Effects of Spirulina platensis and/or Allium sativum on Antioxidant Status, Immune Response, Gut Morphology, and Intestinal Lactobacilli and Coliforms of Heat-Stressed Broiler Chicken
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Garlic Powder and Spirulina platensis
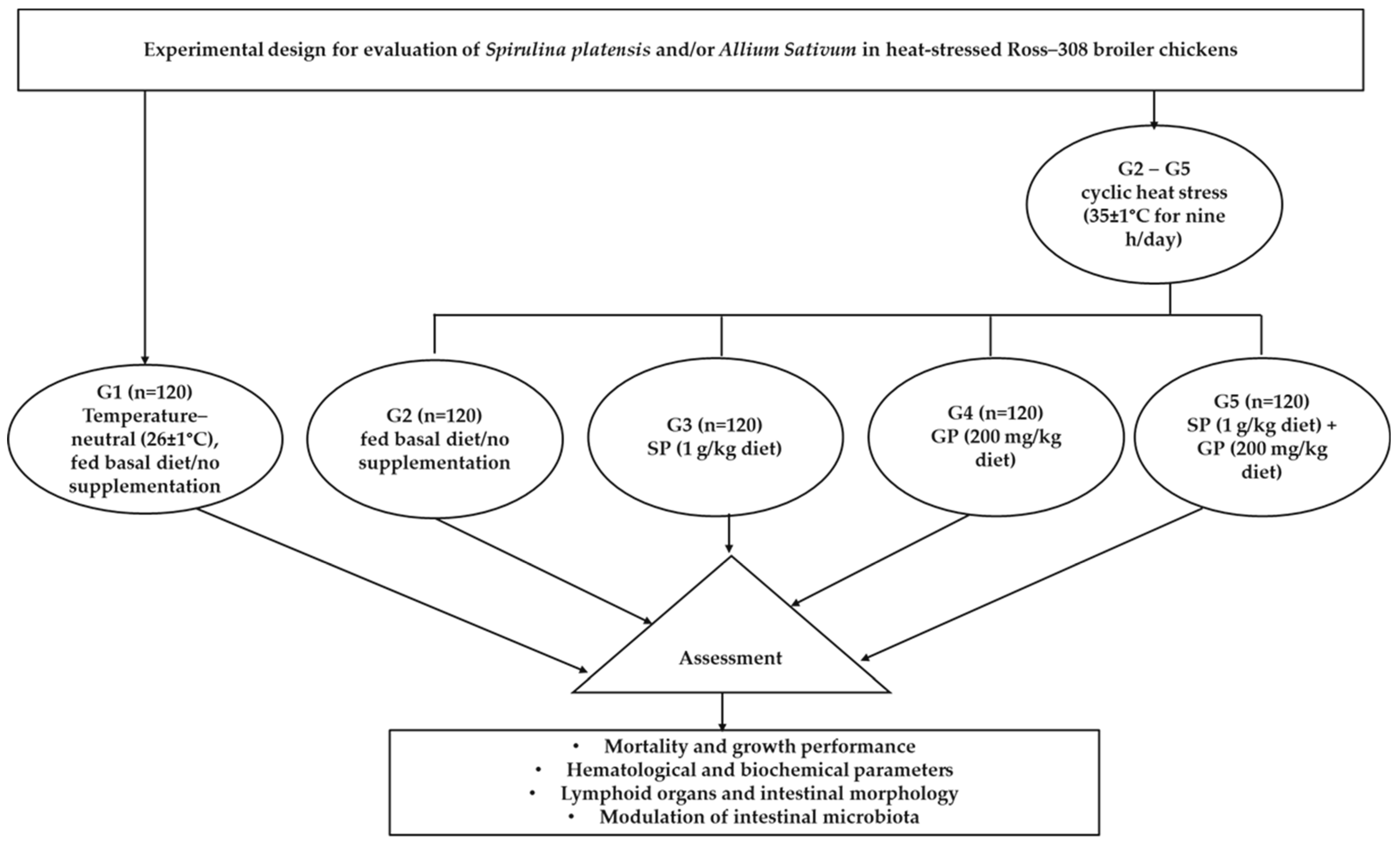
2.2. Chickens and Experimental Design
2.3. Assessment Parameters
2.3.1. Mortality and Growth Performance
2.3.2. Hematogram and Biochemical Parameters
2.3.3. Lymphoid Organs and Intestinal Morphology
2.3.4. Modulatory Effect on Lactobacilli and Total Coliforms Microflora
2.4. Statistical Analysis
3. Results
3.1. Analysis of S. platensis
3.2. Performance Parameters
3.3. Lymphoid Organ Weights and Hematological Blood Variables
3.4. Serum Metabolites and Oxidative Blood Markers
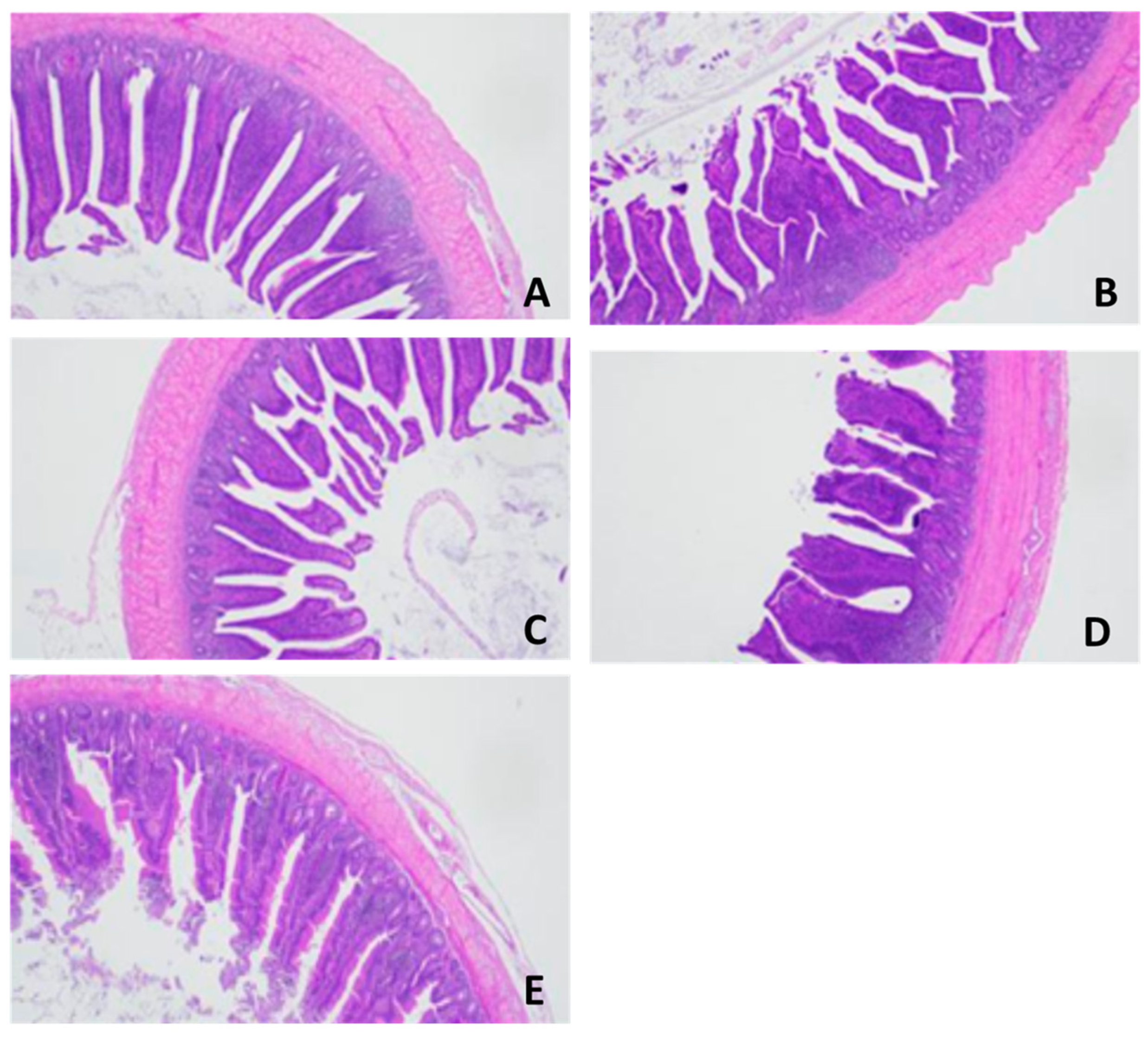
3.5. Intestinal Microbiota and Morphology
3.6. Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basiouni, S.; Tellez-Isaias, G.; Latorre, D.G.; Graham, D.B.; Petrone-Garcia, M.W.; El-Sweedi, H.; Yalçın, S.; Wahab, A.; Visscher, C.; May-Simera, L.M.; et al. Anti-Inflammatory and antioxidative phytogenic substances against secret killers in poultry: Current Status and Prospects. Vet. Res. 2022, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Attia, Y.; Khafaga, A.F.; Farooq, M.Z.; El-Seedi, H.R.; Eisenreich, W.; Tellez-Isaias, G. Restoring healthy gut microbiome in poultry using alternative feed additives with particular attention to phytogenic substances: Challenges and prospects. Ger. J. Vet. Res. 2022, 2, 32–42. [Google Scholar] [CrossRef]
- Tellez-Isaias, G.; Eisenreich, W.; Shehata, A.A. Nutraceuticals to mitigate the secret killers in animals. Vet. Sci. 2022, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Isaias, G.; Eisenreich, W.; Petrone-Garcia, V.M.; Hernandez-Velasco, X.; Castellanos-Huerta, C.-H.; Tellez, G., Jr.; Latorre, J.D.; Bottje, W.G.; Senas-Cuesta, R.; Coles, M.E.; et al. Effects of chronic stress and intestinal inflammation on commercial poultry health and performance: A Review. Ger. J. Vet. Res. 2023, 3, 38–57. [Google Scholar] [CrossRef]
- Settar, P.; Yalcin, S.; Turkmut, L.; Ozkan, S.; Cahanar, A. Season by Genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999, 78, 1353–1358. [Google Scholar] [CrossRef]
