Indication of West Nile Virus (WNV) Lineage 2 Overwintering among Wild Birds in the Regions of Peloponnese and Western Greece
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. WNV Surveillance in Greece
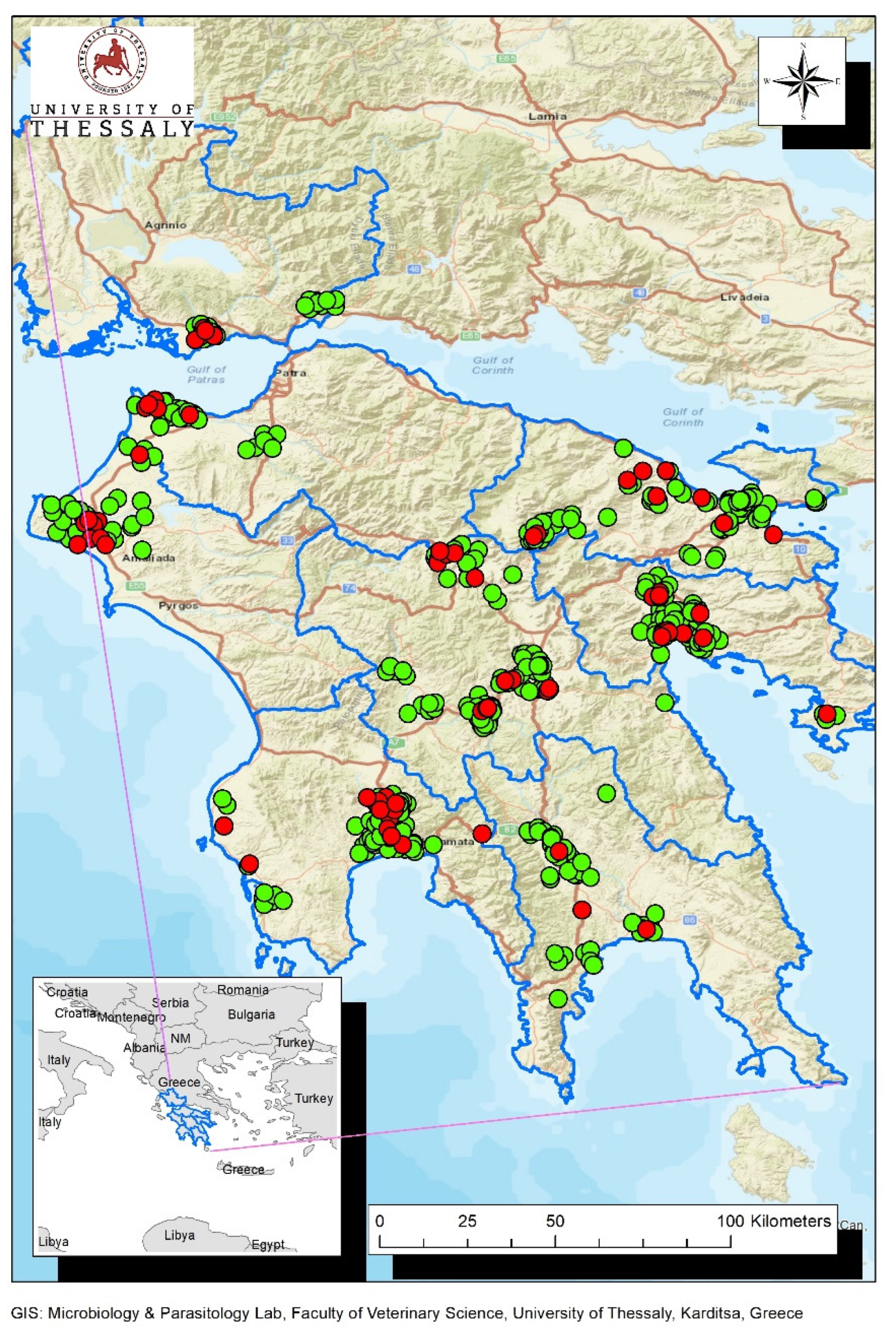
2.2. Study Area and Collection of Biological Material from Wild Birds
2.3. Molecular Detection of WNV in Wild Birds
2.4. Phylogenetic Analysis
2.5. Environmental Variables
2.6. Statistical Analysis
3. Results
3.1. Molecular Detection of WNV in Wild Birds
3.2. Phylogenetic Analysis
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A Global Perspective on the Epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M.Á. Experimental Infections of Wild Birds with West Nile Virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. 1940, 20, 471–472. [Google Scholar] [CrossRef]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean Basin: 1950–2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar] [CrossRef]
- Kramer, L.D.; Ciota, A.T.; Kilpatrick, A.M. Introduction, Spread, and Establishment of West Nile Virus in the Americas. J. Med. Entomol. 2019, 56, 1448–1455. [Google Scholar] [CrossRef]
- Papa, A. Emerging Arboviral Human Diseases in Southern Europe. J. Med. Virol. 2017, 89, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of West Nile Virus Lineage 2 in Europe: A Review on the Introduction and Spread of a Mosquito-Borne Disease. Front. Public Health 2014, 2, 271. [Google Scholar] [CrossRef]
- Marra, P.P.; Griffing, S.; Caffrey, C.; Kilpatrick, M.A.; McLean, R.; Brand, C.; Saito, E.; Dupuis, A.P.; Kramer, L.; Novak, R. West Nile Virus and Wildlife. BioScience 2004, 54, 393–402. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, M.; Banet, C. The Role of Birds in the Ecology of West Nile Virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267, 309–322. [Google Scholar] [CrossRef]
