Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine
Abstract
Simple Summary
Abstract
1. Introduction
2. Venom Source
3. Venom Constituents and Their Biological Activities
3.1. Melittin
3.2. Apamin
3.3. Mast Cell Degranulation Peptide
3.4. Adolapin
3.5. Phospholipase A2
3.6. Hyaluronidase
4. Collection of Bee Venom
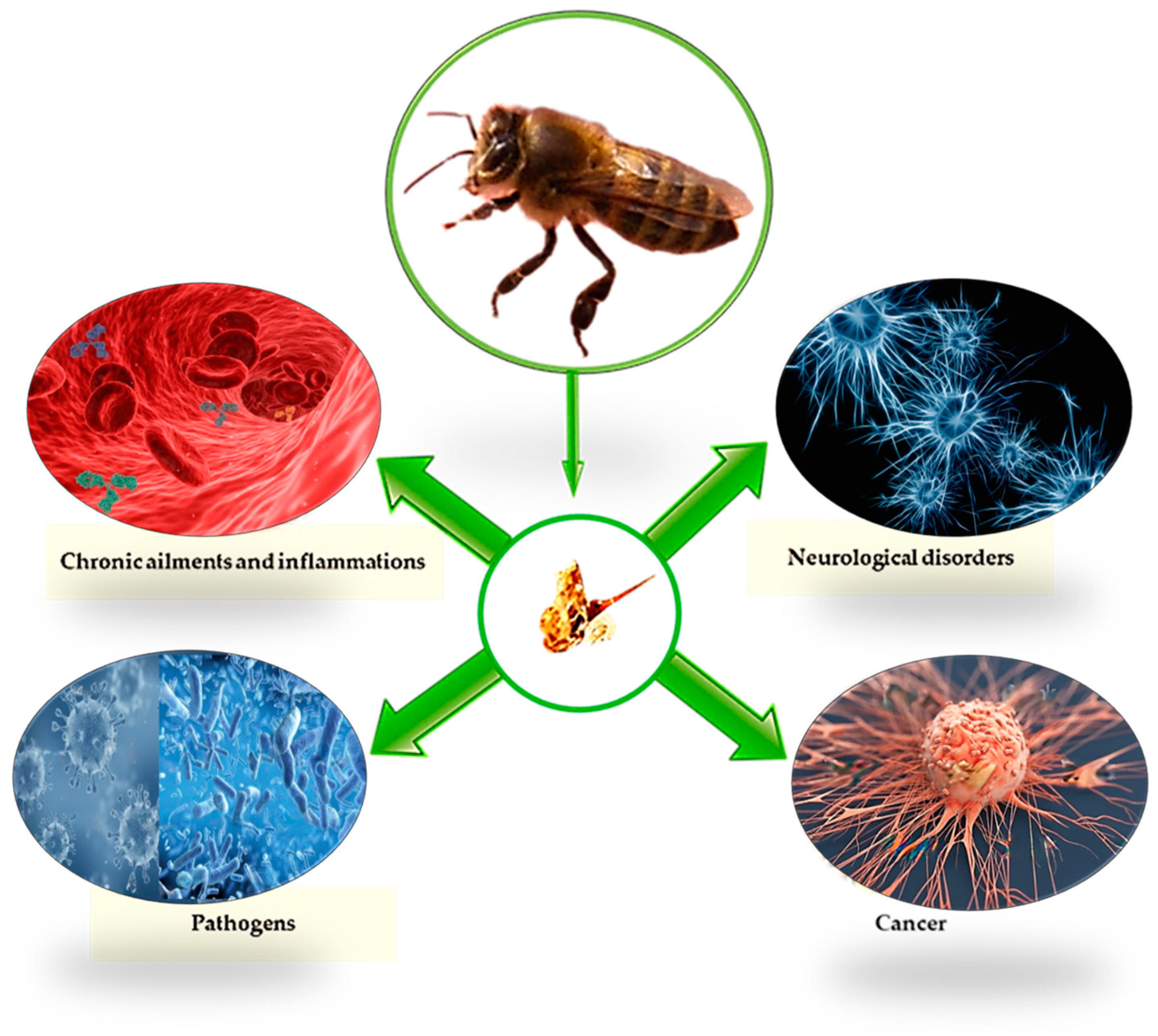
5. Effects and Applications in Veterinary Medicine
5.1. Antioxidant Activity
5.2. Antimicrobial and Antiviral Activity
5.3. Anti-Inflammatory Activity
5.4. Neurodegenerative Disorders
5.5. Anti-Cancer Activity
5.6. Activities on Other Diseases
6. Current Limits in Use
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In vitro evaluation of acute toxicity of five citrus spp. Essential oils towards the parasitic mite varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Castagna, F.; Bava, R.; Piras, C.; Carresi, C.; Musolino, V.; Lupia, C.; Marrelli, M.; Conforti, F.; Palma, E.; Britti, D. Green Veterinary Pharmacology for Honey Bee Welfare and Health: Origanum heracleoticum L.(Lamiaceae) Essential Oil for the Control of the Apis mellifera Varroatosis. Vet. Sci. 2022, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Morse, R.A.; Calderone, N.W. The Value of Honey Bees As Pollinators of U.S. Crops in 2000. Bee Cult. 2000, 128, 1–15. [Google Scholar]
- Abdela, N.; Jilo, K. Bee venom and its therapeutic values: A review. Adv. Life Sci. Technol. 2016, 44, 18–22. [Google Scholar]
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomed. Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Boukraâ, L. Bee products: The rediscovered antibiotics. Anti-Infect. Agents 2015, 13, 36–41. [Google Scholar] [CrossRef]
- Szweda, P.; Kot, B. Bee products and essential oils as alternative agents for treatment of infections caused by S. aureus. Front. Staphylococcus Aureus 2017, 203–223. [Google Scholar]
- Hellner, M.; Winter, D.; von Georgi, R.; Münstedt, K. Apitherapy: Usage and experience in German beekeepers. Evid. Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef]
- Münstedt, K. Bee products—An overview of their pharmacological properties and medicinal applications. Bee Prod. Their Appl. Food Pharm. Ind. 2022, 1–23. [Google Scholar]
- Gokulakrishnaa, R.; Thirunavukkarasu, S. Apitherapy: A valuable gift from honey bee. J. Entomol. Zool. Stud. 2020, 8, 2317–2323. [Google Scholar]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Bogdanov, S. Biological and therapeutic properties of bee venom. Bee Prod. Sci. 2016, 1–23. [Google Scholar]
- Clark, C.C. Encyclopedia of Complementary Health Practice P; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 0826117228. [Google Scholar]
- Crane, E. The past and present importance of bee products to man. In Bee Products; Springer: Berlin/Heidelberg, Germany, 1997; pp. 1–13. [Google Scholar]
- Krell, R. Value-Added Products from Beekeeping; Food and Agriculture Organization: Rome, Italy, 1996; ISBN 9251038198. [Google Scholar]
- Kwon, Y.; Lee, J.; Lee, H.; Han, H.; Mar, W.; Kang, S.; Beitz, A.J.; Lee, J. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef]
- Piek, T. Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1483263703. [Google Scholar]
- Schmidt, J.O. Toxinology of venoms from the honeybee genus Apis. Toxicon 1995, 33, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Tarpy, D.R.; Gilley, D.C.; Seeley, T.D. Levels of selection in a social insect: A review of conflict and cooperation during honey bee (Apis mellifera) queen replacement. Behav. Ecol. Sociobiol. 2004, 55, 513–523. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Zwiener, R.D. Immunology of bee venom. Clin. Rev. Allergy Immunol. 2018, 54, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Singletary, E.; Charlton, N. Methods of honey bee stinger removal: A systematic review of the literature. Cureus 2020, 12, e8078. [Google Scholar] [CrossRef]
- Lensky, Y.; Cassier, P. The alarm pheromones of queen and worker honey bees. Bee World 1995, 76, 119–129. [Google Scholar] [CrossRef]
