The Relationship between Mastitis and Antimicrobial Peptide S100A7 Expression in Dairy Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sample and Goat Mammary Tissue Sample
2.2. Somatic Cell Count Determination
2.3. Bacteriological Culture
2.4. Immunohistochemistry
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Total RNA Isolation and cDNA Synthesis
2.7. q-PCR
2.8. Statistics
3. Results
3.1. The Difference of S100A7 Concentration in Subclinical and Clinical Mastitis Goats
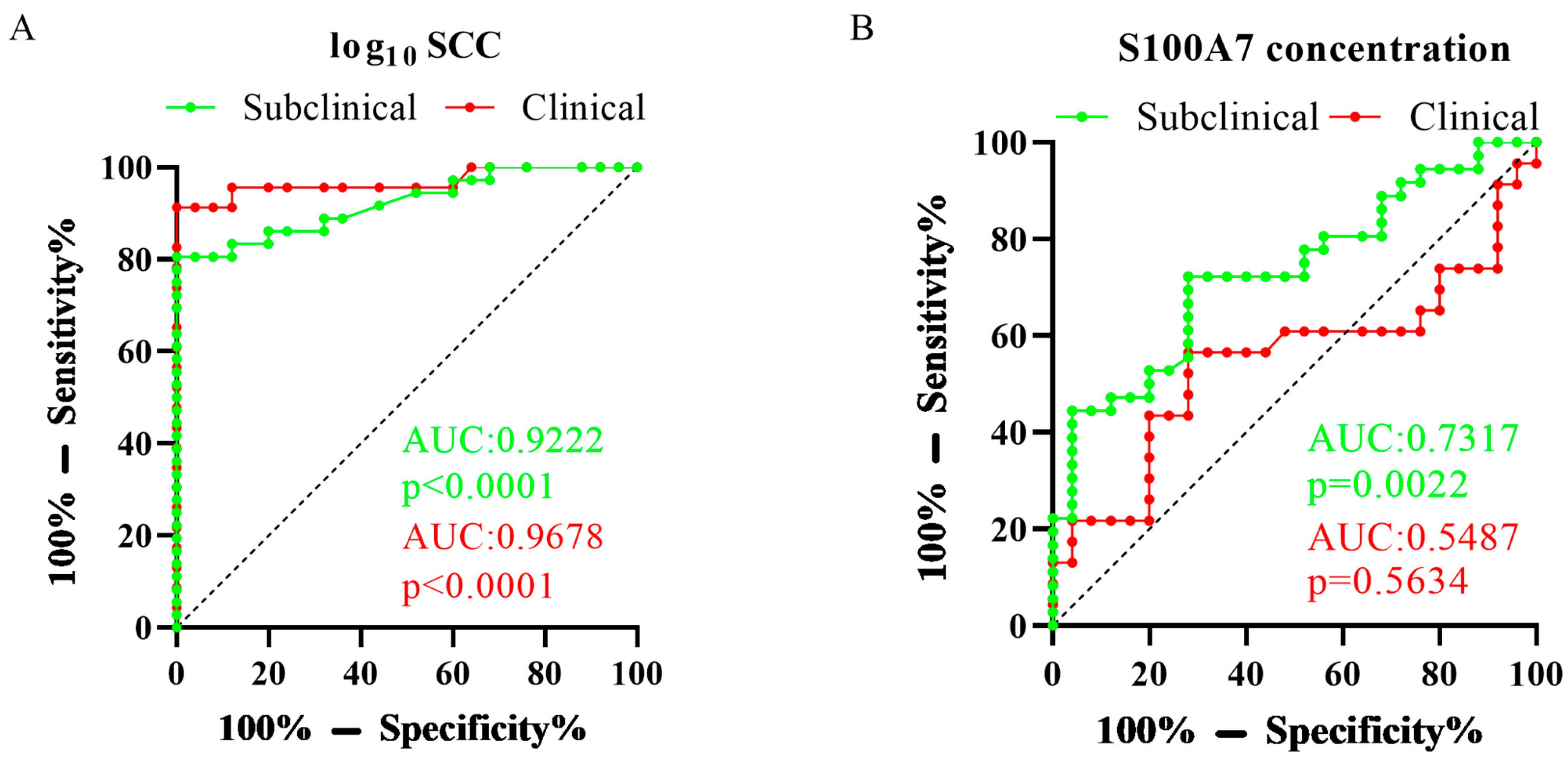
3.2. Test Characteristics of SCC and S100A7 Levels in Subclinical and Clinical Mastitis Goats
3.3. Relationship between the SCC and S100A7 Concentration in Dairy Goat
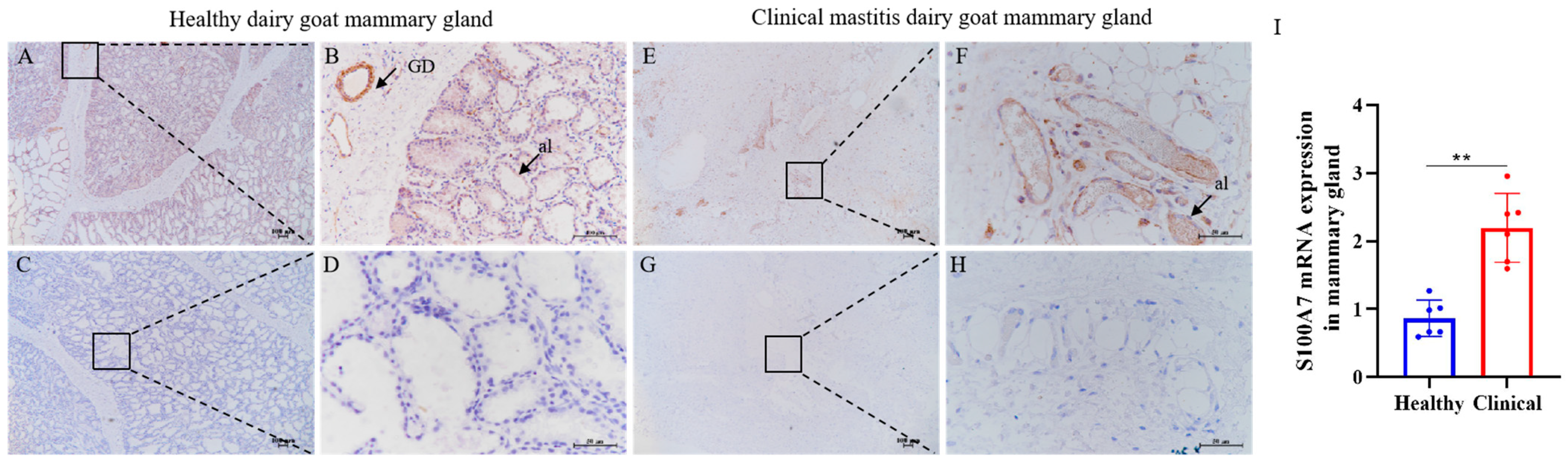
3.4. The Immunohistochemistry and Expression of S100A7 in Healthy and Clinical Mastitis Dairy Goat Gland
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blosser, T.H. Economic losses from and the national research program on mastitis in the United States. J. Dairy Sci. 1979, 62, 119–127. [Google Scholar] [CrossRef]
- Hagnestam, C.; Emanuelson, U.; Berglund, B. Yield losses associated with clinical mastitis occurring in different weeks of lactation. J. Dairy Sci. 2007, 90, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Kayano, M.; Itoh, M.; Kusaba, N.; Hayashiguchi, O.; Kida, K.; Tanaka, Y.; Kawamoto, K.; Gröhn, Y.T. Associations of the first occurrence of pathogen-specific clinical mastitis with milk yield and milk composition in dairy cows. J. Dairy Res. 2018, 85, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Koop, G.; van Werven, T.; Toft, N.; Nielen, M. Estimating test characteristics of somatic cell count to detect Staphylococcus aureus-infected dairy goats using latent class analysis. J. Dairy Sci. 2011, 94, 2902–2911. [Google Scholar] [CrossRef]
- Boireau, C.; Cazeau, G.; Jarrige, N.; Calavas, D.; Madec, J.Y.; Leblond, A.; Haenni, M.; Gay, É. Antimicrobial resistance in bacteria isolated from mastitis in dairy cattle in France, 2006–2016. J. Dairy Sci. 2018, 101, 9451–9462. [Google Scholar] [CrossRef]
- Romero, J.; Benavides, E.; Meza, C. Assessing Financial Impacts of Subclinical Mastitis on Colombian Dairy Farms. Front Vet. Sci. 2018, 5, 273. [Google Scholar] [CrossRef]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, B.E.; Lewis, M.J.; Boonyayatra, S.; Maxwell, M.L.; Saxton, A.; Oliver, S.P.; Almeida, R.A. Short communication: Evaluation of bulk tank milk microbiological quality of nine dairy farms in Tennessee. J. Dairy Sci. 2012, 95, 4275–4279. [Google Scholar] [CrossRef]