- Gamino, V.; Höfle, U. Pathology and Tissue Tropism of Natural West Nile Virus Infection in Birds: A Review. Vet. Res. 2013, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, G.; Touloudi, A.; Athanasiou, L.V.; Giannakopoulos, A.; Iacovakis, C.; Birtsas, P.; Spyrou, V.; Dalabiras, Z.; Petrovska, L.; Billinis, C. Serological and Molecular Investigation into the Role of Wild Birds in the Epidemiology of West Nile Virus in Greece. Virol. J. 2012, 9, 266. [Google Scholar] [CrossRef]
- Valiakos, G.; Touloudi, A.; Iacovakis, C.; Athanasiou, L.; Birtsas, P.; Spyrou, V.; Billinis, C. Molecular Detection and Phylogenetic Analysis of West Nile Virus Lineage 2 in Sedentary Wild Birds (Eurasian Magpie), Greece, 2010. Eurosurveillance 2011, 16, 19862. [Google Scholar] [CrossRef]
- Valiakos, G.; Touloudi, A.; Athanasiou, L.V.; Giannakopoulos, A.; Iacovakis, C.; Birtsas, P.; Spyrou, V.; Dalabiras, Z.; Petrovska, L.; Billinis, C. Exposure of Eurasian Magpies and Turtle Doves to West Nile Virus during a Major Human Outbreak, Greece, 2011. Eur. J. Wildl. Res. 2012, 58, 749–753. [Google Scholar] [CrossRef]
- Valiakos, G.; Plavos, K.; Vontas, A.; Sofia, M.; Giannakopoulos, A.; Giannoulis, T.; Spyrou, V.; Tsokana, C.N.; Chatzopoulos, D.; Kantere, M.; et al. Phylogenetic Analysis of Bird-Virulent West Nile Virus Strain, Greece. Emerg. Infect. Dis. 2019, 25, 2323–2325. [Google Scholar] [CrossRef]
- Sofia, M.; Giannakopoulos, A.; Giantsis, I.A.; Touloudi, A.; Birtsas, P.; Papageorgiou, K.; Athanasakopoulou, Z.; Chatzopoulos, D.C.; Vrioni, G.; Galamatis, D.; et al. West Nile Virus Occurrence and Ecological Niche Modeling in Wild Bird Species and Mosquito Vectors: An Active Surveillance Program in the Peloponnese Region of Greece. Microorganisms 2022, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- West Nile Virus. NPHO. Available online: https://eody.gov.gr/en/disease/west-nile-virus/ (accessed on 25 May 2023).
