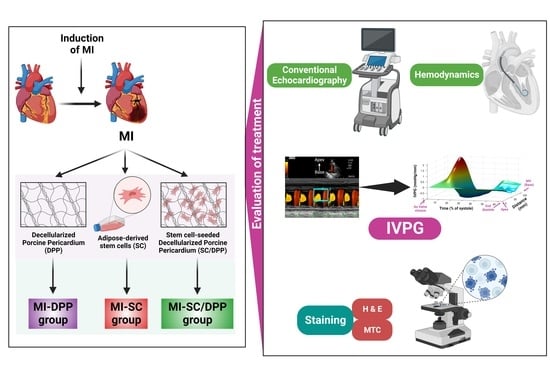
Adipose Stem Cell-Seeded Decellularized Porcine Pericardium: A Promising Functional Biomaterial to Synergistically Restore the Cardiac Functions Post-Myocardial Infarction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Animals of the Study
2.2. Study Design
2.3. Isolation and Characterization of the r-AdMSCs
2.4. Decellularization and Characterization of DPP Biomaterial
2.5. Preparation of Stem Cell-Seeded DPP
2.6. Induction and Treatment of Myocardial Infarction
2.7. Cardiac Function Assessment
2.7.1. Conventional Echocardiography
2.7.2. Intraventricular Pressure Gradient (IVPG)
2.7.3. Intra-Ventricular Catheter-Based Hemodynamics Analysis
2.8. Histological Evaluations
2.9. Statistical Analysis
3. Results
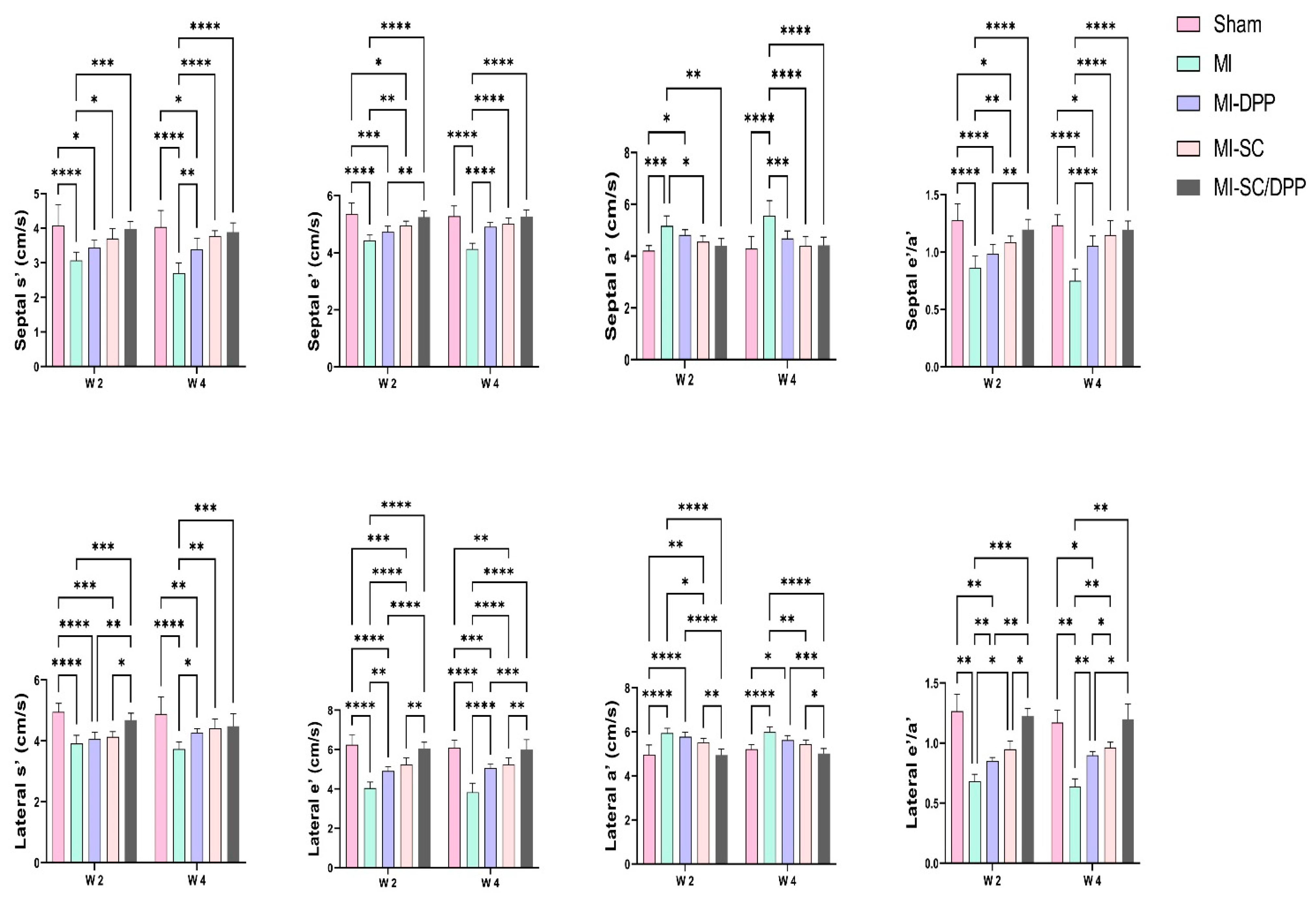
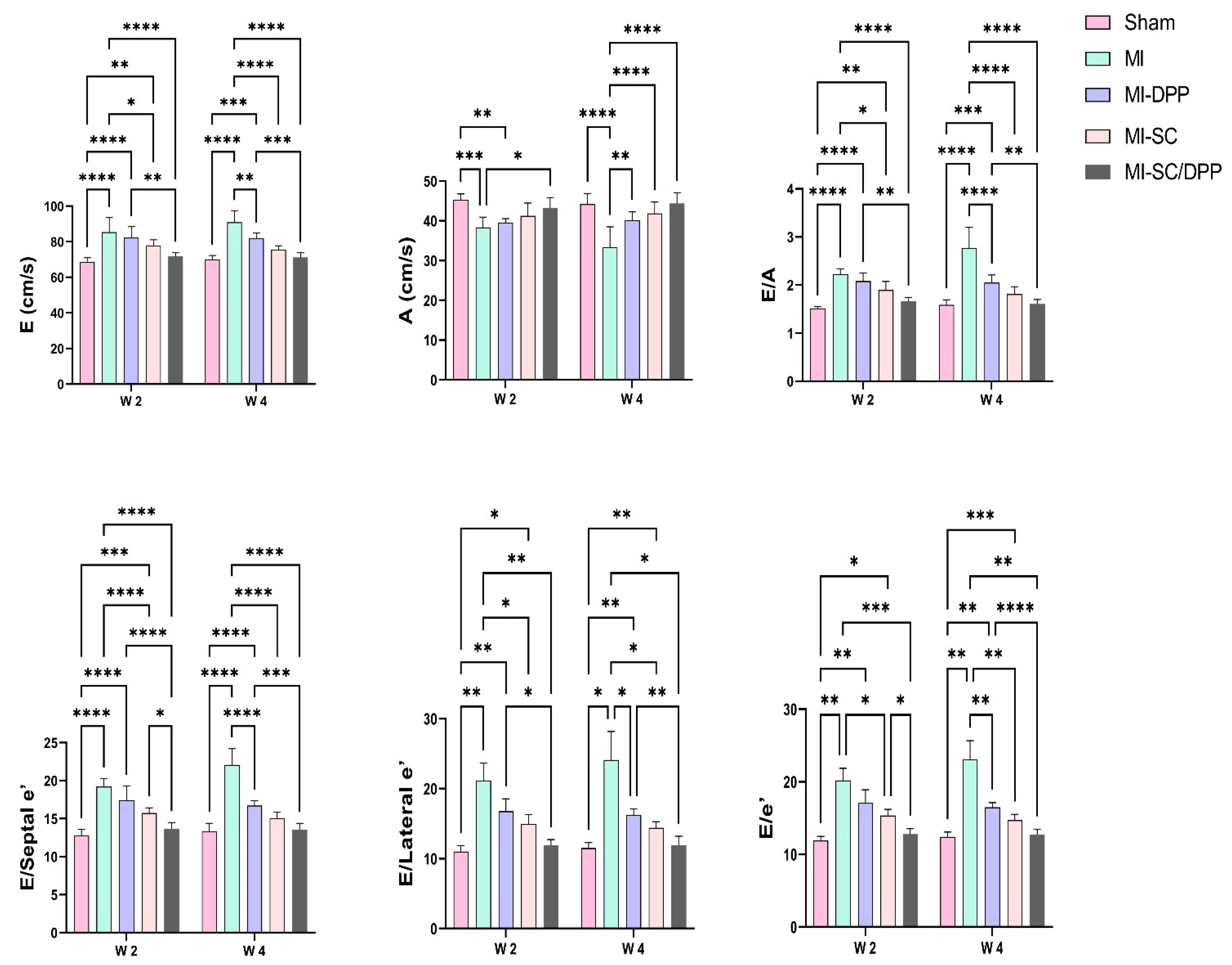
3.1. Echocardiography
3.2. The Intraventricular Pressure Gradient (IVPG)
3.3. Intra-Ventricular Catheter-Based Hemodynamics Analysis
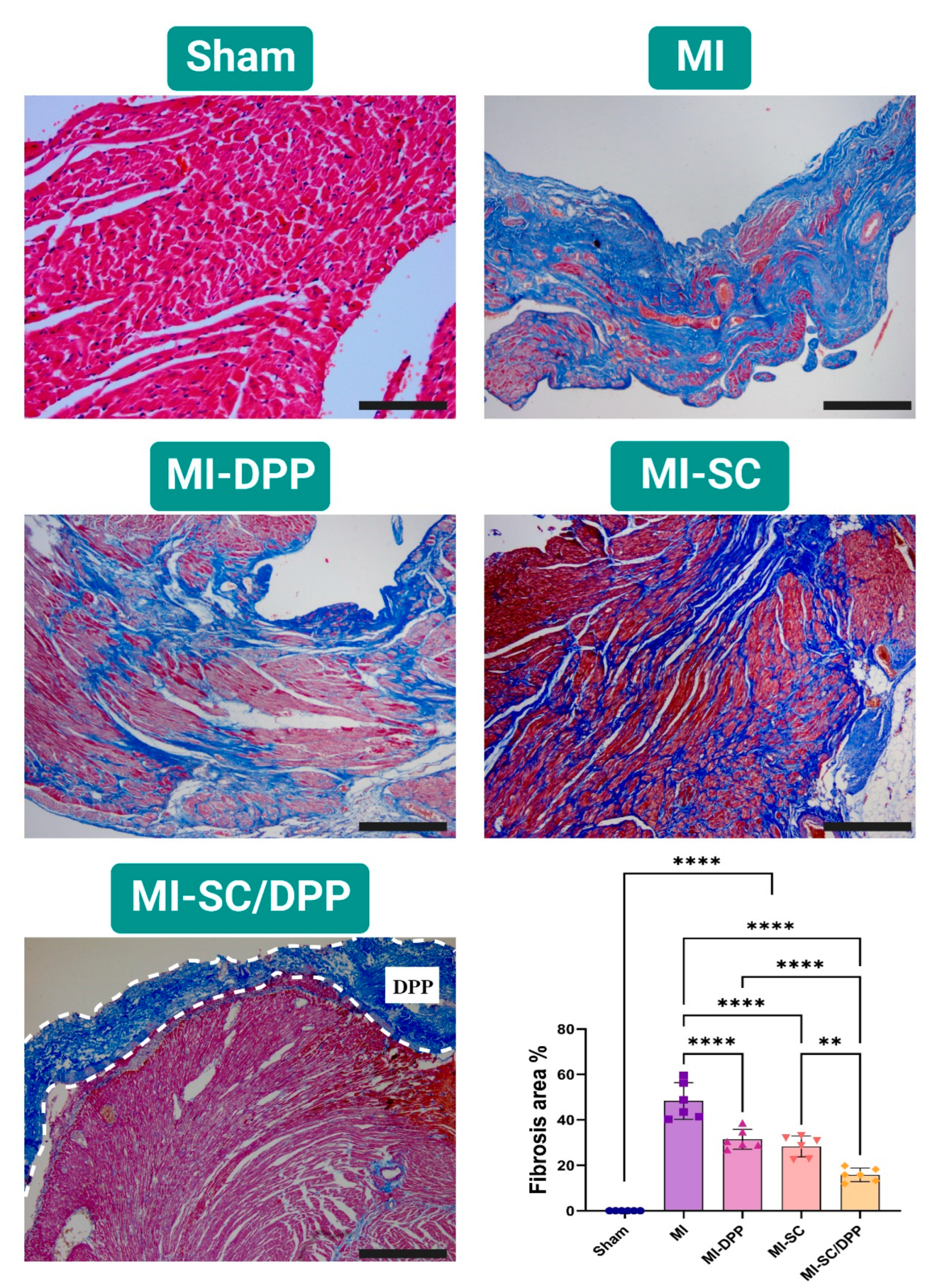
3.4. Histological Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.T.; Kim, H.; Yun, J.; Chung, J.S.; Kwon, S.-M. Promising Therapeutic Strategies for Mesenchymal Stem Cell-Based Cardiovascular Regeneration: From Cell Priming to Tissue Engineering. Stem Cells Int. 2017, 2017, 3945403. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Doghish, A.S.; Tanaka, R. Stimuli-responsive hydrogels: Smart state of-the-art platforms for cardiac tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1174075. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Kaneda, M.; Mady, E.A.; Yoshida, T.; Doghish, A.S.; Tanaka, R. Impact of Adipose Tissue Depot Harvesting Site on the Multilineage Induction Capacity of Male Rat Adipose-Derived Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 7513. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Helal, M.A.Y.; Tanaka, R. The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Veter. Sci. 2022, 9, 648. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Kaneda, M.; Shimada, K.; Nakazawa, Y.; Usui, T.; Elbadawy, M.; Ishihara, Y.; Hirose, M.; Kamei, Y.; et al. Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications. Pharmaceutics 2023, 15, 1906. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef]