- Jayarao, B.M.; Pillai, S.R.; Sawant, A.A.; Wolfgang, D.R.; Hegde, N.V. Guidelines for monitoring bulk tank milk somatic cell and bacterial counts. J. Dairy Sci. 2004, 87, 3561–3573. [Google Scholar] [CrossRef]
- Lafi, S.Q.; Al-Majali, A.M.; Rousan, M.D.; Alawneh, J.M. Epidemiological studies of clinical and subclinical ovine mastitis in Awassi sheep in northern Jordan. Prev. Vet. Med. 1998, 33, 171–181. [Google Scholar] [CrossRef]
- Schaeren, W.; Maurer, J. Prevalence of subclinical udder infections and individual somatic cell counts in three dairy goat herds during a full lactation. Schweiz. Arch. Tierheilkd. 2006, 148, 641–648. [Google Scholar] [CrossRef]
- Paape, M.J.; Capuco, A.V. Cellular defense mechanisms in the udder and lactation of goats. J. Anim. Sci. 1997, 75, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Poutrel, B.; de Crémoux, R.; Ducelliez, M.; Verneau, D. Control of intramammary infections in goats: Impact on somatic cell counts. J. Anim. Sci. 1997, 75, 566–570. [Google Scholar] [CrossRef]
- Contreras, A.; Sierra, D.; Corrales, J.C.; Sanchez, A.; Marco, J.L.A.; Contreras, J.A.; Sierramercado, D.; Corrales, J.; Alvarezsanchez, A.; Marco, J.H. Physiological threshold of somatic cell count and California Mastitis Test for diagnosis of caprine subclinical mastitis. Small Rumin. Res. J. Int. Goat Assoc. 1996, 21, 259–264. [Google Scholar] [CrossRef]
- Contreras, A.; Luengo, C.; Sánchez, A.; Corrales, J.C. The role of intramammary pathogens in dairy goats. Livest. Prod. Sci. 2003, 79, 273–283. [Google Scholar] [CrossRef]
- Hall, S.M.; Rycroft, A.N. Causative organisms and somatic cell counts in subclinical intramammary infections in milking goats in the UK. Vet. Rec. 2007, 160, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Sears, P.M.; Smith, B.S.; English, P.B.; Herer, P.S.; Gonzalez, R.N. Shedding pattern of Staphylococcus aureus from bovine intramammary infections. J. Dairy Sci. 1990, 73, 2785–2789. [Google Scholar] [CrossRef]
- Pieterse, R.; Todorov, S.D. Bacteriocins-exploring alternatives to antibiotics in mastitis treatment. Braz. J. Microbiol. 2010, 41, 542–562. [Google Scholar] [CrossRef] [PubMed]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Olsen, E.; Etzerodt, M.; Madsen, P.; Thøgersen, H.C.; Kruse, T.; Celis, J.E. Psoriasin binds calcium and is upregulated by calcium to levels that resemble those observed in normal skin. J. Investig. Dermatol. 1994, 103, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.; Rasmussen, H.H.; Leffers, H.; Honoré, B.; Dejgaard, K.; Olsen, E.; Kiil, J.; Walbum, E.; Andersen, A.H.; Basse, B.; et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J. Investig. Dermatol. 1991, 97, 701–712. [Google Scholar] [CrossRef]
