In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
- Synthesis of methyl 4-(1H-benz[d]imidazol-2-yl) benzoate (1)
- Synthesis of methyl 4-(1H-benz[d]imidazol-2-yl) benzohydrazide (2)
- Synthesis of N-methyl/ethyl-2-[4-(1H-benzimidazol-2-yl)benzoyl]hydrazine-1-carbothioamide (3a, 3b)
- Synthesis of 3-(4-(1H-benzimidazol-2-yl)phenyl)-5-mercapto-2-(4-(4-methyl/ethyl)-4H-1,2,4-triazole (4a–4b)
- Synthesis of target compounds (5a–5h)
2.2. Culture of the Leishmania tropica Isolate
2.3. Molecular Docking
2.4. Molecular Dynamic Simulations
3. Results
3.1. Chemistry
3.2. Antileishmanial Activity
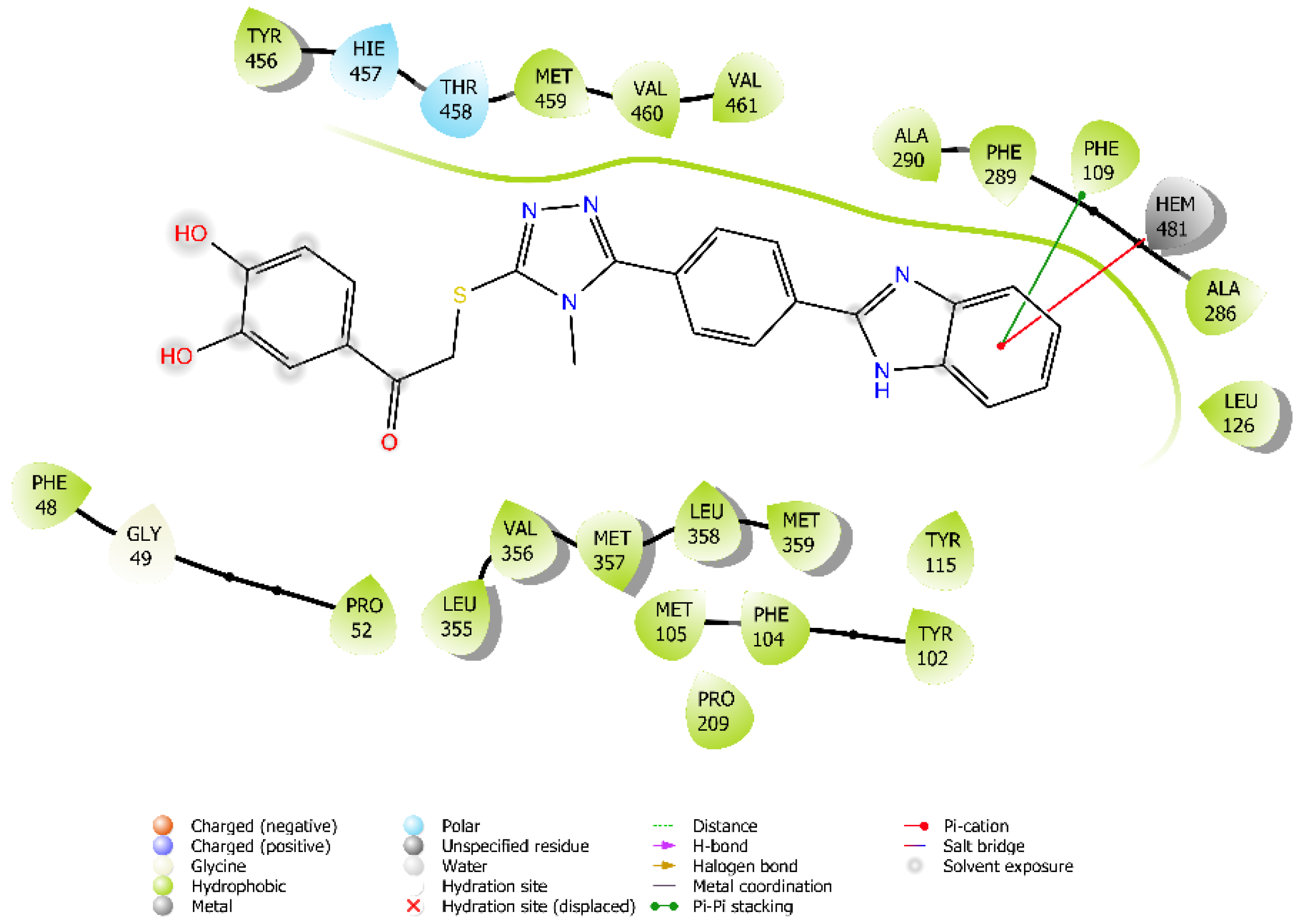
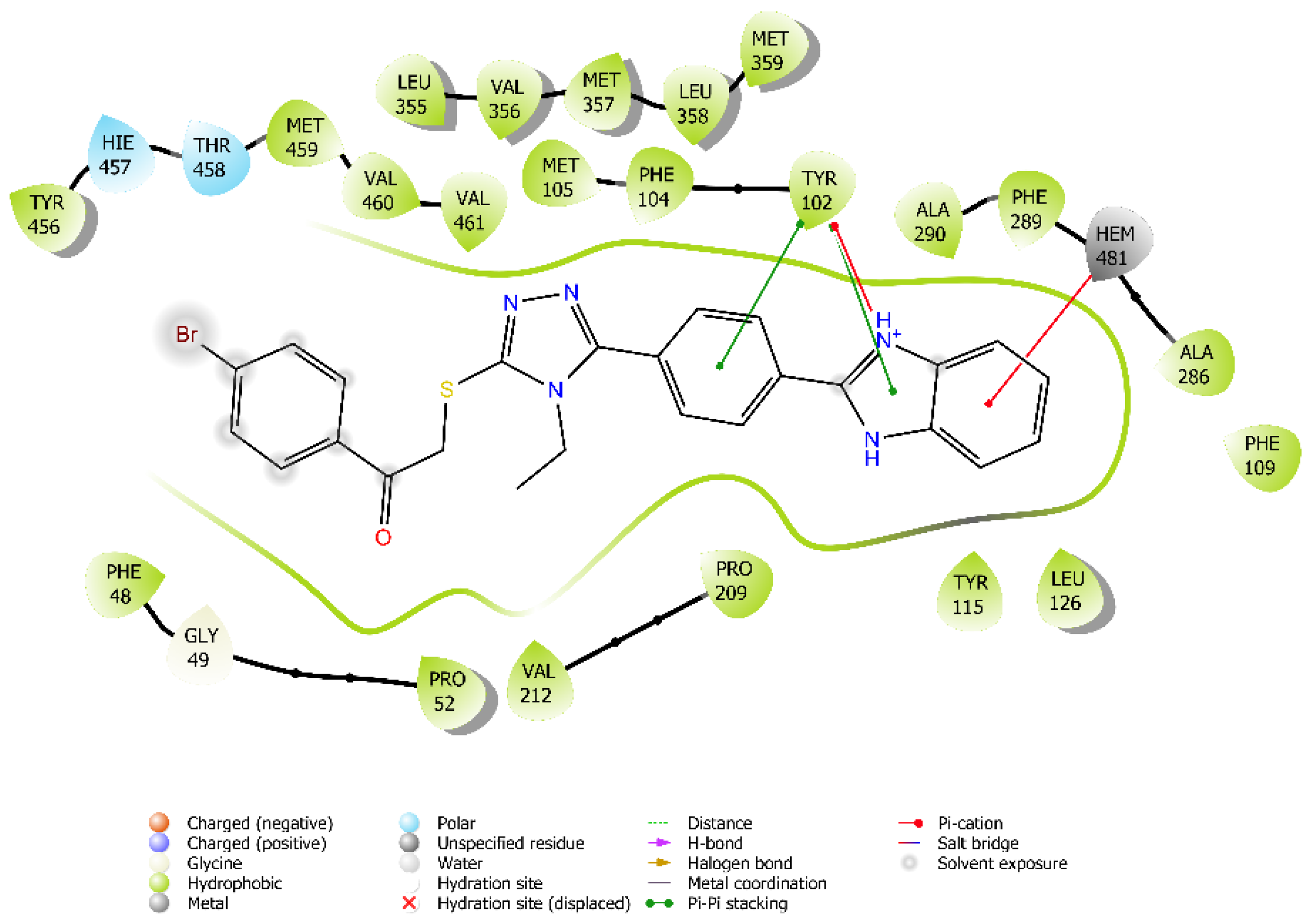
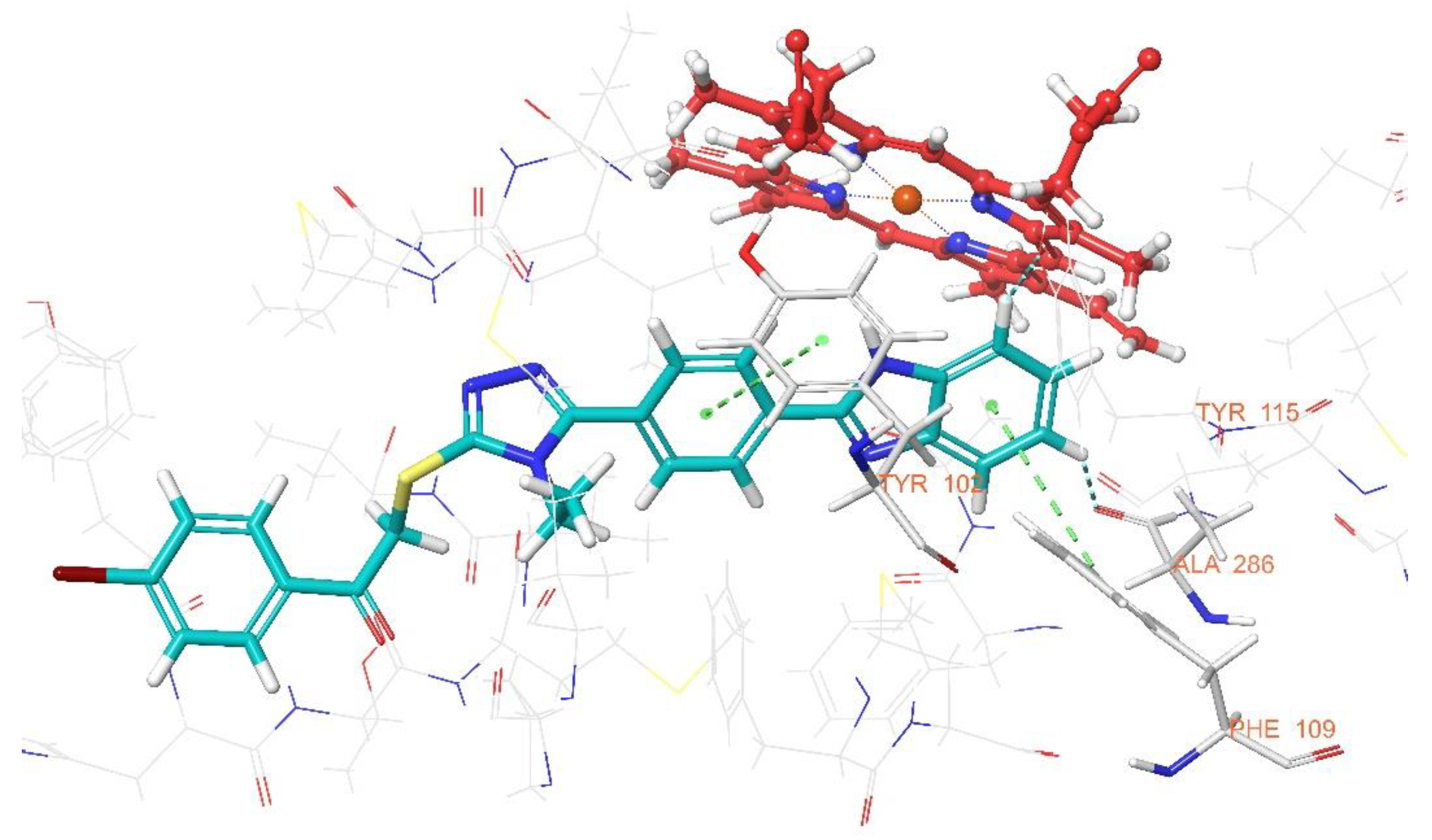