- Annila, I.T.; Karjalainen, E.S.; Annila, P.A.; Kuusisto, P.A. Bee and wasp sting reactions in current beekeepers. Ann. Allergy Asthma Immunol. 1996, 77, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.T.; Flood, A.A. Hymenoptera stings. Clin. Technol. Small Anim. Pract. 2006, 21, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C. Honeybee venom: A rich source of pharmacologically active peptides. Endeavour 1988, 12, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Dotimas, E.M.; Hider, R.C. Honeybee venom. Bee world 1987, 68, 51–70. [Google Scholar] [CrossRef]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Gonçalves, J.; Galante, P. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef]
- Hoffman, D.R. Hymenoptera venom proteins. Nat. Toxins 2 1996, 169–186. [Google Scholar]
- Guralnick, M.W.; Mulfinger, L.M.; Benton, A.W. Collection and standardization of Hymenoptera venoms. Folia Allergol. Immunol. Clin 1986, 33, 9–18. [Google Scholar]
- White, J.; Meier, J. Handbook of Clinical Toxicology of Animal Venoms and Poisons; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1351443143. [Google Scholar]
- Habermann, E.; Jentsch, J. Sequenzanalyse des Melittins aus den tryptischen und peptischen Spaltstücken. Physiol. Chem. 1967, 348, 37–50. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Eisenberg, D. The structure of melittin. I. Structure determination and partial refinement. J. Biol. Chem. 1982, 257, 6010–6015. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Rudy, B. Interactions between membranes and cytolytic peptides. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1986, 864, 123–141. [Google Scholar] [CrossRef]
- Dempsey, C.E. The actions of melittin on membranes. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1990, 1031, 143–161. [Google Scholar] [CrossRef]
- Sansom, M.S. The biophysics of peptide models of ion channels. Prog. Biophys. Mol. Biol. 1991, 55, 139–235. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.; Habermann, E.; Hansen, H. Differentiation of two hemolytic factors in the bee’s venom. Naunyn. Schmiedebergs. Arch. Exp. Pathol. Pharmakol. 1953, 217, 130–143. [Google Scholar] [PubMed]
- Terwilliger, T.C.; Eisenberg, D. The structure of melittin. II. Interpret. J. Biol. Chem. 1982, 257, 6016–6022. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Kajita, S.; Iizuka, H. Melittin-induced alteration of epidermal adenylate cyclase responses. Acta Derm. Venereol. 1987, 67, 295–300. [Google Scholar]
- Zhang, S.; Chen, Z. Melittin exerts an antitumor effect on non-small cell lung cancer cells. Mol. Med. Rep. 2017, 16, 3581–3586. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.D.; Lee, G. Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models. Antioxidants 2021, 10, 1654. [Google Scholar] [CrossRef]
- Fennell, J.F.; Shipman, W.H.; Cole, L.J. Antibacterial action of melittin, a polypeptide from bee venom. Proc. Soc. Exp. Biol. Med. 1968, 127, 707–710. [Google Scholar] [CrossRef]
- Azam, M.N.K.; Ahmed, M.N.; Biswas, S.; Ara, N.; Rahman, M.M.; Hirashima, A.; Hasan, M.N. A review on bioactivities of honey bee venom. Annu. Res. Rev. Biol. 2018, 1–13. [Google Scholar] [CrossRef]
- Gu, H.; Han, S.M.; Park, K.-K. Therapeutic effects of apamin as a bee venom component for non-neoplastic disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Mourre, C.; Nehlig, A.; Lazdunski, M. Cerebral glucose utilization after administration of apamin, a toxin active on Ca2+-dependent K+ channels. Brain Res. 1988, 451, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, K.-H.; Lee, W.-R.; An, H.-J.; Lee, S.-J.; Han, S.-M.; Lee, K.-G.; Park, Y.-Y.; Kim, K.-S.; Lee, Y.-S. Apamin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration through suppressions of activated Akt and Erk signaling pathway. Vascul. Pharmacol. 2015, 70, 8–14. [Google Scholar] [CrossRef]
- Walde, P.; Jäckle, H.; Luisi, P.L.; Dempsey, C.J.; Banks, B.E.C. Spectroscopic investigations of peptide 401 from bee venom. Biopolym. Orig. Res. Biomol. 1981, 20, 373–385. [Google Scholar] [CrossRef]