- Lara, L.; Rostagno, M. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Monson, M.S.; Van Goor, A.G.; Ashwell, C.M.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. Immunomodulatory effects of heat stress and lipopolysaccharide on the bursal transcriptome in two distinct chicken lines. BMC Genom. 2018, 19, 643. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat Stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Abo Ghanima, M.M.; Abd El-Hack, M.E.; Othman, S.I.; Taha, A.E.; Allam, A.A.; Eid Abdel-Moneim, A.-M. Impact of different rearing systems on growth, carcass traits, oxidative stress biomarkers, and humoral immunity of broilers Exposed to Heat Stress. Poult. Sci. 2020, 99, 3070–3078. [Google Scholar] [CrossRef]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Furlan, R.L.; Macari, M.; Malheiros, E.B.; Secato, E.R.; Guerreiro, J.R. Effect of indomethacin on hyperthermia induced by heat stress in broiler chickens. Int. J. Biometeorol. 1998, 42, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Le, H.H. Deleterious effects of heat stress on poultry production: Unveiling the benefits of betaine and polyphenols. Poultry 2022, 1, 147–156. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Abd El-Hac, M.E.; Dhama, K. Nutritional and healthical aspects of Spirulina (arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2015, 12, 36–51. [Google Scholar] [CrossRef]
- Hassan, F.; Mobarez, S.; Mohamed, M.; Attia, Y.; Mekawy, A.; Mahrose, K. Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.A.; Hassan, E.A.; Abou-Shehema, B.; Abu El-Hassan, S.; El-Gbaly, M.; Assar, M.; Morsy, S.; Zayed, S.; Gorgy, M. the protective role of algae (Spirulina platensis) and Garlic (Allium sativum), and their combination on alleviation of heat stress in broiler chicken. JKAU Met. Env. Arid. Land. Agric. Sci. 2023, 32, 117–145. [Google Scholar]
- Calella, P.; Di Dio, M.; Cerullo, G.; Di Onofrio, V.; Gallé, F.; Liguori, G. Antioxidant, immunomodulatory, and anti-inflammatory effects of spirulina in disease conditions: A systematic review. Int. J. Food Sci. Nutr. 2022, 73, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Ahmed, A.; Ijaz, H.; Abushouk, A.I.; Ahmed, H.; Negida, A.; Aleya, L.; Bungau, S.G. Influence of Spirulina platensis and ascorbic acid on amikacin-induced nephrotoxicity in rabbits. Environ. Sci. Pollut. Res. 2019, 26, 8080–8086. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Khafaga, A.F.; Abd El-Hack, M.E.; Al-Gabri, N.A.; Abukhalil, M.H.; Alfwuaires, M.A.; Bin-Jumah, M.; Alkahtani, S.; Abdel-Daim, M.M.; Aleya, L.; et al. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti- inflammatory, and immune stimulatory properties. Sci. Total Environ. 2020, 701, 134879. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.-M.E.; Selim, D.A.; Basuony, H.A.; Sabic, E.M.; Saleh, A.A.; Ebeid, T.A. Effect of dietary supplementation of bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 2020, 52, 671–680. [Google Scholar] [CrossRef]