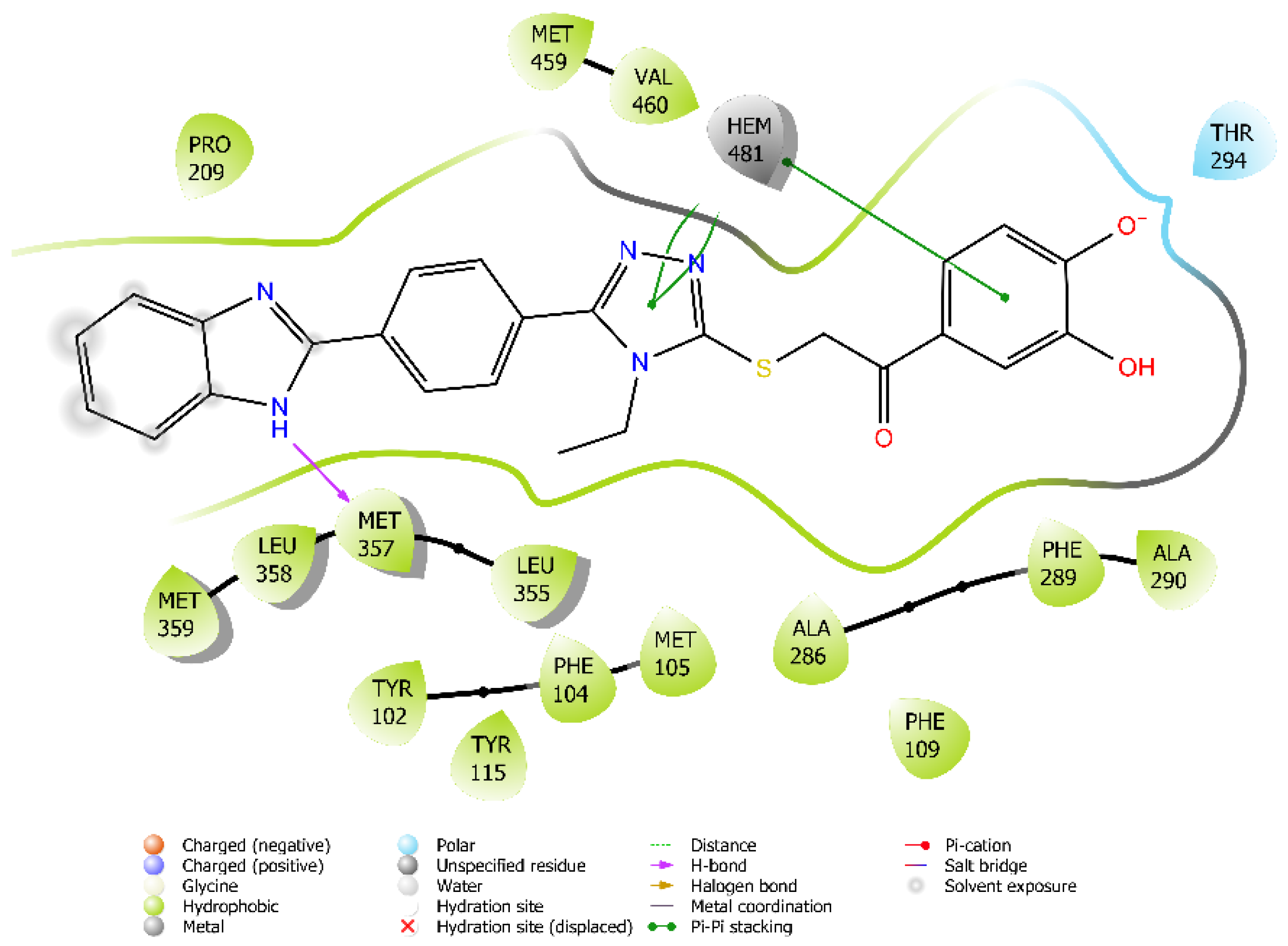
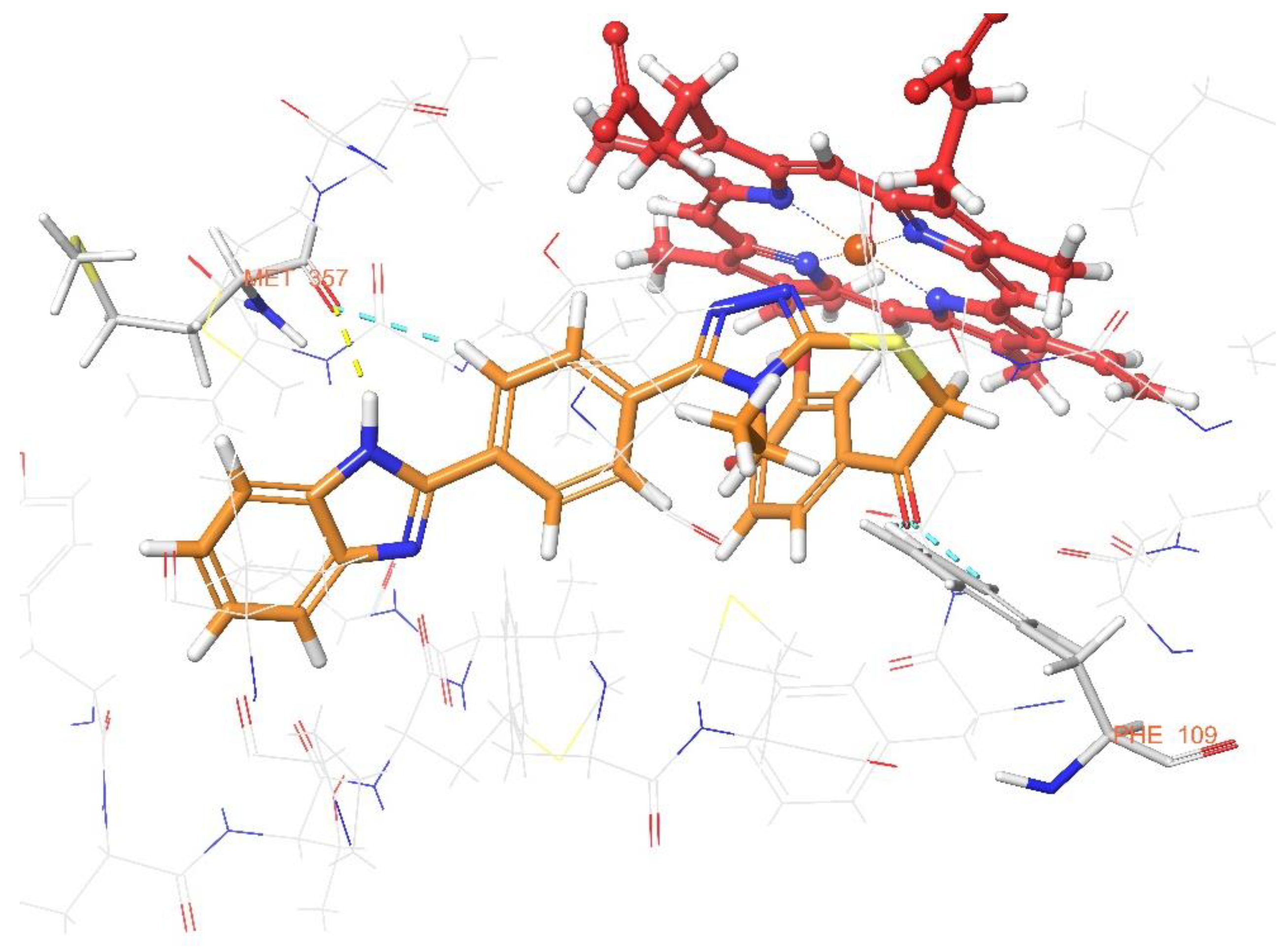
3.3. Molecular Docking
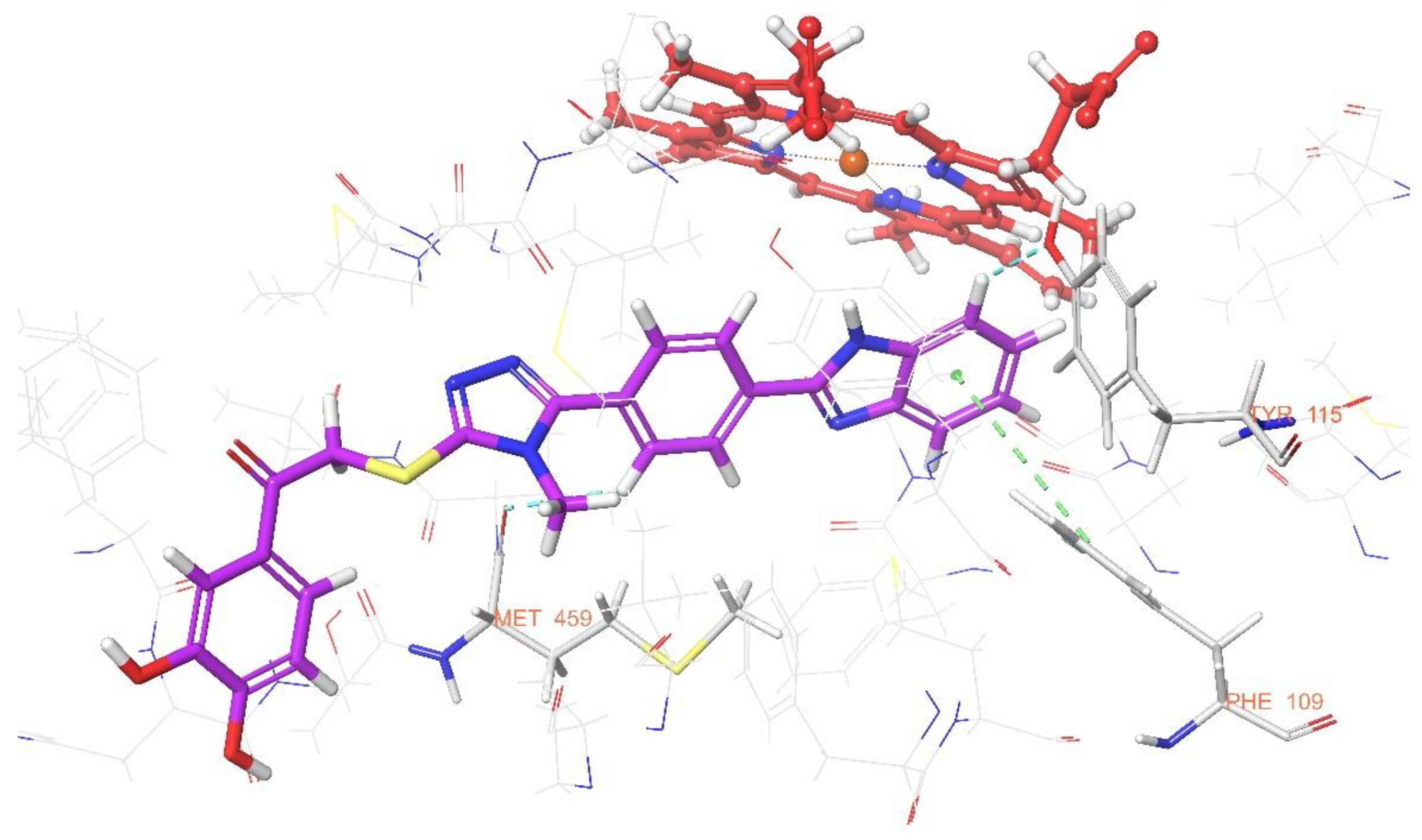
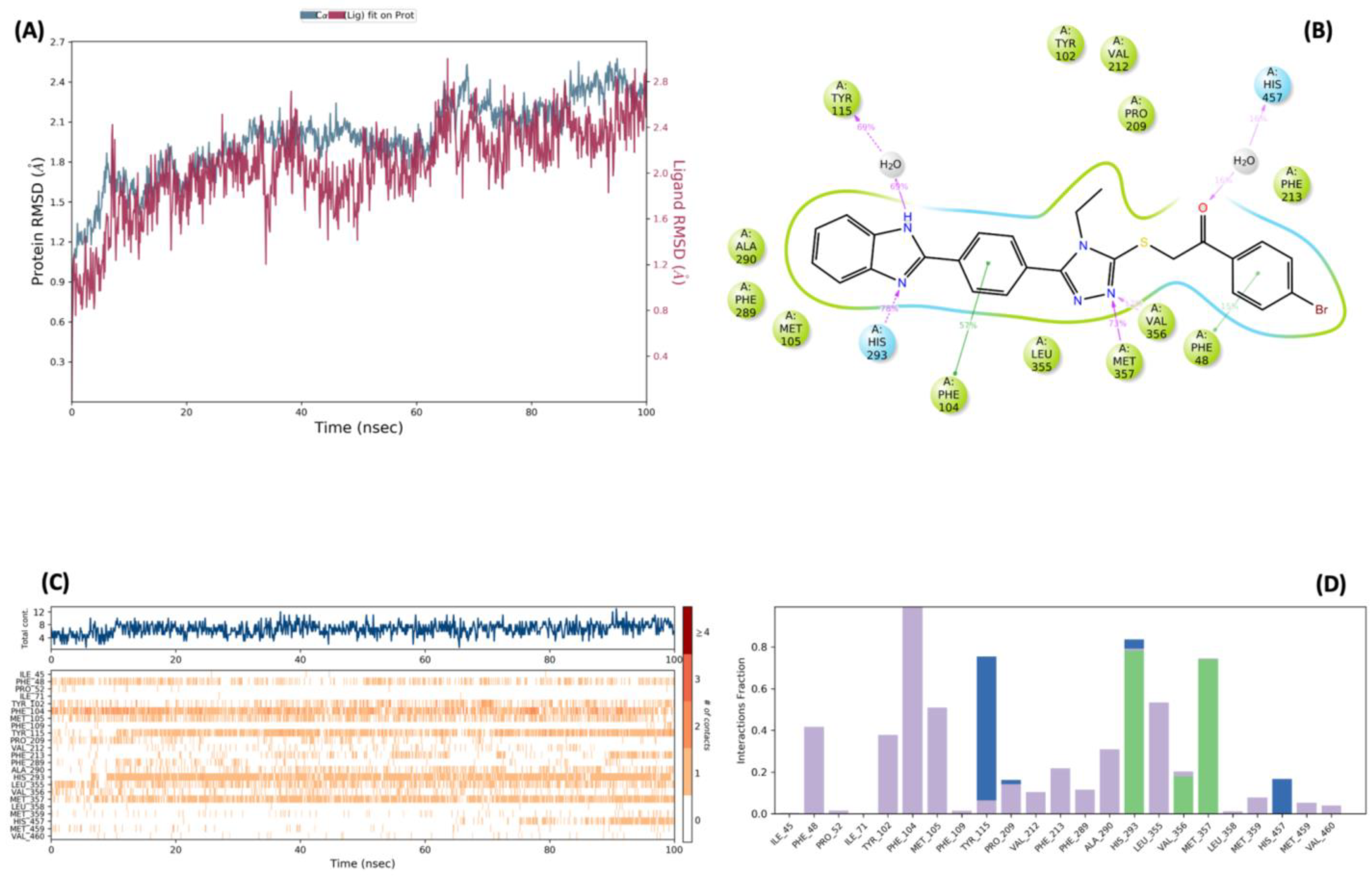
3.4. Molecular Dynamic Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 12 January 2023).
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in Leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Chernin, J. Parasitology; Taylor & Francis: London, UK, 2000; ISBN 0-7484-0817-7. [Google Scholar]
- Rohmer, M.; Bouvier, P.; Ourisson, G. Molecular Evolution of Biomembranes: Structural Equivalents and Phylogenetic Precursors of Sterols. Proc. Natl. Acad. Sci. USA 1979, 76, 847–851. [Google Scholar] [CrossRef]