- Mencattelli, G.; Iapaolo, F.; Polci, A.; Marcacci, M.; Di Gennaro, A.; Teodori, L.; Curini, V.; Di Lollo, V.; Secondini, B.; Scialabba, S.; et al. West Nile Virus Lineage 2 Overwintering in Italy. Trop. Med. Infect. Dis. 2022, 7, 160. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, É.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618. [Google Scholar] [CrossRef]
- Chaskopoulou, A.; L’Ambert, G.; Petric, D.; Bellini, R.; Zgomba, M.; Groen, T.A.; Marrama, L.; Bicout, D.J. Ecology of West Nile Virus across Four European Countries: Review of Weather Profiles, Vector Population Dynamics and Vector Control Response. Parasites Vectors 2016, 9, 482. [Google Scholar] [CrossRef]
- Danis, K.; Papa, A.; Theocharopoulos, G.; Dougas, G.; Athanasiou, M.; Detsis, M.; Baka, A.; Lytras, T.; Mellou, K.; Bonovas, S.; et al. Outbreak of West Nile Virus Infection in Greece, 2010. Emerg. Infect. Dis. 2011, 17, 1868–1872. [Google Scholar] [CrossRef]
- Pervanidou, D.; Kefaloudi, C.N.; Vakali, A.; Tsakalidou, O.; Karatheodorou, M.; Tsioka, K.; Evangelidou, M.; Mellou, K.; Pappa, S.; Stoikou, K.; et al. The 2022 West Nile Virus Season in Greece; A Quite Intense Season. Viruses 2023, 15, 1481. [Google Scholar] [CrossRef]
- Papa, A.; Tsioka, K.; Gewehr, S.; Kalaitzopouou, S.; Pervanidou, D.; Vakali, A.; Kefaloudi, C.; Pappa, S.; Louka, X.; Mourelatos, S. West Nile Fever Upsurge in a Greek Regional Unit, 2020. Acta Trop. 2021, 221, 106010. [Google Scholar] [CrossRef]
- Komar, N. West Nile Virus Surveillance Using Sentinel Birds. Ann. N. Y. Acad. Sci. 2001, 951, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Gossner, C.M.; Marrama, L.; Carson, M.; Allerberger, F.; Calistri, P.; Dilaveris, D.; Lecollinet, S.; Morgan, D.; Nowotny, N.; Paty, M.-C.; et al. West Nile Virus Surveillance in Europe: Moving towards an Integrated Animal-Human-Vector Approach. Euro Surveill. 2017, 22, 30526. [Google Scholar] [CrossRef]
- Πρόγραμμα Επιτήρησης Του Πυρετού Του Δυτικού Νείλου Στα Άγρια Πτηνά Και Στα Ιπποειδή. Available online: https://www.minagric.gr/for-citizen-2/nosimata-zoon/602-progrepitdneilou17?highlight=WyJcdTAzYjkiLCJcdTAzYjlcdTAzYmZcdTAzYzUiLCJcdTAzYjlcdTAzY2NcdTAzYzIiLCJcdTAzYjlcdTAzYmZcdTAzY2QiLCJcdTAzYjlcdTAzYmZcdTAzY2RcdTAzYzIiLCJcdTAzYjlcdTAzYmZcdTAzYzIiLCJcdTAzYjlcdTAzYjciLCJcdTAzYjRcdTAzYzVcdTAzYzRcdTAzYjlcdTAzYmFcdTAzYmZcdTAzY2QiLCJcdTAzYjRcdTAzYzVcdTAzYzRcdTAzYjlcdTAzYmFcdTAzYWMiLCJcdTAzYjRcdTAzYzVcdTAzYzRcdTAzYjlcdTAzYmFcdTAzY2MiLCJcdTAzYjRcdTAzYzVcdTAzYzQiLCJcdTAzYjRcdTAzYzVcdTAzYzRcdTAzY2VcdTAzYmQiLCJcdTAzYjRcdTAzYzVcdTAzYzRcdTAzYjlcdTAzYmFcdTAzY2VcdTAzYmQiLCJcdTAzYmRcdTAzYjVcdTAzYWZcdTAzYmJcdTAzYmZcdTAzYzUiXQ== (accessed on 2 November 2023).