- Gernat, A.A.; Santos, F.B.O.; Grimes, J.L. Alternative Approaches to antimicrobial use in the turkey industry: Challenges and perspectives. Ger. J. Vet. Res. 2021, 1, 37–47. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Lee, H.-J.; Yoon, D.-K.; Ji, D.-S.; Kim, J.-H.; Lee, C.-H. Antioxidant and Antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Adjei-Mensah, B.; Koranteng, A.A.A.; Hamidu, J.A.; Tona, K. Antibacterial activities of garlic (Allium sativum) in broiler and laying hens production. World’s Poult. Sci. J. 2023, 79, 155–176. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Ibrahim, N.S.; Shehata, A.M.; Mohamed, N.G.; Abdel-Moneim, A.-M.E. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53, 115. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Kamasani, J.R.; Mudili, V. Assessment of detoxification efficacy of irradiation on zearalenone mycotoxin in various fruit juices by response surface methodology and elucidation of its in-vitro toxicity. Front. Microbiol. 2018, 9, 2937. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and dpph-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Nusairat, B.; Odetallah, N.; Wang, J.-J. Live performance and microbial load modulation of broilers fed a direct-fed microbials (DFM) and xylanase combination. Vet. Sci. 2022, 9, 142. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Jalukar, S.; Oppy, J.; Rath, N.C.; Packialakshmi, B. The effects of yeast feed supplementation on turkey performance and pathogen colonization in a transport stress/Escherichia coli challenge. Poult. Sci. 2013, 92, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Azhar, K.; Zulkifli, I.; Bejo, M.H.; Kamyab, A. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poult. Sci. 2012, 91, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Tarabees, R.; Gafar, K.M.; EL-Sayed, M.S.; Shehata, A.A.; Ahmed, M. Effects of dietary supplementation of probiotic mix and prebiotic on growth performance, cecal microbiota composition, and protection against Escherichia coli O78 in broiler chickens. Probiotics Antimicrob. Proteins 2019, 11, 981–989. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS/STAT User’s Guide: Statistics; SAS Institute Inc.: Gary, IN, USA, 2004. [Google Scholar]
- Lawson, L.D.; Bauer, R. (Eds.) Phytomedicines of Europe: Chemistry and Biological Activity; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; Volume 691, ISBN 978-0-8412-3559-5. [Google Scholar]
- Khan, S.; Mobashar, M.; Mahsood, F.K.; Javaid, S.; Abdel-Wareth, A.A.; Ammanullah, H.; Mahmood, A. Spirulina inclusion levels in a broiler ration: Evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020, 52, 3233–3240. [Google Scholar] [CrossRef]