- Hanson, J.M.; Morley, J.; Soria-Herrera, C. Anti-inflammatory property of 401 (MCD-peptide), a peptide from the venom of the bee Apis mellifera (L.). Br. J. Pharmacol. 1974, 50, 383. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Bellik, Y. Bee venom: Its potential use in alternative medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Koburova, K.L.; Michailova, S.G.; Shkenderov, S.V. Further investigation on the antiinflammatory properties of adolapin—Bee venom polypeptide. Acta Physiol. Pharmacol. Bulg. 1985, 11, 50–55. [Google Scholar] [PubMed]
- Shipolini, R.A.; Callewaert, G.L.; Cottrell, R.C.; Vernon, C.A. The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur. J. Biochem. 1974, 48, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, L.I. Heparin-induced inhibition of the hemolytic activity of bee venom. Uch. Gor’k. Gos. Univ 1974, 175, 130. [Google Scholar]
- Dudler, T.; Chen, W.-Q.; Wang, S.; Schneider, T.; Annand, R.R.; Dempcy, R.O.; Crameri, R.; Gmachl, M.; Suter, M.; Gelb, M.H. High-level expression in Escherichia coli and rapid purification of enzymatically active honey bee venom phospholipase A2. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1992, 1165, 201–210. [Google Scholar] [CrossRef]
- Hossen, M.; Gan, S.H.; Khalil, M. Melittin, a potential natural toxin of crude bee venom: Probable future arsenal in the treatment of diabetes mellitus. J. Chem. 2017, 2017, 4035626. [Google Scholar] [CrossRef]
- Marković-Housley, Z.; Miglierini, G.; Soldatova, L.; Rizkallah, P.J.; Müller, U.; Schirmer, T. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure 2000, 8, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Bala, E.; Hazarika, R.; Singh, P.; Yasir, M.; Shrivastava, R. A biological overview of Hyaluronidase: A venom enzyme and its inhibition with plants materials. Mater. Today Proc. 2018, 5, 6406–6412. [Google Scholar] [CrossRef]
- Markovic, O.; Mollnar, L. Isolation of and determination of bee venom. Chem. Zvesti 1954, 8, 80–90. [Google Scholar]
- Benton, A.W.; Morse, R.A.; Stewart, J.D. Venom collection from honey bees. Science 1963, 142, 228–230. [Google Scholar] [CrossRef]
- Fakhim-Zadeh, K. Improved device for venom extraction. Bee World 1998, 79, 52–56. [Google Scholar] [CrossRef]
- Simics, M. Commercial bee venom collection. Bee Biz 1998, 7, 19–20. [Google Scholar]
- Ali, M. Studies on bee venom and its medical uses. Int. J. Adv. Res. Technol. 2012, 1, 69–83. [Google Scholar]
- Krivtzov, N.; Lebedev, V. Bienenprodukte; Editing House: Niwa Niwa, Russia, 1995. [Google Scholar]
- Müller, U.; Fricker, M.; Wymann, D.; Blaser, K.; Crameri, R. Increased specificity of diagnostic tests with recombinant major bee venom allergen phospholipase A2. Clin. Exp. Allergy 1997, 27, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Khan, M.A.; Naz, R.; Zeb, S. Extraction of venom from honey bee in district swat, Khyber Pakhtunkhwa, Pakistan. Extraction 2019, 4. [Google Scholar]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee venom: An updating review of its bioactive molecules and its health applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Müller, U.; Akdis, C.A.; Fricker, M.; Akdis, M.; Blesken, T.; Bettens, F.; Blaser, K. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J. Allergy Clin. Immunol. 1998, 101, 747–754. [Google Scholar] [CrossRef]
- Gu, H.; Kim, W.; An, H.; Kim, J.; Gwon, M.; Han, S.M.; Leem, J.; Park, K. Therapeutic effects of bee venom on experimental atopic dermatitis. Mol. Med. Rep. 2018, 18, 3711–3718. [Google Scholar] [CrossRef]
- Skenderov, S.; Und Ivanov, T. Bienenprodukte Zemizdat Verlag, Sofia (Bulg). 1983.