- Volkman, J.K. Sterols and other Triterpenoids: Source Specificity and Evolution of Biosynthetic Pathways. Org. Geochem. 2005, 36, 139–159. [Google Scholar] [CrossRef]
- Nes, W.R.; McKean, M.L. Biochemistry of Steroids and Other Isoprenoids; University Park Press: Baltimore, MD, USA, 1977. [Google Scholar]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14-alpha-demethylase Cytochrome P450 (CYP51), a P450 in all Biological Kingdoms. Biochim. Biophys. Acta 2007, 1770, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.A.; Ericsson, J. Sterols and Isoprenoids: Signaling Molecules Derived from the Cholesterol Biosynthetic Pathway. Annu. Rev. Biochem. 1999, 68, 157–185. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; McLeodm, R.; Rice, D.W.; Ginger, M.; Chance, M.L.; Goad, L.J. Fatty Acid and Sterol Metabolism: Potential Antimicrobial Targets in Apicomplexan and Trypanosomatid Parasitic Protozoa. Mol. Biochem. Parasitol. 2003, 126, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cross, G.A.; Nes, W.D. Cholesterol Import Fails to Prevent Catalyst-Based Inhibition of Ergosterol Synthesis and Cell Proliferation of Trypanosoma brucei. J. Lipid Res. 2007, 48, 665–673. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Park, H.W.; Hargrove, T.Y.; Vanhollebeke, B.; Wawrzak, Z.; Harp, J.M.; Sundaramoorthy, M.; Nes, W.D.; Pays, E.; Chaudhuri, M.; et al. Crystal