- Houtgraaf, J.H.; Dekker, W.K.D.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef]
- Penn, M.S.; Mangi, A.A. Genetic Enhancement of Stem Cell Engraftment, Survival, and Efficacy. Circ. Res. 2008, 102, 1471–1482. [Google Scholar] [CrossRef]
- Kanda, M.; Nagai, T.; Kondo, N.; Matsuura, K.; Akazawa, H.; Komuro, I.; Kobayashi, Y. Pericardial Grafting of Cardiac Progenitor Cells in Self-Assembling Peptide Scaffold Improves Cardiac Function After Myocardial Infarction. Cell Transplant. 2023, 32, 09636897231174078. [Google Scholar] [CrossRef]
- Tanaka, T.; Hara, S.; Hendawy, H.; El-Husseiny, H.M.; Tanaka, R.; Asakura, T. Development of Small-Diameter Artificial Vascular Grafts Using Transgenic Silk Fibroin. Prosthesis 2023, 5, 763–773. [Google Scholar] [CrossRef]
- Texakalidis, P.; Giannopoulos, S.; Charisis, N.; Giannopoulos, S.; Karasavvidis, T.; Koullias, G.; Jabbour, P. A meta-analysis of randomized trials comparing bovine pericardium and other patch materials for carotid endarterectomy. J. Vasc. Surg. 2018, 68, 1241–1256.e1. [Google Scholar] [CrossRef]
- Kameli, S.M.; Khorramirouz, R.; Eftekharzadeh, S.; Fendereski, K.; Daryabari, S.S.; Tavangar, S.M.; Kajbafzadeh, A. Application of tissue-engineered pericardial patch in rat models of myocardial infarction. J. Biomed. Mater. Res. Part A 2018, 106, 2670–2678. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.-M.; Tafti, S.H.A.; Khorramirouz, R.; Sabetkish, S.; Kameli, S.M.; Orangian, S.; Rabbani, S.; Oveisi, N.; Golmohammadi, M.; Kashani, Z. Evaluating the role of autologous mesenchymal stem cell seeded on decellularized pericardium in the treatment of myocardial infarction: An animal study. Cell Tissue Bank. 2017, 18, 527–538. [Google Scholar] [CrossRef]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, H.; Yuan, Y.; Hu, B.; Gu, N. Recent fabrications and applications of cardiac patch in myocardial infarction treatment. View 2022, 3, 20200153. [Google Scholar] [CrossRef]
- Yao, Y.; Li, A.; Wang, S.; Lu, Y.; Xie, J.; Zhang, H.; Zhang, D.; Ding, J.; Wang, Z.; Tu, C.; et al. Multifunctional elastomer cardiac patches for preventing left ventricle remodeling after myocardial infarction in vivo. Biomaterials 2022, 282, 121382. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Wu, T.; Zhao, X.; Yang, R.; Wang, H.; Liu, W. Combined intramyocardial injectable hydrogel and pericardial adhesive hydrogel patch therapy strategy to achieve gene/ion/gas delivery for improving cardiac function. Nano Today 2023, 50, 101861. [Google Scholar] [CrossRef]