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phyther. Res. 2021, 35, 743–750. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Niedbała, G.; Alqarni, M.; Zirintunda, G.; Ssempijja, F.; Musinguzi, S.P.; Usman, I.M.; Matama, K.; Hetta, H.F.; Mbiydzenyuy, N.E. Bee venom—A potential complementary medicine candidate for SARS-CoV-2 infections. Front. Public Health 2020, 8, 594458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-D.; Park, H.-J.; Chae, Y.; Lim, S. An overview of bee venom acupuncture in the treatment of arthritis. Evid. Based Complement. Altern. Med. 2005, 2, 79–84. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First characterization of the venom from apis mellifera syriaca, a honeybee from the middle east region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pavel, C.I.; Mărghitaş, L.A.; Dezmirean, D.S.; Tomoş, L.I.; Bonta, V.; Şapcaliu, A.; Buttstedt, A. Comparison between local and commercial royal jelly—Use of antioxidant activity and 10-hydroxy-2-decenoic acid as quality parameter. J. Apic. Res. 2014, 53, 116–123. [Google Scholar] [CrossRef]
- Rekka, E.; Kourounakis, L.; Kourounakis, P. Antioxidant activity of and interleukin production affected by honey bee venom. Arzneimittelforschung 1990, 40, 912–913. [Google Scholar] [PubMed]
- El-Hanoun, A.; El-Komy, A.; El-Sabrout, K.; Abdella, M. Effect of bee venom on reproductive performance and immune response of male rabbits. Physiol. Behav. 2020, 223, 112987. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, A.; El-Hanoun, A.; Abdella, M.; El-Sabrout, K. Improving the reproductive, immunity and health status of rabbit does using honey bee venom. J. Anim. Physiol. Anim. Nutr. 2021, 105, 975–983. [Google Scholar] [CrossRef] [PubMed]
- El-Speiy, M.; Elsawy, M.; Sadaka, T.; Elkomy, A.; Hassan, S. Impact of bee venom and oxytetracycline on blood parameters, antioxidant, immunity status and bacterial count of weaning rabbits. Egypt. J. Rabbit Sci. 2022, 32, 181–199. [Google Scholar] [CrossRef]
- Kim, D.; Han, S.; Choi, Y.-S.; Kang, H.-K.; Lee, H.-G.; Lee, K. Effects of dietary bee venom on serum characteristic, antioxidant activity and liver fatty acid composition in broiler chickens. Korean J. Poult. Sci. 2019, 46, 39–46. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Jasovský, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance—A threat to the world’s sustainable development. Ups. J. Med. Sci. 2016, 121, 159–164. [Google Scholar] [CrossRef]
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stärk, K.D.C.; Häsler, B. Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.C.; Weissman, L.; Eisenberg, D. The structure of melittin in the form I crystals and its implication for melittin’s lytic and surface activities. Biophys. J. 1982, 37, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yeo, J.; Baek, H.; Lin, S.-M.; Meyer, S.; Molan, P. Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J. Asian Nat. Prod. Res. 2009, 11, 796–804. [Google Scholar] [CrossRef]
- Socarras, K.M.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial activity of bee venom and melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef]
- Lubke, L.L.; Garon, C.F. The antimicrobial agent melittin exhibits powerful in vitro inhibitory effects on the Lyme disease spirochete. Clin. Infect. Dis. 1997, 25, S48–S51. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.; Yeo, J.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef]
- Issam, A.-A.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar]
- El-Seedi, H.; Abd El-Wahed, A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A. Antimicrobial properties of Apis mellifera’s bee venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Hwang, S.J.; Chenoweth, P.J.; Pak, S.C. Somatic cell count in milk of bee venom treated dairy cows with mastitis. J. ApiProduct ApiMed Sci. 2009, 1, 104–109. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jun, H.-K.; Kim, S.; You, M.-J.; Jun, M.-H.; Kim, D.-H. Therapeutic effect of injection-acupuncture with bee-venom (apitoxin) in cases of canine otitis externa. J. Vet. Clin. 2008, 25, 159–164. [Google Scholar]