- Schäfer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, D.E.; Etzerodt, M.; Madsen, P.; Celis, J.E.; Thøgersen, H.C.; Nyborg, J.; Kjeldgaard, M. EF-hands at atomic resolution: The structure of human psoriasin (S100A7) solved by MAD phasing. Structure 1998, 6, 477–489. [Google Scholar] [CrossRef]
- Donato, R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1999, 1450, 191–231. [Google Scholar] [CrossRef]
- Regenhard, P.; Leippe, M.; Schubert, S.; Podschun, R.; Kalm, E.; Grötzinger, J.; Looft, C. Antimicrobial activity of bovine psoriasin. Vet. Microbiol. 2009, 136, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.W.; Lai, S.J.; Yoshimura, Y.; Isobe, N. Messenger RNA expression and immunolocalization of psoriasin in the goat mammary gland and its milk concentration after an intramammary infusion of lipopolysaccharide. Vet. J. 2014, 202, 89–93. [Google Scholar] [CrossRef]
- Zhang, G.W.; Lai, S.J.; Yoshimura, Y.; Isobe, N. Expression of cathelicidins mRNA in the goat mammary gland and effect of the intramammary infusion of lipopolysaccharide on milk cathelicidin-2 concentration. Vet. Microbiol. 2014, 170, 125–134. [Google Scholar] [CrossRef]
- Tedde, V.; Bronzo, V.; Puggioni, G.M.G.; Pollera, C.; Casula, A.; Curone, G.; Moroni, P.; Uzzau, S.; Addis, M.F. Milk cathelicidin and somatic cell counts in dairy goats along the course of lactation. J. Dairy Res. 2019, 86, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Akamatsu, H.; Obayashi, T.; Nagahata, H.; Higuchi, H.; Iwano, H.; Oshida, T.; Yoshimura, Y.; Isobe, N. Relationship between concentration of lingual antimicrobial peptide and somatic cell count in milk of dairy cows. Vet. Immunol. Immunopathol. 2013, 153, 298–301. [Google Scholar] [CrossRef]
- Addis, M.F.; Tedde, V.; Puggioni, G.M.G.; Pisanu, S.; Casula, A.; Locatelli, C.; Rota, N.; Bronzo, V.; Moroni, P.; Uzzau, S. Evaluation of milk cathelicidin for detection of bovine mastitis. J. Dairy Sci. 2016, 99, 8250–8258. [Google Scholar] [CrossRef]
- Wolf, R.; Howard, O.M.; Dong, H.F.; Voscopoulos, C.; Boeshans, K.; Winston, J.; Divi, R.; Gunsior, M.; Goldsmith, P.; Ahvazi, B.; et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J. Immunol. 2008, 181, 1499–1506. [Google Scholar] [CrossRef]