Structures of Trypanosoma brucei Sterol 14alpha-demethylase and Implications for Selective Treatment of Human Infections. J. Biol. Chem. 2010, 285, 1773–1780. [Google Scholar] [CrossRef]
- Yao, C.; Wilson, M.E. Dynamics of Sterol Synthesis during Development of Leishmania spp. Parasites to Their Virulent form. Parasit. Vectors 2016, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hsu, F.F.; Baykal, E.; Huang, J.; Zhang, K. Sterol Biosynthesis is Required for Heat Resistance but not Extracellular Survival in Leishmania. PLoS Pathog. 2014, 10, e1004427. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A. Recent Advances in Antifungal Chemotherapy. Int. J. Antimicrob. Agents 2007, 30, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zonios, D.I.; Bennet, J.E. Update on Azole Antifungals. Semin. Respir. Crit. Care Med. 2008, 29, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Moreno, S.N.; Turrens, J.F.; Katzin, A.M.; Gonzalez-Cappa, S.M.; Stoppani, A.O. Biochemical and Ultrastructural Alterations Produced by Miconazole and Econazole in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1981, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Beach, D.H.; Goad, L.J.; Holz, G.G. Effects of Ketoconazole on Sterol Biosynthesis by Trypanosoma cruzi Epimastigotes. Biochem. Biophys. Res. Commun. 1986, 136, 851–856. [Google Scholar] [CrossRef]