- Meyer, T.; Cebotari, S.; Brandes, G.; Hartung, D.; Wacker, F.; Theis, M.; Kaufeld, T.; Tudorache, I.; Nolte, I.; Haverich, A.; et al. Decellularized Porcine Pericardium Enhances Autologous Vascularized Matrix as a Prosthesis for Left Ventricular Full-Wall Myocardial Reconstruction. Prosthesis 2023, 5, 113–129. [Google Scholar] [CrossRef]
- Hashizume, R.; Fujimoto, K.L.; Hong, Y.; Guan, J.; Toma, C.; Tobita, K.; Wagner, W.R. Biodegradable elastic patch plasty ameliorates left ventricular adverse remodeling after ischemia–reperfusion injury: A preclinical study of a porous polyurethane material in a porcine model. J. Thorac. Cardiovasc. Surg. 2013, 146, 391–399.e1. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-J.; Chen, S.-C.; Chang, Y.; Hwang, S.-M.; Lin, W.-W.; Lai, P.-H.; Chiang, H.K.; Hsu, L.-F.; Yang, H.-H.; Sung, H.-W. Porous acellular bovine pericardia seeded with mesenchymal stem cells as a patch to repair a myocardial defect in a syngeneic rat model. Biomaterials 2006, 27, 5409–5419. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H. Evaluation of Some Prosthetic Implants for Surgical Management of Different Varieties of Hernias in Domestic Animals; Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Benha University: Banha, Egypt, 2017; pp. 42–43. [Google Scholar]
- El-Husseiny, M.H. Platelet Rich Fibrin Augmented Versus Non-Augmented Glycerolized Bovine Pericardium and Polypropylene Mesh for Repairing of Large Abdominal Wall Defects. Eur. J. Med. Nat. Sci. 2020, 3, 33–48. [Google Scholar] [CrossRef]
- Badylak, S.F.; Kochupura, P.V.; Cohen, I.S.; Doronin, S.V.; Saltman, A.E.; Gilbert, T.W.; Kelly, D.J.; Ignotz, R.A.; Gaudette, G.R. The Use of Extracellular Matrix as an Inductive Scaffold for the Partial Replacement of Functional Myocardium. Cell Transplant. 2006, 15, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.A.; Sellke, F.W.; Bianchi, C.; Khan, T.A.; Faro, R.; Zhang, J.-P.; Cohn, W.E. Endogenous myocardial angiogenesis and revascularization using a gastric submucosal patch. Ann. Thorac. Surg. 2003, 75, 1443–1449. [Google Scholar] [CrossRef]