- Gross, S.R.; Sin, C.G.; Barraclough, R.; Rudland, P.S. Joining S100 proteins and migration: For better or for worse, in sickness and in health. Cell Mol. Life Sci. 2014, 71, 1551–1579. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Hutchison, B.L.; Mitchell, E.A. Mastitis in the first year postpartum. Birth 1999, 26, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Michie, C.; Lockie, F.; Lynn, W. The challenge of mastitis. Arch. Dis. Child. 2003, 88, 818–821. [Google Scholar] [CrossRef]
- Amir, L.H.; Forster, D.A.; Lumley, J.; McLachlan, H. A descriptive study of mastitis in Australian breastfeeding women: Incidence and determinants. BMC Public Health 2007, 7, 62. [Google Scholar] [CrossRef]
- Scott, J.A.; Robertson, M.; Fitzpatrick, J.; Knight, C.; Mulholland, S. Occurrence of lactational mastitis and medical management: A prospective cohort study in Glasgow. Int. Breastfeed J. 2008, 3, 21. [Google Scholar] [CrossRef]
- Contreras, G.A.; Rodríguez, J.M. Mastitis: Comparative etiology and epidemiology. J. Mammary Gland Biol. Neoplasia 2011, 16, 339–356. [Google Scholar] [CrossRef]
- Koop, G.; Islam, M.N.; Rahman, M.M.; Khatun, M.; Ferdous, J.; Sayeed, M.A.; Islam, S.; Ahaduzzaman, M.; Akter, S.; Mannan, A.; et al. Risk factors and therapy for goat mastitis in a hospital-based case-control study in Bangladesh. Prev. Vet. Med. 2016, 124, 52–57. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Wu, Y.; Yan, Y.; Zhao, X.; Wei, Q.; Ma, B. NF-κB-Dependent Snail Expression Promotes Epithelial-Mesenchymal Transition in Mastitis. Animals 2021, 11, 3422. [Google Scholar] [CrossRef]
- Windria, S.; Salasia, S.I.O.; Nugroho, W.; Widayanti, R.; Indarjulianto, S. Development of ELISA against milk haptoglobin for diagnosis of subclinical mastitis in goats. Heliyon 2021, 7, e06314. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, C.; Martínez, J.R.; Carrledo, J.A.; San Primitivo, F. Fossomatic cell-counting on ewe milk: Comparison with direct microscopy and study of variation factors. J. Dairy Sci. 2003, 86, 138–145. [Google Scholar] [CrossRef]
- Wicks, H.C.; Leaver, J.D. Influence of genetic merit and environment on somatic cell counts of Holstein-Friesian cows. Vet. J. 2006, 172, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Lôbo, R.N.B.; El Faro, L.; Dos Santos, G.G.; Bruneli, F.