- Chaskopoulou, A.; Dovas, C.; Chaintoutis, S.; Bouzalas, I.; Ara, G.; Papanastassopoulou, M. Evidence of Enzootic Circulation of West Nile Virus (Nea Santa-Greece-2010, Lineage 2), Greece, May to July 2011. Euro Surveill. 2011, 16, 19933. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Kumar, S. Evolutionary Distance Estimation Under Heterogeneous Substitution Pattern Among Lineages. Mol. Biol. Evol. 2002, 19, 1727–1736. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- SRTM 90m Digital Elevation Database v4.1. CGIAR-CSI. 2017. Available online: https://cgiarcsi.community/data/srtm-90m-digital-elevation-database-v4-1/ (accessed on 25 May 2023).
- HydroSHEDS. Available online: https://www.hydrosheds.org/ (accessed on 25 May 2023).
- Datasets—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/data/#c0=5&c11=&c5=all&b_start=0 (accessed on 25 May 2023).
- Copernicus Land Monitoring Service—Corine Land Cover—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/data/copernicus-land-monitoring-service-corine (accessed on 25 May 2023).
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 1989; ISBN 978-0-471-61553-8. [Google Scholar]
- Gill, F.; Donsker, D.; Rasmussen, P. (Eds.) World Bird List (v 13.1)_red. Available online: http://www.worldbirdnames.org/ (accessed on 25 May 2023). [CrossRef]
- Το Κόκκινο Βιβλίο των Aπειλούμενων Ζώων της Ελλάδας. Available online: https://www.ornithologiki.gr/el/enhmerwsh-ekpaideush/enimerosi/yliko-enimerosis-ekdoseis/155-to-kokkino-vivlio-ton-apeiloymenon-zoon-tis-elladas (accessed on 13 March 2023).
- Lelli, R.; Calistri, P.; Bruno, R.; Monaco, F.; Savini, G.; Di Sabatino, D.; Corsi, I.; Pascucci, I. West Nile Transmission in Resident Birds in Italy. Transbound. Emerg. Dis. 2012, 59, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, G.; Papaspyropoulos, K.; Giannakopoulos, A.; Birtsas, P.; Tsiodras, S.; Hutchings, M.R.; Spyrou, V.; Pervanidou, D.; Athanasiou, L.V.; Papadopoulos, N.; et al. Use of Wild Bird Surveillance, Human Case Data and GIS Spatial Analysis for Predicting Spatial Distributions of West Nile Virus in Greece. PLoS ONE 2014, 9, e96935. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Bakonyi, T.; Xanthopoulou, K.; Vázquez, A.; Tenorio, A.; Nowotny, N. Genetic Characterization of West Nile Virus Lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011, 17, 920–922. [Google Scholar] [CrossRef]
- Tsioka, K.; Gewehr, S.; Pappa, S.; Kalaitzopoulou, S.; Stoikou, K.; Mourelatos, S.; Papa, A. West Nile Virus in Culex Mosquitoes in Central Macedonia, Greece, 2022. Viruses 2023, 15, 224. [Google Scholar] [CrossRef]
- Napp, S.; Montalvo, T.; Piñol-Baena, C.; Gómez-Martín, M.B.; Nicolás-Francisco, O.; Soler, M.; Busquets, N. Usefulness of Eurasian Magpies (Pica Pica) for West Nile Virus Surveillance in Non-Endemic and Endemic Situations. Viruses 2019, 11, 716. [Google Scholar] [CrossRef]
- Vakali, A.; Beleri, S.; Tegos, N.; Fytrou, A.; Mpimpa, A.; Sergentanis, T.N.; Pervanidou, D.; Patsoula, E. Entomological Surveillance Activities in Regions in Greece: Data on Mosquito Species Abundance and West Nile Virus Detection in Culex Pipiens Pools (2019–2020). Trop. Med. Infect. Dis. 2022, 8, 1. [Google Scholar] [CrossRef]