- Tudorache, I.; Kostin, S.; Meyer, T.; Teebken, O.; Bara, C.; Hilfiker, A.; Haverich, A.; Cebotari, S. Viable vascularized autologous patch for transmural myocardial reconstruction. Eur. J. Cardio-Thorac. Surg. 2009, 36, 306–311. [Google Scholar] [CrossRef]
- Nazari, H.; Heirani-Tabasi, A.; Esmaeili, E.; Kajbafzadeh, A.-M.; Hassannejad, Z.; Boroomand, S.; Alavijeh, M.H.S.; Mishan, M.A.; Tafti, S.H.A.; Warkiani, M.E.; et al. Decellularized human amniotic membrane reinforced by MoS2-Polycaprolactone nanofibers, a novel conductive scaffold for cardiac tissue engineering. J. Biomater. Appl. 2022, 36, 1527–1539. [Google Scholar] [CrossRef]
- Rane, A.A.; Christman, K.L. Biomaterials for the Treatment of Myocardial Infarction. J. Am. Coll. Cardiol. 2011, 58, 2615–2629. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, J.-Y.; Zheng, Z.; Zhang, H.; Hu, S.-S. A novel vascularized patch enhances cell survival and modifies ventricular remodeling in a rat myocardial infarction model. J. Thorac. Cardiovasc. Surg. 2010, 140, 1388–1396.e3. [Google Scholar] [CrossRef]
- Araña, M.; Gavira, J.J.; Peña, E.; González, A.; Abizanda, G.; Cilla, M.; Pérez, M.M.; Albiasu, E.; Aguado, N.; Casado, M.; et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials 2014, 35, 143–151. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.S.; Go, J.L.; Hennessy, R.R.; Tefft, B.J.; Jana, S.; Stoyles, N.J.; Al-Hijji, M.A.; Thaden, J.J.; Pislaru, S.V.; Simari, R.D.; et al. Recellularization of a novel off-the-shelf valve following xenogenic implantation into the right ventricular outflow tract. PLoS ONE 2017, 12, e0181614. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Go, J.L.; Noble, C.; Morse, D.; Lerman, A.; Young, M.D. In Vivo Response of Acellular Porcine Pericardial for Tissue Engineered Transcatheter Aortic Valves. Sci. Rep. 2019, 9, 1094. [Google Scholar] [CrossRef]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Hou, D.; Youssef, E.A.-S.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L.; et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112, I-150–I-156. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.J.; Luo, J.; Chiu, R.C.; Shum-Tim, D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: Implications for cellular cardiomyoplasty. J. Thorac. Cardiovasc. Surg. 2006, 132, 628–632. [Google Scholar] [CrossRef]