- Perumal Samy, R.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.V.; Bow, H.; Yap, E.H.; Thong, T.W.J. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Boutrin, M.-C.; Foster, H.A.; Pentreath, V.W. The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp. Parasitol. 2008, 119, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, C.; Calzada, C.; Lagarde, M.; Schreével, J.; Deregnaucourt, C. Interplay between lipoproteins and bee venom phospholipase A2 in relation to their anti-plasmodium toxicity. J. Lipid Res. 2006, 47, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B. Antifungal activity of bee venom and sweet bee venom against clinically isolated Candida albicans. J. Pharmacopunct. 2016, 19, 45. [Google Scholar] [CrossRef]
- Hegazi, A.G.; El-Fadaly, H.A.; Barakat, A.M.; Abou-El-Doubal, S.K.A. In vitro effects of some bee products on T. gondii Tachyzoites. Glob. Vet. 2014, 13, 1043–1050. [Google Scholar]
- Uddin, M.B.; Lee, B.-H.; Nikapitiya, C.; Kim, J.-H.; Kim, T.-H.; Lee, H.-C.; Kim, C.G.; Lee, J.-S.; Kim, C.-J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Chaturvedi, P.K.; Chun, S.N.; Lee, Y.G.; Ahn, W.S. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line. Oncology Reports 2015, 33.4, 1675–1682. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.-M.; Kim, J.-H.; Cho, C.-W.; Jeon, J.-W.; Park, J.-K.; Lee, S.-H.; Jung, B.-G.; Lee, B.-J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018, 200, 40–51. [Google Scholar] [CrossRef]
- Scicutella, F.; Mannelli, F.; Daghio, M.; Viti, C.; Buccioni, A. Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review. Antibiotics 2021, 10, 1010. [Google Scholar] [CrossRef]
- Kim, D.-H.; Han, S.-M.; Keum, M.C.; Lee, S.; An, B.-K.; Lee, S.-R.; Lee, K.-W. Evaluation of bee venom as a novel feed additive in fast-growing broilers. Br. Poult. Sci. 2018, 59, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Oh, B.Y.; Kim, B.S.; Lee, W.; Baek, H.J.; Kim, S.T.; Hwang, S.J.; Pak, S.C. Effects of honeybee venom supplementation in drinking water on growth performance of broiler chickens. Poult. Sci. 2010, 89, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Hwang, S.J.; Jang, C.H.; Chenoweth, P.J.; Pak, S.C. Effects of bee venom treatment on growth performance of young pigs. Am. J. Chin. Med. 2009, 37, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Yang, E.J.; Lee, M.S.; Kim, Y.-S.; Huh, Y.; Cho, I.-H.; Kang, S.; Koh, H.-K. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int. J. Neurosci. 2011, 121, 209–217. [Google Scholar] [CrossRef]
- Yang, E.J.; Jiang, J.H.; Lee, S.M.; Yang, S.C.; Hwang, H.S.; Lee, M.S.; Choi, S.-M. Bee venom attenuates neuroinflammatory events and extends survival in amyotrophic lateral sclerosis models. J. Neuroinflamm. 2010, 7, 69. [Google Scholar] [CrossRef]
- Cai, M.; Lee, J.H.; Yang, E.J. Bee venom ameliorates cognitive dysfunction caused by neuroinflammation in an animal model of vascular dementia. Mol. Neurobiol. 2017, 54, 5952–5960. [Google Scholar] [CrossRef]
- Chung, E.S.; Lee, G.; Lee, C.; Ye, M.; Chung, H.; Kim, H.; Bae, S.S.; Hwang, D.-S.; Bae, H. Bee venom phospholipase A2, a novel Foxp3+ regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of Parkinson’s disease. J. Immunol. 2015, 195, 4853–4860. [Google Scholar] [CrossRef]
- Park, H.J.; Son, D.J.; Lee, C.W.; Choi, M.S.; Lee, U.S.; Song, H.S.; Lee, J.M.; Hong, J.T. Melittin inhibits inflammatory target gene expression and mediator generation via interaction with IκB kinase. Biochem. Pharmacol. 2007, 73, 237–247. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, G.-Y. Regulation of inflammatory cytokine production by bee venom in rat chondrocytes. J. Physiol. Pathol. Korean Med. 2011, 25, 132–137. [Google Scholar] [CrossRef]