- Chaudhary, A.; Mishra, P.; Amaz, S.A.; Mahato, P.L.; Das, R.; Jha, R.; Mishra, B. Dietary supplementation of microalgae mitigates the negative effects of heat stress in broilers. Poult. Sci. 2023, 102, 102958. [Google Scholar] [CrossRef]
- Mishra, P.; Das, R.; Chaudhary, A.; Mishra, B.; Jha, R. Effects of microalgae, with or without xylanase supplementation, on growth performance, organs development, and gut health parameters of broiler chickens. Poult. Sci. 2023, 102, 103056. [Google Scholar] [CrossRef]
- Mirzaie, S.; Zirak-Khattab, F.; Hosseini, S.A.; Donyaei-Darian, H. Effects of Dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018, 31, 556–563. [Google Scholar] [CrossRef]
- Siegel, H.S. Physiological Stress in Birds. Bio. Sci. 1995, 30, 529–533. [Google Scholar] [CrossRef]
- Rehman, Z.; Munir, M.T. Effect of Garlic on the Health and Performance of Broilers. Vet. J. 2015, 3, 32–39. [Google Scholar]
- Zhang, H.Q.; Lin, A.P.; Sun, Y.; Deng, Y.M. Chemo- and radio-protective effects of polysaccharide of Spirulina platensis on hemopoietic system of mice and dogs. Acta Pharmacol. Sin. 2001, 22, 1121–1124. [Google Scholar]
- Huang, Z.; Mei, X.; Xu, D.; Xu, S.; Lv, J. Protective effects of polysacchride of Spirulina platensis and Sargassum thunbeergii on vascular of alloxan induced diabetic rats. Zhongguo Zhong Yao Za Zhi 2005, 30, 211–215. [Google Scholar]
- Mohamed, F. Nutrition and Immunity on Poultry. Egy. Poult. Sci. 1998, 443–448. [Google Scholar]
- Eid, K.M.; Iraqi, M.M. Effect of Garlic Powder on Growth Performance and Immune Response for Newcastle and Avian Influenza Virus Diseases in Broiler of Chickens; Benha University: Benha, Egypt, 2014; pp. 8–12. [Google Scholar]
- Dorhoi, A.; Dobrean, V.; Zăhan, M.; Virag, P. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother. Res. 2006, 20, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A Review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased fat synthesis and limited apolipoprotein b cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Colla, L.M.; Muccillo-Baisch, A.L.; Costa, J.A.V. Spirulina platensis effects on the levels of total cholesterol, HDL and triacylglycerols in rabbits fed with a hypercholesterolemic diet. Braz. Arch. Biol. Technol. 2008, 51, 405–411. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Maulik, S.K. Effect of garlic on cardiovascular disorders: A review. Nutr. J. 2002, 1, 4. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Chowdhury, S.D.; Smith, T.K. Effects of dietary garlic on cholesterol metabolism in laying hens. Poult. Sci. 2002, 81, 1856–1862. [Google Scholar] [CrossRef]