- Ciampi, Q.; Villari, B. Role of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunction. Cardiovasc. Ultrasound 2007, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Takahashi, K.; Tanaka, R. Intraventricular pressure gradient: A novel colour M-mode echocardiographic-derived imaging modality to assess and predict the alterations following acute myocardial infarction. Eur. Heart J. 2023, 44, ehac779-004. [Google Scholar] [CrossRef]
- El-Husseiny, H.M. In Vitro and In Vivo Evaluation of Stem Cell-Seeded Biomaterials for Cardiac Tissue Engineering after Myocardial Infarction; Tokyo University of Agriculture and Technology: Fuchu, Japan, 2023. [Google Scholar]
- Ramm, R.; Goecke, T.; Theodoridis, K.; Hoeffler, K.; Sarikouch, S.; Findeisen, K.; Ciubotaru, A.; Cebotari, S.; Tudorache, I.; Haverich, A.; et al. Decellularization combined with enzymatic removal of N-linked glycans and residual DNA reduces inflammatory response and improves performance of porcine xenogeneic pulmonary heart valves in an ovine in vivo model. Xenotransplantation 2020, 27, e12571. [Google Scholar] [CrossRef]
- Tan, M.Y.; Zhi, W.; Wei, R.Q.; Huang, Y.C.; Zhou, K.P.; Tan, B.; Deng, L.; Luo, J.C.; Li, X.Q.; Xie, H.Q.; et al. Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials 2009, 30, 3234–3240. [Google Scholar] [CrossRef]
- Sharma, N.M.; Cunningham, C.J.; Zheng, H.; Liu, X.; Patel, K.P. Hypoxia-Inducible Factor-1α Mediates Increased Sympathoexcitation via Glutamatergic N-Methyl-d-Aspartate Receptors in the Paraventricular Nucleus of Rats with Chronic Heart Failure. Circ. Heart Fail. 2016, 9, e003423. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, R.Y.; Park, B.-W.; Lee, S.; Choi, S.W.; Park, J.-H.; Choi, J.J.; Kim, S.-W.; Jang, J.; Cho, D.-W.; et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019, 10, 3123. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.-J.; Park, J.-H.; Lee, S.; Park, B.-W.; Lee, S.M.; Hwang, J.-W.; Kim, J.-J.; Kang, B.; Sim, W.-S.; et al. Enhancement strategy for effective vascular regeneration following myocardial infarction through a dual stem cell approach. Exp. Mol. Med. 2022, 54, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Ayres, N.; Cuneo, B.; Gotteiner, N.; Hornberger, L.; Spevak, P.J.; Van Der Veld, M. American society of echocardiography guidelines and standards for performance of the fetal echocardiogram. J. Am. Soc. Echocardiogr. 2004, 17, 803–810. [Google Scholar] [CrossRef]