- Moon, D.-O.; Park, S.-Y.; Lee, K.-J.; Heo, M.-S.; Kim, K.-C.; Kim, M.-O.; Lee, J.-D.; Choi, Y.H.; Kim, G.-Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007, 7, 1092–1101. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Schindler, J.F.; Monahan, J.B.; Smith, W.G. p38 pathway kinases as anti-inflammatory drug targets. J. Dent. Res. 2007, 86, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Nebreda, A.R. Roles of p38α mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015, 282, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, W.-R.; Kim, K.-H.; An, H.-J.; Chang, Y.-C.; Han, S.-M.; Park, Y.-Y.; Pak, S.C.; Park, K.-K. Effects of bee venom against Propionibacterium acnes-induced inflammation in human keratinocytes and monocytes. Int. J. Mol. Med. 2015, 35, 1651–1656. [Google Scholar] [CrossRef]
- Lee, W.-R.; Kim, K.-H.; An, H.-J.; Kim, J.; Chang, Y.-C.; Chung, H.; Park, Y.-Y.; Lee, M.-L.; Park, K. The protective effects of Melittin on Propionibacterium acnes–induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Shin, J.-M.; Bae, Y.-S.; Cho, H.-J.; Park, K.-K.; Choe, J.-Y.; Han, S.-M.; Moon, S.-K.; Kim, W.-J.; Choi, Y.H. Melittin has a chondroprotective effect by inhibiting MMP-1 and MMP-8 expressions via blocking NF-κB and AP-1 signaling pathway in chondrocytes. Int. Immunopharmacol. 2015, 25, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Oh, M.J.; Lee, D.H.; Lee, Y.S.; Lee, J.; Kim, D.-H.; Choi, C.-H.; Song, M.J.; Song, H.S.; Hong, J.T. Anti-inflammatory effect of bee venom in phthalic anhydride-induced atopic dermatitis animal model. Inflammopharmacology 2020, 28, 253–263. [Google Scholar] [CrossRef]
- Ozturk, A.B.; Bayraktar, R.; Gogebakan, B.; Mumbuc, S.; Bayram, H. Comparison of inflammatory cytokine release from nasal epithelial cells of non-atopic non-rhinitic, allergic rhinitic and polyp subjects and effects of diesel exhaust particles in vitro. Allergol. Immunopathol. 2017, 45, 473–481. [Google Scholar] [CrossRef]
- Lorenzetti, O.J. Influence of bee venom in the adjuvant-induced arthritic rat model. Res. Commun. Chem. Pathol. Pharmacol. 1972, 4, 339–352. [Google Scholar]
- Zurier, R.B.; Mitnick, H.; Bloomgarden, D.; Weissmann, G. Effect of bee venom on experimental arthritis. Ann. Rheum. Dis. 1973, 32, 466. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Bliven, M.L. Anti-arthritic effect of bee venom. Agents Actions 1979, 9, 205–211. [Google Scholar] [CrossRef]
- Eiseman, J.L.; Von Bredow, J.; Alvares, A.P. Effect of honeybee (Apis mellifera) venom on the course of adjuvant-induced arthritis and depression of drug metabolism in the rat. Biochem. Pharmacol. 1982, 31, 1139–1146. [Google Scholar] [CrossRef]
- Vick, J.A.; Mehlman, B.; Brooks, R.; Phillips, S.J.; Shipman, W. Effect of bee venom and melittin on plasma cortisol in the unanesthetized monkey. Toxicon 1972, 10, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Vick, J.A.; Warren, G.B.; Brooks, R.B. The effect of treatment with whole bee venom on cage activity and plasma cortisol levels in the arthritic dog. Inflammation 1976, 1, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Short, T.; Jackson, R.; Beard, G. Usefulness of bee venom therapy in canine arthritis. NAAS Proc. 1979, 2, 13–17. [Google Scholar]
- Jeong, C.H.; Cheng, W.N.; Bae, H.; Lee, K.W.; Han, S.M.; Petriello, M.C.; Lee, H.G.; Seo, H.G.; Han, S.G. Bee venom decreases LPS-induced inflammatory responses in bovine mammary epithelial cells. J. Microbiol. Biotechnol. 2017, 27, 1827–1836. [Google Scholar] [CrossRef]
- Von Bredow, J.; Bradford, C.; Froehlich, H.; Vick, J. Treatment of Equine Arthritis with Bee Venom. Proc. Apiotherapy Conf. 1978, 76–79. [Google Scholar]
- Kim, D.-H.; Liu, J.-Z.; Choi, S.-H.; MacManus, P.; Jennings, P.; Darcy, K.; Burke, F.; Leorald, N.; Rogers, P.A.M. Acupuncture treatment in a case with equine laminitis. J. Vet. Clin. 2006, 23, 6–8. [Google Scholar]
- Mizrahi, A.; Fulder, S.; Sheinman, N.; Sheinman, N. Potentiating Health and the Crisis of the Immune System; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; ISBN 0306456028. [Google Scholar]
- Mohammadi-Rad, M.; Ghasemi, N.; Aliomrani, M. Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Res. Pharm. Sci. 2019, 14, 424. [Google Scholar]