- Gebhardt, R.; Beck, H.; Wagner, K.G. Inhibition of cholesterol biosynthesis by allicin and ajoene in rat hepatocytes and HepG2 cells. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1994, 1213, 57–62. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of heat-shock proteins mediates chicken cell survival against thermal stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.I.; Kim, I.H. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018, 97, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Pourali, M.; Kermanshahi, H.; Golian, A.; Razmi, G.R.; Soukhtanloo, M. Antioxidant and anticoccidial effects of garlic powder and sulfur amino acids on eimeria-infected and uninfected broiler chickens. IJVR 2014, 15, 227–232. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Nazareno, M.A.; Fusari, C.M.; Camargo, A.B. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017, 220, 219–224. [Google Scholar] [CrossRef]
- Song, J.; Jiao, L.F.; Xiao, K.; Luan, Z.S.; Hu, C.H.; Shi, B.; Zhan, X.A. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed. Sci. Technol. 2013, 185, 175–181. [Google Scholar] [CrossRef]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, M.; Singh, N.; Kaur, P.; Sethi, A.P.S.; Sikka, S.S. Effect of sun dried whole bulb garlic powder on nutrient utilization, blood parameters, duodenum morphology and faecal microbial load in broiler chickens. Indian. J. Anim. Sci. 2017, 87, 195–198. [Google Scholar] [CrossRef]
- Mariey, Y.; Samak, H.; Ibrahem, M. Effect of using Spirulina platensis algae as a feed additive for poultry diets: 1-productive and reproductive performances of local laying hens. Egypt. Poult. Sci. J. 2012, 32, 201–215. [Google Scholar]
- Mariey, Y.A.; Samak, H.R.; Abou-Khashba, H.A.; Sayed, M.A.M.; Abou-Zeid, A.E. Effect of using Spirulina platensis Algae as a feed additives for poultry diets. Egypt. Poult. Sci. J. 2014, 34, 245–258. [Google Scholar]
- Cai, B.; Yi, X.; Han, Q.; Pan, J.; Chen, H.; Sun, H.; Wan, P. Structural characterization of oligosaccharide from spirulina platensis and its effect on the faecal microbiota in vitro. Food Sci. Hum. Wellness 2022, 11, 109–118. [Google Scholar] [CrossRef]
- Pradhan, J.; Das, B.K.; Sahu, S.; Marhual, N.P.; Swain, A.K.; Mishra, B.K.; Eknath, A.E. Traditional antibacterial activity of freshwater microalga Spirulina platensis to aquatic pathogens. Aquac. Res. 2012, 43, 1287–1295. [Google Scholar] [CrossRef]
- Oleforuh-Okoleh, V.U.; Chukwu, G.C.; Adeolu, A.I. Effect of ground ginger and garlic on the growth performance, carcass quality and economics of production of broiler chickens. Glob. J. Biosci. Biotechnol. 2014, 3, 225–229. [Google Scholar]
- Rahimi, S.; Zadeh, Z.T.; Torshizi, M.A.K.; Omidbaigi, R.; Rokni, H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Tech. 2011, 13, 527–539. [Google Scholar]
