Extracutaneous Melanotic Melanoma with Nervous System Involvement in a Buffalo (Bubalus bubalis)
Abstract
:Simple Summary
Abstract
1. Introduction
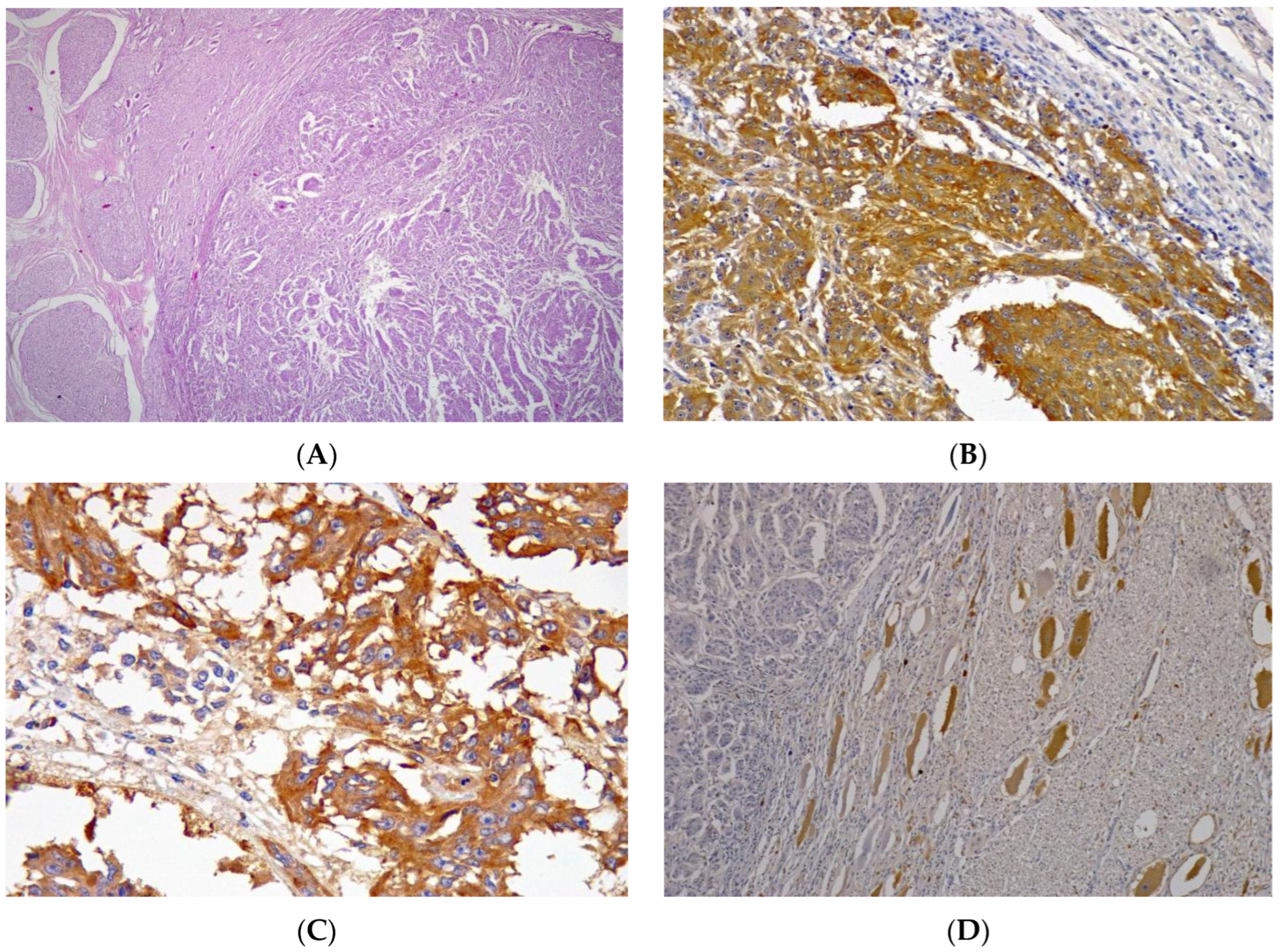
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- I.B.B.E.—Instituto Brasileiro de Geografia e Estatística; de Bubalinos, R. (Búfalos) no Brasil. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/bubalinos/br (accessed on 1 October 2023).