- Lauriano, A.; Rossi, A.; Galletti, G.; Casadei, G.; Santi, A.; Rubini, S.; Carra, E.; Lelli, D.; Calzolari, M.; Tamba, M. West Nile and Usutu Viruses’ Surveillance in Birds of the Province of Ferrara, Italy, from 2015 to 2019. Viruses 2021, 13, 1367. [Google Scholar] [CrossRef]
- Haussig, J.M.; Young, J.J.; Gossner, C.M.; Mezei, E.; Bella, A.; Sirbu, A.; Pervanidou, D.; Drakulovic, M.B.; Sudre, B. Early Start of the West Nile Fever Transmission Season 2018 in Europe. Euro Surveill. 2018, 23, 1800428. [Google Scholar] [CrossRef]
- Paz, S. Climate Change Impacts on West Nile Virus Transmission in a Global Context. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20130561. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, M.; Rizzoli, A.; Metz, M.; Rosà, R.; Marini, G.; Chadwick, E.; Neteler, M. Identifying the Environmental Conditions Favouring West Nile Virus Outbreaks in Europe. PLoS ONE 2015, 10, e0121158. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnejad, F.; Otarod, V.; Fathnia, A.; Ahmadabadi, A.; Fallah, M.H.; Zavareh, A.; Miandehi, N.; Durand, B.; Sabatier, P. Impact of Climate and Environmental Factors on West Nile Virus Circulation in Iran. J. Arthropod Borne Dis. 2016, 10, 315–327. [Google Scholar] [PubMed]
- Mavrakis, A.; Papavasileiou, C.; Alexakis, D.; Papakitsos, E.C.; Salvati, L. Meteorological Patterns and the Evolution of West Nile Virus in an Environmentally Stressed Mediterranean Area. Environ. Monit. Assess. 2021, 193, 227. [Google Scholar] [CrossRef]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The Role of Birds of Prey in West Nile Virus Epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.S.; Vineyard, M.P.; Woods, L.W.; Reisen, W.K. Dynamics of West Nile Virus Persistence in House Sparrows (Passer Domesticus). PLoS Neglected Trop. Dis. 2012, 6, e1860. [Google Scholar] [CrossRef]
- Aguilera-Sepúlveda, P.; Napp, S.; Llorente, F.; Solano-Manrique, C.; Molina-López, R.; Obón, E.; Solé, A.; Jiménez-Clavero, M.Á.; Fernández-Pinero, J.; Busquets, N. West Nile Virus Lineage 2 Spreads Westwards in Europe and Overwinters in North-Eastern Spain (2017–2020). Viruses 2022, 14, 569. [Google Scholar] [CrossRef]
- Rudolf, I.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Šebesta, O.; Mendel, J.; Bakonyi, T.; Schaffner, F.; Nowotny, N.; et al. West Nile Virus in Overwintering Mosquitoes, Central Europe. Parasites Vectors 2017, 10, 452. [Google Scholar] [CrossRef]
- Kampen, H.; Tews, B.A.; Werner, D. First Evidence of West Nile Virus Overwintering in Mosquitoes in Germany. Viruses 2021, 13, 2463. [Google Scholar] [CrossRef]
- Montecino-Latorre, D.; Barker, C.M. Overwintering of West Nile Virus in a Bird Community with a Communal Crow Roost. Sci. Rep. 2018, 8, 6088. [Google Scholar] [CrossRef]