Â.T.; Peixoto, M. Genetic parameters for somatic cell count (SCC) and milk production traits of Guzerá cows using data normalized by different procedures. Trop Anim. Health Prod. 2020, 52, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Paape, M.J.; Di Carlo, A.L.; Miller, R.H.; Rainard, P. Evaluation of selected antibiotic residue screening tests for milk from individual goats. J. Dairy Sci. 1997, 80, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Persson, Y.; Olofsson, I. Direct and indirect measurement of somatic cell count as indicator of intramammary infection in dairy goats. Acta Vet. Scand. 2011, 53, 15. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, K.; Fan, M.; Wan, W.; Zhao, X.; Pan, M.; Ma, B.; Wei, Q. Immunolocalization of antibacterial peptide S100A7 in mastitis goat mammary gland and lipopolysaccharide induces the expression and secretion of S100A7 in goat mammary gland epithelial cells via TLR4/NFκB signal pathway. Anim. Biotechnol. 2023, 34, 2701–2713. [Google Scholar] [CrossRef]
- Samara, E.M.; Ayadi, M.; Aljumaah, R.S. Feasibility of utilising an infrared-thermographic technique for early detection of subclinical mastitis in dairy camels (Camelus dromedarius). J. Dairy Res. 2014, 81, 38–45. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Murdough, P.; Pankey, W.; Delaney, C.; Barlow, J.; Scruton, D. Relationships among somatic cell count, California mastitis test, impedance and bacteriological status of milk in goats and sheep in early lactation. Small Rumin. Res. 2001, 40, 245–254. [Google Scholar] [CrossRef]
- Franceschi, P.; Faccia, M.; Malacarne, M.; Formaggioni, P.; Summer, A. Quantification of Cheese Yield Reduction in Manufacturing Parmigiano Reggiano from Milk with Non-Compliant Somatic Cells Count. Foods 2020, 9, 212. [Google Scholar] [CrossRef]
- Schalm, O.W.; Noorlander, D.O. Experiments and observations leading to development of the California mastitis test. J Am. Vet. Med. Assoc. 1957, 130, 199–204. [Google Scholar]
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet. World 2018, 11, 562–577. [Google Scholar] [CrossRef]
- Isobe, N. Control mechanisms for producing antimicrobial factors in ruminant mammary gland. Anim. Sci. J. 2017, 88, 937–943. [Google Scholar] [CrossRef]
- Tsugami, Y.; Suzuki, N.; Nii, T.; Isobe, N. Sodium Acetate and Sodium Butyrate Differentially Upregulate Antimicrobial Component Production in Mammary Glands of Lactating Goats. J. Mammary Gland Biol. Neoplasia 2022, 27, 133–144. [Google Scholar] [CrossRef]
- Purba, F.Y.; Nii, T.; Yoshimura, Y.; Isobe, N. Short communication: Production of antimicrobial peptide S100A8 in the goat mammary gland and effect of intramammary infusion of lipopolysaccharide on S100A8 concentration in milk. J. Dairy Sci. 2019, 102, 4674–4681. [Google Scholar] [CrossRef]