- Garcia, A.R. Conforto térmico na reprodução de bubalinos criados em condições tropicais. Rev. Bras. Reprodução Anim. 2013, 37, 121–130. [Google Scholar]
- Rajão, D.S.; Bastianetto, E.; Reis, J.K.P.; Oliveira, D.A.A.; Lago, L.A.; Leite, R.C. Estudo da infecção pelo vírus da leucose bovina em bubalinos (Bubalus bubalis) no estado de Minas Gerais. Rev. Bras. Med. Vet. 2010, 32, 42–45. [Google Scholar]
- Chaves, N.P.; Bezerra, D.C.; Santos, L.S.; Sá, J.S.; Santos, H.P.; Pereira, H.M. Intercorrência entre leucose enzoótica e brucelose em búfalos (Bubalus bubalis) em sistema de produção extensivo. Pesqui. Veterinária Bras. 2012, 32, 131–134. [Google Scholar] [CrossRef]
- Awadin, W.; Mosbah, E. Histopathology of tumor and tumor-like lesions in twelve female water buffaloes. J. Vet. Sci. Med. Diagn. 2013, 2, 2–4. [Google Scholar]
- Gupta, P.P.; Singh, B.; Gill, B.S. Some uncommon neoplasms of Indian water buffaloes (Bubalus bubalis). Zentralblatt Veterinärmedizin Reihe A 1977, 24, 511–519. [Google Scholar] [CrossRef]
- Damé, M.C.F.; Marcolongo-Pereira, C.; Fiss, L.; Adrién, M.L.; Schild, A.L. Malignant melanoma in albino water buffalo (Bubalus bubalis). Semin. Ciências Agrárias 2015, 36, 3239–3244. [Google Scholar] [CrossRef]
- Maiolino, P.; Ozkul, A.; Sepici-Dincel, A.; Roperto, F.; Yücel, G.; Russo, V.; Urraro, C.; Lucà, R.; Riccardi, M.G.; Martano, M.; et al. Bovine papillomavirus type 2 infection and microscopic patterns of urothelial tumors of the urinary bladder in water buffaloes. BioMed Res. Int. 2013, 146, 937918. [Google Scholar] [CrossRef]
- Mandal, P.C.; Iyer, P.K.R. Mammary intraductal carcinoma in a buffalo (Bubalus bubalis). Pathol. Vet. 1969, 6, 534–537. [Google Scholar] [CrossRef]
- Kanitakis, J. Anatomy, histology, and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar]
- Van der Weyden, L.; Brenn, T.; Patton, E.E.; Wood, G.A.; Adams, D.J. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J. Pathol. 2020, 252, 4–21. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, L.C.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, J.T.S.A.; Biscarde, C.E.A.; Oliveira, R.S.; Ferreira, E.A.; Pedroso, P.M.O. Carcinoma de células escamosas na região frontal da cabeça em cabra. Acta Sci. Vet. 2013, 41, 1–4. [Google Scholar]
- Melo-Neto, G.B.; Correia, D.A.B.; Mesquita, E.P.; Torres, M.B.M.A. Melanoma metastático em caprino. Acta Sci. Vet. 2019, 47, 419. [Google Scholar]
- Teixeira, C.; Pires, I.; Ferreira, S.; Vieira-Pinto, M. Lesões melanocíticas em suínos abatidos para consumo. Arq. Bras. Med. Veterinária Zootec. 2013, 65, 783–791. [Google Scholar] [CrossRef]