- Tsai, L.-C.; Lin, Y.-W.; Hsieh, C.-L. Effects of bee venom injections at acupoints on neurologic dysfunction induced by thoracolumbar intervertebral disc disorders in canines: A Randomized, Controlled Prospective Study. Biomed Res. Int. 2015, 2015, 363801. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Oh, H.; Han, J.; Lee, H.; Jeong, S.-M.; Choi, S.-H.; Kim, C.M.-H.; Kim, D.-H. Therapeutic effect of bee-venom and dexamethasone in dogs with facial nerve paralysis. J. Vet. Clin. 2007, 24, 503–508. [Google Scholar]
- Sung, H.-J.; Park, H.-M. Therapeutic Trial of Bee Venom Acupuncture for Idiopathic Facial Paralysis in a Dog. J. Vet. Clin. 2013, 30, 107–110. [Google Scholar]
- Li, D.; Chung, G.; Kim, S.K. The involvement of central noradrenergic pathway in the analgesic effect of bee venom acupuncture on vincristine-induced peripheral neuropathy in rats. Toxins 2020, 12, 775. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Lee, J.-D.; Park, D.-S. The Anti-Cancer Effect of Apamin in Bee-Venom on Melanoma cell line SK-MEL-2 and Inhibitory Effect on the MAP-Kinase Signal Pathway. J. Acupunct. Res. 2001, 18, 101–115. [Google Scholar]
- Hait, W.N.; Grais, L.; Benz, C.; Cadman, E.C. Inhibition of growth of leukemic cells by inhibitors of calmodulin: Phenothiazines and melittin. Cancer Chemother. Pharmacol. 1985, 14, 202–205. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Ip, S.; Chu, Y.; Yu, C.; Chen, P.; Ho, H.; Yang, J.; Huang, H.; Chueh, F.; Lai, T.; Chung, J. Bee venom induces apoptosis through intracellular Ca2+-modulated intrinsic death pathway in human bladder cancer cells. Int. J. Urol. 2012, 19, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Hsieh, C.-L. Clinical applications of bee venom acupoint injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Jeong, Y.-J.; Park, K.-K.; Park, Y.-Y.; Chung, I.-K.; Lee, K.-G.; Yeo, J.-H.; Han, S.-M.; Bae, Y.-S.; Chang, Y.-C. Bee venom suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and NF-κB-dependent mechanisms. J. Ethnopharmacol. 2010, 127, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-E.; Baek, Y.-H.; Lee, M.-H.; Choi, D.-Y.; Park, D.-S.; Lee, J.-D. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010, 292, 98–110. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef]
- Lee, C.; Bae, S.-J.S.; Joo, H.; Bae, H. Melittin suppresses tumor progression by regulating tumor-associated macrophages in a Lewis lung carcinoma mouse model. Oncotarget 2017, 8, 54951. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Molecular details on the intermediate states of melittin action on a cell membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2234–2241. [Google Scholar] [CrossRef]
- Soliman, C.; Eastwood, S.; Truong, V.K.; Ramsland, P.A.; Elbourne, A. The membrane effects of melittin on gastric and colorectal cancer. PLoS ONE 2019, 14, e0224028. [Google Scholar] [CrossRef]
- Daniluk, K.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Strojny, B.; Szczepaniak, J.; Bałaban, J.; Sosnowska, M.; Chwalibog, A.; Sawosz, E. Use of selected carbon nanoparticles as melittin carriers for MCF-7 and MDA-MB-231 human breast cancer cells. Materials 2019, 13, 90. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Abd-Elhakim, Y.M.; Ismail, S.A.A. Involvement of the anti-inflammatory, anti-apoptotic, and anti-secretory activity of bee venom in its therapeutic effects on acetylsalicylic acid-induced gastric ulceration in rats. Toxicology 2019, 419, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Kim, J.H.; Jo, M.; Xue, C.; kyu Park, J.; Lee, J.K. Bee wax coated water-soluble fraction of bee venom improved altered glucose homeostasis in streptozotocin-induced diabetic rats. J. Tradit. Chin. Med. 2019, 39, 842. [Google Scholar]