- Shartova, N.; Mironova, V.; Zelikhina, S.; Korennoy, F.; Grishchenko, M. Spatial Patterns of West Nile Virus Distribution in the Volgograd Region of Russia, a Territory with Long-Existing Foci. PLoS Neglected Trop. Dis. 2022, 16, e0010145. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Hanafi-Bojd, A.A.; Asghari, S.; Chavshin, A.R. The Potential of West Nile Virus Transmission Regarding the Environmental Factors Using Geographic Information System (GIS), West Azerbaijan Province, Iran. J. Arthropod Borne Dis. 2019, 13, 27–38. [Google Scholar] [PubMed]
- Giesen, C.; Herrador, Z.; Fernandez-Martinez, B.; Figuerola, J.; Gangoso, L.; Vazquez, A.; Gómez-Barroso, D. A Systematic Review of Environmental Factors Related to WNV Circulation in European and Mediterranean Countries. One Health 2023, 16, 100478. [Google Scholar] [CrossRef] [PubMed]


| Environmental Variable | Code |
|---|---|
| Annual mean temperature (°C) | clima1 |
| Maximum temperature of warmest month (°C) | clima2 |
| Heat stress index 1 | HI |
| Altitude (m) | dem |
| Distance from water collections and hydrographic network (m) | waterdis |
| Distance from livestock farms (sheep, goats and cattle, m) | farmdis |
| Land uses (44 classes) | landcorine |
| Human population density (people/km2) | popden |
| Scientific Name | Common Name | Status a | Regional Units of Peloponnese Region | Regional Units of Western Greece Region | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Argolis | Arcadia | Corinthia | Laconia | Messenia | Achaia | Ilia | Aitoloakarnania | |||
| Anas (Mareca) penelope | Eurasian wigeon | WV, PM | 0/1 | - | - | - | - | - | - | - |
| Anas platyrhynchos | Mallard | WV, r | 0/1 | - | - | - | - | - | - | - |
| Ardea cinerea | Grey heron | R, PM | 0/2 Ε | - | - | - | - | - | - | - |
| Ardeola ralloides | Squacco heron | SV, PM | 0/1 Ε | - | - | - | - | - | - | - |
| Buteo buteo | Common buzzard | R, WV | 0/1 D | 0/1 D | - | - | - | - | - | - |
| Calidris spp. | - | PM, WV | 0/3 E | - | - | - | - | - | - | - |
| Carduelis carduelis | European goldfinch | R, wv | 0/1 | - | - | - | 0/2 | - | - | - |
| Cettia cetti | Cetti’s warbler | R | - | - | - | - | 0/2 | - | - | - |
| Charadius dubius | Little ringed plover | SV, PM | 0/3 E | - | - | - | - | - | - | - |
| Columba livia | Rock dove | R | 0/1 | - | - | - | 0/4 + 1 D | - | - | - |
| Corvus cornix | Hooded crow | R | 0/2 | 0/5 | 0/11 | 1/17 | 1/7 | - | - | - |
| Corvus (Coloeus) monedula | Western jackdaw | R | - | 0/6 | - | - | - | - | - | - |
| Egretta garzetta | Little egret | PM, R | 0/3 E | - | - | - | - | - | - | - |
| Erithacus rubecula | European robin | WV, r | 0/2 | 0/1 + 1 D | 0/1 | - | 0/1 | - | - | - |
| Fringilla coelebs | Common chaffinch | R, WV | - | 0/1 D | - | - | 0/1 D | - | - | - |
| Gallinago gallinago | Common snipe | WV, PM | 0/1 | - | - | - | - | - | - | - |
| Garrulus glandarius | Eurasian jay | R | 0/6 | 0/3 | 1/1 | - | 0/3 | - | - | - |
| Himantopus himantopus | Black-winged stilt | PM, SV | 1/6 | - | - | - | - | - | - | - |
| Hirundo rustica | Barn swallow | SV, PM | - | - | 0/1 D | - | - | - | - | - |
| Induna (Iduna) pallida | Eastern olivaceous warbler | SV | - | - | - | 0/3 | 0/4 | - | - | - |
| Lanius collurio | Red-backed shrike | SV, PM | - | 0/1 | - | - | - | - | - | - |