- Cid, G.C.; Oliveira, M.C.; Pires, A.P.C.; Costa, S.Z.R.; Santos, B.B.N.; Avila, M.S.D.; Nogueira, V.A.; Peixoto, P.V.; França, T.N. Metastatic cutaneous amelanotic melanoma in a rabbit ultrastructural, morphological, and immunohistochemical aspects. J. Exot. Pet Med. 2022, 40, 45–46. [Google Scholar] [CrossRef]
- Busato, E.M.; Sousa, R.S.; Silva-Meirelles, J.R.; Castro, M.L.; Deconto, I.; Dornbusch, P.T. Compressão de medula espinhal ocasionada por melanoma em cavalo de pelagem castanha. Arq. Bras. Med. Veterinária Zootec. 2017, 69, 1346–1350. [Google Scholar] [CrossRef]
- Brito, M.F.; França, T.N.; Jabour, F.F.; Seixas, J.N.; Andrade, G.B.; Oliveira, L.I.; Peixoto, P.V. Metastasizing oral melanoma in a cow. Ciência Rural. 2009, 39, 1248–1252. [Google Scholar] [CrossRef]
- Jesus, J.; Bortolini, A.; Almeida, G.C.; Oliveira, B.C.; Bruzamarelo, A.; Rech, R.R.; Barros, C.S.L.; Alves, C.E.F.; Elias, F. Diffuse melanosis secondary to metastatic melanoma in a Nelore bull. Braz. J. Vet. Pathol. 2021, 14, 191–198. [Google Scholar] [CrossRef]
- Madheswaran, R.; Shahana, S.; Gopal, K.; Sankar, P. A case report of non-systemic highly aggressive melanoma in a cow. Indian J. Vet. Pathol. 2019, 43, 124–126. [Google Scholar] [CrossRef]
- Anoop, S.; Venugopal, S.K.; Sarangom, S.B.; Khan, B.A.; Aswin, K.; Karthika, P.; Basil, K.G.; Mathai, V.M.; Krishna, B.D. Melanoma in a crossbred cow. Blue Cross Book 2016, 33, 74–76. [Google Scholar]
- Winslow, C.M.; Wood, J.; Gilliam, J.N.; Breshears, M.A. Congenital amelanotic melanoma in a crossbred heifer calf. J. Vet. Diagn. Investig. 2017, 29, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Beytut, E.; Kılıç, E.; Yayla, S. Histopathological and immunohistochemical evaluation of congenital cutaneous melanomas in calves (3 cases). Ankara Univ Vet Fak. 2018, 65, 425–432. [Google Scholar]
- Sabri, M.A.; Shahzad, M.; Qayyum, A. Ocular melanoma in a buffalo: A clinical case recorded under field conditions. Buffalo Bull. 2010, 29, 235–237. [Google Scholar]
- Riet-Correa, F.; Riet-Correa, G.; Schild, A.L. Importância do exame clínico para o diagnóstico das enfermidades do sistema nervoso em ruminantes e equídeos. Pesqui. Veterinária Bras. 2002, 22, 161–168. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Goldschmidt, K.H. Epithelial and melanocytic tumors of the skin. In Tumors in Domestic Animals, 5th ed.; Wiley Blacwell: Ames, IA, USA, 2017; pp. 88–142. [Google Scholar]
- Reis, M.D.O.; Slaviero, M.; Lorenzett, M.P.; Cruz, R.A.; Guimarães, L.L.; Pavarini, S.P.; Driemeier, D.; Sonne, L. Neoplasmas bovinos diagnosticados no setor de Patologia Veterinária da UFRGS, Porto Alegre (2005–2014). Pesqui. Veterinária Bras. 2017, 37, 105–109. [Google Scholar] [CrossRef]
- Van Beek, E.J.; Balm, A.J.; Nieweg, O.E.; Hamming-Vrieze, O.; Lohuis, P.J.; Klop, W.M. Treatment of regional metastatic melanoma of unknown primary origin. Cancers 2015, 7, 1543–1553. [Google Scholar] [CrossRef]
