoratensis and Candida tropicalis Isolated from the Gastrointestinal Tract of Fish Exhibited Inhibitory Effects against Pathogenic Bacteria of Nile Tilapia" Veterinary Sciences 10, no. 2: 129. https://doi.org/10.3390/vetsci10020129
APA StyleSiangpro, N., Chuakrut, S., Sirimanapong, W., Tanasupawat, S., Phongsopitanun, W., Meksiriporn, B., Boonnorat, J., Sarin, S., Kucharoenphaibul, S., & Jutakanoke, R. (2023). Lactiplantibacillus argentoratensis and Candida tropicalis Isolated from the Gastrointestinal Tract of Fish Exhibited Inhibitory Effects against Pathogenic Bacteria of Nile Tilapia. Veterinary Sciences, 10(2), 129. https://doi.org/10.3390/vetsci10020129