- Zacchigna, S.; Paldino, A.; Falcão-Pires, I.; Daskalopoulos, E.P.; Ferro, M.D.; Vodret, S.; Lesizza, P.; Cannatà, A.; Miranda-Silva, D.; Lourenço, A.P.; et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar] [CrossRef]
- Shi, K.; Zhao, W.; Chen, Y.; Ho, W.T.; Yang, P.; Zhao, Z.J. Cardiac hypertrophy associated with myeloproliferative neoplasms in JAK2V617F transgenic mice. J. Hematol. Oncol. 2014, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Verma, P.; Hourigan, K.; Banerjee, R. Myocardial infarction: Stem cell transplantation for cardiac regeneration. Regen. Med. 2015, 10, 1025–1043. [Google Scholar] [CrossRef]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Lang, I.; Dettke, M.; Beran, G.; Graf, S.; Sochor, H.; Nyolczas, N.; Charwat, S.; Hemetsberger, R.; Christ, G.; et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: The MYSTAR prospective, randomized study. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 70–81. [Google Scholar] [CrossRef]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic Delivery of Bone Marrow–Derived Mesenchymal Stem Cells to the Infarcted Myocardium. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef]
- Janssens, S.; Dubois, C.; Bogaert, J.; Theunissen, K.; Deroose, C.; Desmet, W.; Kalantzi, M.; Herbots, L.; Sinnaeve, P.; Dens, J.; et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet 2006, 367, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; López, J. Methods of stem cell delivery in cardiac diseases. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S110–S113. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Martens, T.P.; Viles-Gonzalez, J.F.; Siminiak, T. Catheter-based delivery of cells to the heart. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Sarig, U.; Sarig, H.; De-Berardinis, E.; Chaw, S.-Y.; Nguyen, E.B.; Ramanujam, V.S.; Thang, V.D.; Al-Haddawi, M.; Liao, S.; Seliktar, D.; et al. Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomater. 2016, 44, 209–220. [Google Scholar] [CrossRef]