- Küsters-Vandevelde, H.V.; Küsters, B.; van Engen-van Grunsven, A.C.; Groenen, P.J.; Wesseling, P.; Blokx, W.A. Primary melanocytic tumors of the central nervous system: A review with focus on molecular aspects. Brain Pathol. 2015, 25, 209–226. [Google Scholar] [CrossRef]
- Ramos-Vara, J.Á.; Borst, L.B. Immunohistochemistry: Fundamentals and applications in oncology. In Tumors in Domestic Animals, 5th ed.; Wiley Blacwell: Ames, IA, USA, 2017; pp. 44–87. [Google Scholar]
- Tzanavaris, K.; Pettas, E.; Thermos, G.; Georgaki, M.; Piperi, E.; Nikitakis, N.G. Base of tongue metastasis of cutaneous malignant melanoma with rhabdoid and neuroendocrine features: Report of a rare case and review of the literature. Head Neck Pathol. 2022, 16, 1230–1241. [Google Scholar] [CrossRef]
- Bianchi, R.M.; Panziera, W.; Galiza, G.J.N.D.; Kommers, G.D.; Fighera, R.A. Surtos de raiva em bubalinos no Rio Grande do Sul, Brasil. Ciência Rural. 2017, 47, 01–05. [Google Scholar]
- Barbosa, J.D.; Tokarnia, C.H.; Albernaz, T.T.; Oliveira, C.M.C.; Silva, N.D.S.; Silveira, J.A.S.D.; Belo-Reis, A.S.; Lima, D.H.D.S. Intoxicação natural por Ipomoea asarifolia (Convolvulaceae) em búfalos na Ilha de Marajó, Pará. Pesqui. Veterinária Bras. 2012, 32, 869–871. [Google Scholar] [CrossRef]
- Colodel, E.M.; Nakazato, L.; Weiblen, R.; Mello, R.M.; Silva, R.R.P.D.; Souza, M.D.A.; Filho, J.A.O.; Caron, L. Meningoencefalite necrosante em bovinos causada por herpesvírus bovino no estado de Mato Grosso, Brasil. Ciência Rural. 2002, 32, 293–298. [Google Scholar] [CrossRef]
- Prado, R.G.S.; Domiciano, T.A.O.; Paredes, L.J.A.; Bezerra, P.S.; Pereira, W.L.A.; Cerqueira, V.D.; Driemeier, D.; Riet-Correa, G. Listeriose nervosa em búfalos. Pesqui. Veterinária Bras. 2019, 39, 299–303. [Google Scholar] [CrossRef]
- Barbosa, J.D.; Bomjardim, H.D.A.; Campos, K.F.; Duarte, M.D.; Bezerra Júnior, P.S.; Gava, A.; Salvarani, F.M.; Oliveira, C.M.C. Intoxicação por chumbo em bovinos e galinhas no estado do Pará. Pesqui. Veterinária Bras. 2014, 34, 1077–1080. [Google Scholar] [CrossRef]
- Duarte, M.D.; Bezerra Júnior, P.S.; Lima, D.H.S.; Bomjardim, H.A.; Oliveira, C.M.C.; Silva, N.D.S.; Faial, K.C.F.; Barbosa, J.D. Surto de intoxicação por sal em ovinos no estado do Pará. Pesqui. Veterinária Bras. 2014, 34, 1061–1068. [Google Scholar] [CrossRef]
- Lima, E.F.; Riet-Correa, F.; Castro, R.S.D.; Gomes, A.A.B.; Lima, F.D.S. Sinais clínicos, distribuição das lesões no sistema nervoso e epidemiologia da raiva em herbívoros na região Nordeste do Brasil. Pesqui. Veterinária Bras. 2005, 25, 250–264. [Google Scholar] [CrossRef]
- Sanches, A.W.D.; Langohr, I.M.; Stigger, A.L.; Barros, C.S.L. Doenças do sistema nervoso central em bovinos no Sul do Brasil. Pesqui. Veterinária Bras. 2000, 20, 113–118. [Google Scholar] [CrossRef]