| Larus michahellis | Yellow-legged gull | R | 0/2 E + 1 D | - | - | - | - | - | - | - |
| Larus (Chroicocephalus) ridibundus | Black-headed gull | WV, r | 0/5 E | - | 0/3 | - | - | - | - | - |
| Numerius (Numenius) arquata | Eurasian curlew | WV, PM | 0/1 E | - | - | - | - | - | - | - |
| Otus scops | Eurasian scops owl | PLM | 0/1D | - | 0/1 | - | - | - | - | - |
| Parus major | Great tit | R | - | - | - | 1/4 | 0/3 | - | - | - |
| Passer domesticus | House sparrow | R | 0/4 | 0/7 | 0/13 + 1 D | 0/2 | 1/6 | - | - | - |
| Phalacrocorax carbo | Great cormorant | WV, r | 0/1 E | - | - | - | - | - | - | - |
| Pica pica | Magpie | R | 9/47 + 2 D | 15/58 + 2 D | 4/36 | 0/4 | 9/37 | 5/22 + 1 D | 9/38 | 5/14 |
| Scolopax rusticola | Eurasian woodcock | WV, r | - | - | 0/2 | 0/1 | - | - | - | - |
| Streptopelia decaocto | Eurasian collared dove | R | - | - | 0/8 | - | - | - | - | - |
| Strix aluco | Tawny owl | R | - | - | 0/2 D | - | - | - | - | - |
| Sturnus vulgaris | Common starling | WV, R | - | 0/1 | 1/1 | - | 1/3 | 0/8 | - | 0/7 |
| Sylvia (Curruca) communis | Common whitethroat | SV, PM | - | 0/1 | - | - | - | - | - | - |
| Sylvia (Curruca) melanocephala | Sardinian warbler | R | - | - | 0/2 | 0/1 | 0/2 | - | - | - |
| Turdus merula | Common blackbird | R, WV | - | - | 0/1 | 1/4 | 0/1 | - | - | - |
| Turdus philomelos | Song thrush | WV, r | 2/8 | 0/4 | 3/10 | 0/8 + 1 D | 1/4 | - | - | 0/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasakopoulou, Z.; Sofia, M.; Skampardonis, V.; Giannakopoulos, A.; Birtsas, P.; Tsolakos, K.; Spyrou, V.; Chatzopoulos, D.C.; Satra, M.; Diamantopoulos, V.; et al. Indication of West Nile Virus (WNV) Lineage 2 Overwintering among Wild Birds in the Regions of Peloponnese and Western Greece. Vet. Sci. 2023, 10, 661. https://doi.org/10.3390/vetsci10110661
Athanasakopoulou Z, Sofia M, Skampardonis V, Giannakopoulos A, Birtsas P, Tsolakos K, Spyrou V, Chatzopoulos DC, Satra M, Diamantopoulos V, et al. Indication of West Nile Virus (WNV) Lineage 2 Overwintering among Wild Birds in the Regions of Peloponnese and Western Greece. Veterinary Sciences. 2023; 10(11):661. https://doi.org/10.3390/vetsci10110661
Chicago/Turabian StyleAthanasakopoulou, Zoi, Marina Sofia, Vassilis Skampardonis, Alexios Giannakopoulos, Periklis Birtsas, Konstantinos Tsolakos, Vassiliki Spyrou, Dimitris C. Chatzopoulos, Maria Satra, Vassilis Diamantopoulos, and et al. 2023. "Indication of West Nile Virus (WNV) Lineage 2 Overwintering among Wild Birds in the Regions of Peloponnese and Western Greece" Veterinary Sciences 10, no. 11: 661. https://doi.org/10.3390/vetsci10110661
APA StyleAthanasakopoulou, Z., Sofia, M., Skampardonis, V., Giannakopoulos, A., Birtsas, P., Tsolakos, K., Spyrou, V., Chatzopoulos, D. C., Satra, M., Diamantopoulos, V., Mpellou, S., Galamatis, D., G. Papatsiros, V., & Billinis, C. (2023). Indication of West Nile Virus (WNV) Lineage 2 Overwintering among Wild Birds in the Regions of Peloponnese and Western Greece. Veterinary Sciences, 10(11), 661. https://doi.org/10.3390/vetsci10110661