- Kaoud, H.A. Effect of Spirulina platensis as a dietary supplement on broiler performance in comparison with prebiotics. Spec. J. Biol. Sci. 2015, 1, 1–6. [Google Scholar]
- Song, D.; Liu, X.; Diao, Y.; Sun, Y.; Gao, G.; Zhang, T.; Chen, K.; Pei, L. Hydrogen-rich solution against myocardial injury and aquaporin expression via the pi3k/akt signaling pathway during cardiopulmonary bypass in rats. Mol. Med. Rep. 2018, 18, 1925–1938. [Google Scholar] [CrossRef]
- Lamm, D.L.; Riggs, D.R. Enhanced immunocompetence by garlic: Role in bladder cancer and other malignancies. J. Nutr. 2001, 131, 1067S–1070S. [Google Scholar] [CrossRef]
- Abbas, A.O.; Gehad, A.E.; Iii, G.L.H.; Gharib, H.B.A.; Mashaly, M.M. The effect of lighting program and melatonin on the alleviation of the negative impact of heat stress on the immune response in broiler chickens. Int. J. Poult. Sci. 2007, 6, 651–660. [Google Scholar] [CrossRef][Green Version]
| Ingredients (%) | Starter Grower (1–21 d) | Finisher (22–35 d) |
|---|---|---|
| Yellow corn | 54.40 | 62.00 |
| Soybean meal 44% | 27.00 | 24.05 |
| Corn gluten meal 60% | 10.00 | 6.19 |
| Soybean oil | 4.55 | 4.00 |
| Limestone | 1.10 | 1.00 |
| Dicalcium phosphate | 2.20 | 2.05 |
| Vitamins and minerals premix 1 | 0.30 | 0.30 |
| DL-methionine | 0.05 | 0.01 |
| L-lysine (HCl) | 0.15 | 0.15 |
| NaCl | 0.25 | 0.25 |
| Total | 100 | 100 |
| Calculated analysis 2 | ||
| Crude protein (%) | 23.03 | 20.02 |
| Metabolizable energy (kcal/kg) | 3204 | 3201 |
| Calcium (%) | 1.05 | 0.97 |
| Available phosphorus (%) | 0.45 | 0.42 |
| Lysine (%) | 1.14 | 1.03 |
| Methionine (%) | 0.52 | 0.41 |
| Total sulfur amino acids (%) | 0.90 | 0.73 |
| Component | Content |
|---|---|
| Total phenols, mg gallic acid equivalent/100 g | 1585 |
| Total flavonoids, mg quercetin equivalent/100 g | 175 |
| DPPH scavenging activity (%) * | 90 |
| Total antioxidant capacity (mg ascorbic acid equivalent/100 g) | 46.50 |
| Parameters Traits ParaP | G1 (Negative Control) | Groups Raised under Heat Stress | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | ||||
| Survival % | 100.0 a | 80.0 c | 98.3 b | 98.3 b | 100.0 a | 0.008 | <0.001 |
| Final body weight (BW) * | 2135 a | 1850 c | 2035 b | 2020 b | 2100 a | 25.22 | 0.005 |
| Daily feed intake (DFI) * | 108 | 103.4 | 104.3 | 104.1 | 105.7 | 10.22 | 0.058 |
| Feed conversion ratio (FCR) * | 1.80 b | 2.00 a | 1.83 b | 1.84 b | 1.80 b | 0.257 | 0.028 |
| EPEI ** | 338.1 a | 229.9 c | 311.5 b | 310.4 b | 334.1 a | 4.528 | 0.005 |
| Parameters | G1 | Groups Raised under Heat Stress | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | |||||
| Lymphoid organs | Bursa (%) | 0.196 a | 0.135 c | 0.168 b | 0.177 b | 0.169 b | 0.025 | 0.024 |