- Shah, M.; Kc, P.; Zhang, G. In Vivo Assessment of Decellularized Porcine Myocardial Slice as an Acellular Cardiac Patch. ACS Appl. Mater. Interfaces 2019, 11, 23893–23900. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Bai, A.; Jiang, W.; Li, X.; Wang, X.; Mao, Y.; Lu, C.; Qian, R.; Guo, F.; et al. Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials 2016, 105, 52–65. [Google Scholar] [CrossRef]
- Passier, R.; van Laake, L.W.; Mummery, C.L. Stem-cell-based therapy and lessons from the heart. Nature 2008, 453, 322–329. [Google Scholar] [CrossRef]
- Iwano, H.; Kamimura, D.; Fox, E.; Hall, M.; Vlachos, P.; Little, W.C. Altered Spatial Distribution of the Diastolic Left Ventricular Pressure Difference in Heart Failure. J. Am. Soc. Echocardiogr. 2015, 28, 597–605.e1. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]




| Time | W 2 | W 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Sham | MI | MI-DPP | MI-SC | MI-SC/DPP | Sham | MI | MI-DPP | MI-SC | MI-SC/DPP |
| Dimensional Parameters: | ||||||||||
| LVIDd (mm) | 6.45 ± 0.43 | 7.83 ± 0.41 **** | 7.37 ± 0.20 *** | 6.88 ± 0.24 †††† | 6.67 ± 0.17 ††††‡‡ | 6.40 ± 0.34 | 8.88 ± 0.41 **** | 7.15 ± 0.17 **†††† | 6.68 ± 0.24 †††† | 6.23 ± 0.20 ††††‡‡‡ |
| IVSd (mm) | 2.07 ± 0.26 | 1.52 ± 0.20 **** | 1.63 ± 0.17 ** | 1.73 ± 0.15 * | 1.97 ± 0.11 ††‡ | 2.10 ± 0.26 | 1.50± 0.13 **** | 1.73 ± 0.15 * | 1.90 ± 0.08 †† | 1.97 ± 0.11 ††† |
| LVPWd (mm) | 1.70 ± 0.21 | 1.90 ± 0.31 | 1.88 ± 0.13 | 1.77 ± 0.11 | 1.68 ± 0.11 | 1.75 ± 0.10 | 2.13 ± 0.29 * | 1.80 ± 0.13 † | 1.68 ± 0.20 †† | 1.63 ± 0.11 ††† |
| LVIDs (mm) | 3.60 ± 0.22 | 5.15 ± 0.28 **** | 4.53 ± 0.15 ****†† | 3.98 ± 0.21 ††††‡ | 3.80 ± 0.16 ††††‡‡‡ | 3.70 ± 0.26 | 6.70 ± 0.49 **** | 4.30 ± 0.13 **†††† | 3.97 ± 0.20 †††† | 3.78 ± 0.29 ††††‡ |
| IVSs (mm) | 2.02 ± 0.11 | 1.70 ± 0.21 * | 1.72 ± 0.14 * | 1.82 ± 0.26 | 1.95 ± 0.10 | 2.07 ± 0.20 | 1.47 ± 0.14 **** | 1.73 ± 0.07 ** | 1.78 ± 0.11 *† | 1.93 ± 0.07 ††† |
| LVPWs (mm) | 2.48 ± 0.16 | 1.95 ± 0.17 **** | 1.95 ± 0.19 **** | 2.10 ± 0.08 ** | 2.15 ± 0.13 * | 2.27 ± 0.17 | 1.60 ± 0.24 **** | 1.92 ± 0.23 *† | 2.15 ± 0.10 †††† | 2.08 ± 0.11 ††† |