- Bhatt, T.; Bhosale, A.; Bajantri, B.; Mathapathi, M.S.; Rizvi, A.; Scita, G.; Majumdar, A.; Jamora, C. Sustained Secretion of the Antimicrobial Peptide S100A7 Is Dependent on the Downregulation of Caspase-8. Cell Rep. 2019, 29, 2546–2555.e4. [Google Scholar] [CrossRef]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005, 6, 57–64. [Google Scholar] [CrossRef]
- Regenhard, P.; Petzl, W.; Zerbe, H.; Sauerwein, H. The antibacterial psoriasin is induced by E. coli infection in the bovine udder. Vet. Microbiol. 2010, 143, 293–298. [Google Scholar] [CrossRef]
- Tetens, J.; Friedrich, J.J.; Hartmann, A.; Schwerin, M.; Kalm, E.; Thaller, G. The spatial expression pattern of antimicrobial peptides across the healthy bovine udder. J. Dairy Sci. 2010, 93, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Tsugami, Y.; Harada, R.; Nii, T.; Suzuki, N.; Isobe, N. Effects of frequent teat stimulation on antimicrobial component production in mammary glands of lactating goats. Vet. Immunol. Immunopathol. 2022, 249, 110431. [Google Scholar] [CrossRef] [PubMed]
- EisenbeiB, D.; Ardebilli, S.; Harder, J.; Lange, H.; Rudolph, B.; Schröder, J.; Weichenthal, M.; Gläser, R. The antimicrobial protein psoriasin (S100A7) is significantly upregulated in the peripheral blood of psoriasis patients. J. Investig. Dermatol. 2006, 126, 61. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhu, K.; Liu, H.; Fan, M.; Zhao, X.; Pan, M.; Ma, B.; Wei, Q. The Relationship between Mastitis and Antimicrobial Peptide S100A7 Expression in Dairy Goats. Vet. Sci. 2023, 10, 653. https://doi.org/10.3390/vetsci10110653
Yan Y, Zhu K, Liu H, Fan M, Zhao X, Pan M, Ma B, Wei Q. The Relationship between Mastitis and Antimicrobial Peptide S100A7 Expression in Dairy Goats. Veterinary Sciences. 2023; 10(11):653. https://doi.org/10.3390/vetsci10110653
Chicago/Turabian StyleYan, Yutong, Kunyuan Zhu, Haokun Liu, Mingzhen Fan, Xiaoe Zhao, Menghao Pan, Baohua Ma, and Qiang Wei. 2023. "The Relationship between Mastitis and Antimicrobial Peptide S100A7 Expression in Dairy Goats" Veterinary Sciences 10, no. 11: 653. https://doi.org/10.3390/vetsci10110653
APA StyleYan, Y., Zhu, K., Liu, H., Fan, M., Zhao, X., Pan, M., Ma, B., & Wei, Q. (2023). The Relationship between Mastitis and Antimicrobial Peptide S100A7 Expression in Dairy Goats. Veterinary Sciences, 10(11), 653. https://doi.org/10.3390/vetsci10110653