| Spleen (%) | 0.221 a | 0.155 c | 0.176 b | 0.185 b | 0.201 ab | 0.036 | 0.001 | |
| Thymus (%) | 0.425 a | 0.302 b | 0.411 a | 0.405 a | 0.415 a | 0.044 | 0.035 | |
| Hematological parameters | RBCs (×106) | 3.65 a | 2.70 d | 3.10b c | 3.05 c | 3.40 b | 0162 | 0.001 |
| WBCs (×103) | 4.12 a | 3.52 c | 4.00 b | 4.05 b | 4.10 b | 0.082 | 0.002 | |
| Heterophils (%) | 23.5 d | 31.6 a | 27.0 b | 25.6 c | 25.0 c | 0.530 | 0.036 | |
| Lymphocytes (%) | 76.3 a | 67.9 d | 72.7 c | 74.0 b | 74.6 ab | 0.426 | 0.001 | |
| Monocyte (%) | 0.16 | 0.35 | 0.23 | 0.30 | 0.25 | 0.122 | 0.625 | |
| Basophil (%) | 0.04 | 0.19 | 0.12 | 0.10 | 0.15 | 0.04 | 0.051 | |
| Stress indices | H/L ratio | 0.308 c | 0.465 a | 0.372 b | 0.346 b | 0.335 bc | 0.02 | 0.025 |
| Corticosterone (nmol/L) | 26.2 b | 29.2 a | 27.0 ab | 26.8 b | 26.2 b | 0.244 | 0.020 | |
| Parameters | G1 (Negative Control) | Groups Raised under Heat Stress | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | |||||
| Lipid metabolites | Triglycerides (mg/dL) | 179.1 b | 196.8 a | 180.2 b | 178.6 b | 177.8 b | 3.274 | 0.001 |
| Cholesterol (mg/dL) | 190.2 bc | 244.5 a | 200.6 b | 195.8 bc | 188.5 c | 3.552 | 0.018 | |
| LDL (mg/dL) | 90.8 bc | 140.5 a | 98.7 b | 85.5 c | 85.0 c | 2.458 | 0.005 | |
| HDL (mg/dL) | 66.5 b | 60.8 c | 69.6 a | 67.5 b | 70.4 a | 2.118 | 0.004 | |
| LDL/HDL | 1.365 b | 2.310 a | 1.418 ab | 1.266 c | 1.207 c | 0.258 | 0001 | |
| Liver leakage enzymes | AST (U/L) | 62.0 b | 66.8 a | 61.8 b | 61.5 b | 61.0 b | 1.008 | 0.025 |
| ALT (U/L) | 70.2 b | 76.8 a | 69.8 b | 70.2 b | 69.0 b | 2.285 | 0.008 | |
| ALT/AST ratio | 1.132 | 1.149 | 1.129 | 1.173 | 1.131 | 0.055 | 0.325 | |
| Renal function | Creatinine (mg/dL) | 0.288 b | 0.528 a | 0.278 b | 0.269 b | 0.275 b | 0.025 | 0.004 |
| Uric acid (mg/dL) | 5.33 b | 6.75 a | 4.58 c | 5.00b c | 4.98b c | 0.145 | 0.025 | |
| Uric acid/creatinine ratio | 18.51 a | 12.78 c | 16.47 b | 18.59 a | 18.11 a | 1.025 | 0.004 | |
| Antioxidants status | MDA (nmol/mL) | 1.96 c | 3.22 a | 2.55 b | 2.38 b | 2.21 b | 0.058 | 0.035 |
| TAC (U/mL) | 8.28 a | 5.36 c | 7.65 b | 7.85 b | 8.10 a | 1.145 | 0.004 | |
| TAC/MDA ratio | 4.224 a | 1.664 c | 3.00 b | 3.298 ab | 3.665 ab | 0.854 | 0.002 | |
| Parameters | G1 (Negative Control) | Groups Raised under Heat Stress | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | |||||
| Cecal bacterial count (log10 CFU/g) | Coliforms | 5.20 b | 7.92 a | 5.05 b | 5.62 b | 4.80 c | 0.155 | 0.024 |
| Lactobacillus | 6.05 b | 5.33 c | 7.51 a | 7.65 a | 7.00 a | 0.224 | 0.035 | |
| Fecal bacterial count (log10 CFU/g) | Coliforms | 5.33 b | 7.80 a | 5.25 b | 5.30 b | 4.77 c | 0.08 | 0.005 |