| LVM (mg) | 667.9 ± 117.47 | 787.52 ± 123.24 | 741.62 ± 65.57 | 660.78 ± 46.03 | 668.71 ± 58.52 | 678.45 ± 63.15 | 1052.83 ± 134.25 **** | 711.89 ± 55.78 †††† | 652.32 ± 51.27 †††† | 590.92 ± 54.69 †††† |
| Flow Parameters: | ||||||||||
| EF (%) | 86.73 ± 1.79 | 57.6 ± 2.88 **** | 60.7 ± 1.33 **** | 66.05 ± 3.83 ****†††‡ | 76.1 ± 2.34 ****††††‡‡‡‡#### | 84.82 ± 2.22 | 54.48 ± 5.09 **** | 65.97 ± 2.74 ****†††† | 72.67 ± 2.11 ****††††‡‡ | 80.32 ± 1.30 ††††‡‡‡‡## |
| FS (%) | 48.53 ± 1.74 | 32.73 ± 4.01 **** | 33.05 ± 2.69 **** | 38.03 ± 2.81 ****†‡ | 42.48 ± 2.23 **††††‡‡‡‡ | 46.68 ± 2.59 | 31.23 ± 1.05 **** | 35.53 ± 2.29 **** | 37.95 ± 2.66 ****†† | 43.55 ± 2.71 ††††‡‡‡‡# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Husseiny, H.M.; Mady, E.A.; Usui, T.; Ishihara, Y.; Yoshida, T.; Kobayashi, M.; Sasaki, K.; Ma, D.; Yairo, A.; Mandour, A.S.; et al. Adipose Stem Cell-Seeded Decellularized Porcine Pericardium: A Promising Functional Biomaterial to Synergistically Restore the Cardiac Functions Post-Myocardial Infarction. Vet. Sci. 2023, 10, 660. https://doi.org/10.3390/vetsci10110660
El-Husseiny HM, Mady EA, Usui T, Ishihara Y, Yoshida T, Kobayashi M, Sasaki K, Ma D, Yairo A, Mandour AS, et al. Adipose Stem Cell-Seeded Decellularized Porcine Pericardium: A Promising Functional Biomaterial to Synergistically Restore the Cardiac Functions Post-Myocardial Infarction. Veterinary Sciences. 2023; 10(11):660. https://doi.org/10.3390/vetsci10110660
Chicago/Turabian StyleEl-Husseiny, Hussein M., Eman A. Mady, Tatsuya Usui, Yusuke Ishihara, Toshinori Yoshida, Mio Kobayashi, Kenta Sasaki, Danfu Ma, Akira Yairo, Ahmed S. Mandour, and et al. 2023. "Adipose Stem Cell-Seeded Decellularized Porcine Pericardium: A Promising Functional Biomaterial to Synergistically Restore the Cardiac Functions Post-Myocardial Infarction" Veterinary Sciences 10, no. 11: 660. https://doi.org/10.3390/vetsci10110660
APA StyleEl-Husseiny, H. M., Mady, E. A., Usui, T., Ishihara, Y., Yoshida, T., Kobayashi, M., Sasaki, K., Ma, D., Yairo, A., Mandour, A. S., Hendawy, H., Doghish, A. S., Mohammed, O. A., Takahashi, K., & Tanaka, R. (2023). Adipose Stem Cell-Seeded Decellularized Porcine Pericardium: A Promising Functional Biomaterial to Synergistically Restore the Cardiac Functions Post-Myocardial Infarction. Veterinary Sciences, 10(11), 660. https://doi.org/10.3390/vetsci10110660