- Rissi, D.R.; Rech, R.R.; Barros, R.R.; Kommers, G.D.; Langohr, I.M.; Pierezan, F.; Barros, C.S.L. Forma nervosa de listeriose em caprinos. Pesqui. Veterinária Bras. 2006, 26, 14–20. [Google Scholar] [CrossRef]
- George, L.W. Listeriosis. In Large Animal Internal Medicine, 3rd ed.; Mosby: St. Louis, MO, USA, 2002; pp. 946–949. [Google Scholar]

| Anticorpos | Clone | Results | |
|---|---|---|---|
| Vimentin | Intermediate filaments of mesenchymal cells | V9 | Positive |
| Melan-A | Melanoma antigen | A103 | Positive |
| PNL2 | Melanoma antigen | PNL-2 | Positive |
| CK Pan | Intermediate filaments of epithelial cells | AE1AE3 | Negative |
| Neurofilament | Marker of neurons and neuronal tumors | 2F11 | Negative |
| NSE | Neuron-specific enolase | BBS/NC/VI-H14 | Negative |
| GFAP | Glial fibrillary acidic protein | Polyclonal | Negative |
| SOX10 | Marker of neural crest tumors, melanomas, and schwannomas | BC34 | Positive |
| Chromogranin | Neuroendocrine cell marker | Polyclonal | Sparsely positive |
| Synaptophysin | Neuroendocrine cell marker | DAK-SYNAP | Sparsely positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, J.D.; Oliveira, M.C.; Oliveira, C.M.C.; Bomjardim, H.d.A.; Ferreira, T.T.A.; Duarte, M.D.; da Silveira, J.A.S.; Silveira, N.d.S.e.S.; Barbosa, C.C.; da Silva, A.O.A.; et al. Extracutaneous Melanotic Melanoma with Nervous System Involvement in a Buffalo (Bubalus bubalis). Vet. Sci. 2023, 10, 662. https://doi.org/10.3390/vetsci10120662
Barbosa JD, Oliveira MC, Oliveira CMC, Bomjardim HdA, Ferreira TTA, Duarte MD, da Silveira JAS, Silveira NdSeS, Barbosa CC, da Silva AOA, et al. Extracutaneous Melanotic Melanoma with Nervous System Involvement in a Buffalo (Bubalus bubalis). Veterinary Sciences. 2023; 10(12):662. https://doi.org/10.3390/vetsci10120662
Chicago/Turabian StyleBarbosa, José Diomedes, Mariana Correia Oliveira, Carlos Magno Chaves Oliveira, Henrique dos Anjos Bomjardim, Tatiane Teles Albernaz Ferreira, Marcos Dutra Duarte, José Alcides Sarmento da Silveira, Natália da Silva e Silva Silveira, Camila Cordeiro Barbosa, Aluízio Otávio Almeida da Silva, and et al. 2023. "Extracutaneous Melanotic Melanoma with Nervous System Involvement in a Buffalo (Bubalus bubalis)" Veterinary Sciences 10, no. 12: 662. https://doi.org/10.3390/vetsci10120662
APA StyleBarbosa, J. D., Oliveira, M. C., Oliveira, C. M. C., Bomjardim, H. d. A., Ferreira, T. T. A., Duarte, M. D., da Silveira, J. A. S., Silveira, N. d. S. e. S., Barbosa, C. C., da Silva, A. O. A., Armién, A., & Brito, M. d. F. (2023). Extracutaneous Melanotic Melanoma with Nervous System Involvement in a Buffalo (Bubalus bubalis). Veterinary Sciences, 10(12), 662. https://doi.org/10.3390/vetsci10120662