| Lactobacillus spp. | 6.25 b | 5.00 c | 6.52 a | 7.42 a | 7.85 a | 0.15 | 0.002 | |
| Intestinal morphology (µm) | Villus height (VH) | 1345 a | 1288 c | 1320 b | 1308 b | 1330 b | 28.255 | 0.001 |
| Crypt depth (CD) | 332 b | 355 a | 330 b | 339 b | 328 b | 2.552 | 0.003 | |
| VH/CD | 4.05 a | 3.63 b | 4.00 a | 3.86 a | 4.05 a | 0.445 | 0.025 | |
| Parameters | Control G1 | Groups Raised under Heat Stress | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | |||||
| Immunoglobulin (Ig) | IgM (mg/dL) | 195 a | 96 c | 145 b | 179 ab | 180 ab | 4.025 | 0.004 |
| IgY (mg/dL) | 819 a | 544 c | 725 b | 760 ab | 781 ab | 5.224 | 0.026 | |
| IgA (mg/dL) | 250 | 248 | 257 | 238 | 250 | 5.125 | 0.485 | |
| Immune response | NDV (Log2) | 8.25 a | 6.45 c | 7.35 b | 7.44 b | 8.05 ab | 0.458 | 0.025 |
| IBD (Log2) | 6.55 a | 4.28 c | 5.98 b | 6.08 ab | 6.38 a | 0.342 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, Y.A.; Hassan, R.A.; Addeo, N.F.; Bovera, F.; Alhotan, R.A.; Al-qurashi, A.D.; Al-Baadani, H.H.; Al-Banoby, M.A.; Khafaga, A.F.; Eisenreich, W.; et al. Effects of Spirulina platensis and/or Allium sativum on Antioxidant Status, Immune Response, Gut Morphology, and Intestinal Lactobacilli and Coliforms of Heat-Stressed Broiler Chicken. Vet. Sci. 2023, 10, 678. https://doi.org/10.3390/vetsci10120678
Attia YA, Hassan RA, Addeo NF, Bovera F, Alhotan RA, Al-qurashi AD, Al-Baadani HH, Al-Banoby MA, Khafaga AF, Eisenreich W, et al. Effects of Spirulina platensis and/or Allium sativum on Antioxidant Status, Immune Response, Gut Morphology, and Intestinal Lactobacilli and Coliforms of Heat-Stressed Broiler Chicken. Veterinary Sciences. 2023; 10(12):678. https://doi.org/10.3390/vetsci10120678
Chicago/Turabian StyleAttia, Youssef A., Reda A. Hassan, Nicola Francesco Addeo, Fulvia Bovera, Rashed A. Alhotan, Adel D. Al-qurashi, Hani H. Al-Baadani, Mohamed A. Al-Banoby, Asmaa F. Khafaga, Wolfgang Eisenreich, and et al. 2023. "Effects of Spirulina platensis and/or Allium sativum on Antioxidant Status, Immune Response, Gut Morphology, and Intestinal Lactobacilli and Coliforms of Heat-Stressed Broiler Chicken" Veterinary Sciences 10, no. 12: 678. https://doi.org/10.3390/vetsci10120678
APA StyleAttia, Y. A., Hassan, R. A., Addeo, N. F., Bovera, F., Alhotan, R. A., Al-qurashi, A. D., Al-Baadani, H. H., Al-Banoby, M. A., Khafaga, A. F., Eisenreich, W., Shehata, A. A., & Basiouni, S. (2023). Effects of Spirulina platensis and/or Allium sativum on Antioxidant Status, Immune Response, Gut Morphology, and Intestinal Lactobacilli and Coliforms of Heat-Stressed Broiler Chicken. Veterinary Sciences, 10(12), 678. https://doi.org/10.3390/vetsci10120678