- Kim, J.-Y.; Leem, J.; Hong, H.-L. Melittin ameliorates endotoxin-induced acute kidney injury by inhibiting inflammation, oxidative stress, and cell death in mice. Oxid. Med. Cell. Longev. 2021, 2021, 8843051. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, S.-J.; Maeng, Y.-I.; Leem, J.; Park, K.-K. Protective effects of bee venom against endotoxemia-related acute kidney injury in mice. Biology 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Leem, J.; Park, K.-K. Antioxidative, antiapoptotic, and anti-inflammatory effects of apamin in a murine model of lipopolysaccharide-induced acute kidney injury. Molecules 2020, 25, 5717. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yim, B.K.; Lee, J.-H.; Lee, S.; Kim, T.-H. Risk associated with bee venom therapy: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126971. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.J.; Armstrong, P.J. Bee sting envenomation resulting in secondary immune-mediated hemolytic anemia in two dogs. J. Am. Vet. Med. Assoc. 1999, 214, 1026–1027. [Google Scholar]
- Nair, R.; Riddle, E.A.; Thrall, M.A. Hemolytic anemia, spherocytosis, and thrombocytopenia associated with honey bee envenomation in a dog. Vet. Clin. Pathol. 2019, 48, 620–623. [Google Scholar] [CrossRef]
- Kaplinsky, E.; Ishay, J.; Ben-Shachar, D.; Gitter, S. Effects of bee (Apis mellifera) venom on the electrocardiogram and blood pressure. Toxicon 1977, 15, 251–256. [Google Scholar] [CrossRef]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef]
| Substance | % | Substance | % |
|---|---|---|---|
| Enzymes | Biogenic amines | ||
| Phospholipase A2 | 10–12 | Histamine | 0.5–2 |
| Hyaluronidase | 1.5–2 | Dopamine | 0.2–1 |
| Phosphatase, glucosidase | 1-2 | Noradrenaline | 0.1–0.5 |
| Proteins | Carbohydrates | ||
| Mast cell degranulating Peptide | 1–2 | Sugar (glucose, fructose) | 2 |
| Melittin | 40–50 | Phospholipids | 5 |
| Amino acids | |||
| Secapine | 0.5 | Aminobutyric acid and α-amino acids | 0.4 |
| Tertiapine, apamin, procamine | 2–5 | Volatile substances (pheromones) | 4–8 |
| Other small peptides | 13–15 | Mineral substances | 3–4 |
| Components | Effect |
|---|---|
| Melittin | Peptide with biological activity. Melittin prevents blood from clotting, works well against germs, shields against radiation. Melittin works as an anti-inflammatory in small dosages. It has a haemolytic action and is clearly cytotoxic. |
| Phospholipase A2 | Phospholipase is the most important allergen and therefore the most harmful component of bee venom. |
| Hyaluronidase | It is an enzyme that allows venom to enter tissues and causes blood vessels to widen and tissues to become more permeable, increasing blood flow. |
| Acid phosphatase | Allergen. |
| Apamin | Biologically active peptide; a neurotoxin. |
| Mast cell degranulating peptide | Peptide that degranulates mast cells by releasing biogenic amines. |
| Protease inhibitor | It has anti-inflammatory and hemorrhagic properties and inhibits the action of various proteases, including trypsin, chymotrypsin, plasmin, and thrombin. |
| Adolapin | Anti-inflammatory, anti-rheumatic, and analgesic. |
| Histamine | It dilates blood vessels and increases capillary permeability. It is an allergen. |
| Dopamine, noradrenaline | Neurotransmitters that affect the behaviour and physiology of the senses. |
| Alarm pheromone | It puts the colony on high alert. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bava, R.; Castagna, F.; Musella, V.; Lupia, C.; Palma, E.; Britti, D. Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine. Vet. Sci. 2023, 10, 119. https://doi.org/10.3390/vetsci10020119
Bava R, Castagna F, Musella V, Lupia C, Palma E, Britti D. Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine. Veterinary Sciences. 2023; 10(2):119. https://doi.org/10.3390/vetsci10020119
Chicago/Turabian StyleBava, Roberto, Fabio Castagna, Vincenzo Musella, Carmine Lupia, Ernesto Palma, and Domenico Britti. 2023. "Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine" Veterinary Sciences 10, no. 2: 119. https://doi.org/10.3390/vetsci10020119
APA StyleBava, R., Castagna, F., Musella, V., Lupia, C., Palma, E., & Britti, D. (2023). Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine. Veterinary Sciences, 10(2), 119. https://doi.org/10.3390/vetsci10020119