- Urbina, J.A.; Lazardi, K.; Aguirre, T.; Piras, M.M.; Piras, R. Antiproliferative Synergism of the Allylamine SF 86–327 and Ketoconazole on Epimastigotes and Amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 1988, 32, 1237–1242. [Google Scholar] [CrossRef]
- Apt, W.; Aguilera, X.; Arribada, A.; Pérez, C.; Miranda, C.; Sánchez, G.; Zulantay, I.; Cortés, P.; Rodriguez, J.; Juri, D. Treatment of Chronic Chagas’ Disease with Itraconazole and Allopurinol. Am. J. Trop. Med. Hyg. 1998, 59, 133–138. [Google Scholar] [CrossRef]
- Araújo, M.S.; Martins-Filho, O.A.; Pereira, M.E.; Brener, Z.J. A Combination of Benzimidazole and Ketoconazole Enhances Efficacy of Chemotherapy of Experimental Chagas’ disease. Antimicrob. Chemother. 2000, 45, 819–824. [Google Scholar] [CrossRef]
- Molina, J.; Martins-Filho, O.; Brener, Z.; Romanha, A.J.; Loebenberg, D.; Urbina, J.A. Activities of the Triazole Derivative SCH 56592 (posaconazole) Against Drug-Resistant Strains of the Protozoan Parasite Trypanosoma (Schizotrypanum) cruzi in Immunocompetent and Immunosuppressed Murine Hosts. Antimicrob. Agents Chemother. 2000, 44, 150–155. [Google Scholar] [CrossRef]
- Buckner, F.S. Sterol 14-demethylase Inhibitors for Trypanosoma cruzi Infections. Adv. Exp. Med. Biol. 2008, 625, 61–80. [Google Scholar]
- Lepesheva, G.I.; Ott, R.D.; Hargrove, T.Y.; Kleshchenko, Y.Y.; Schuster, I.; Nes, W.D.; Hill, G.C.; Villalta, F.; Waterman, M.R. Sterol 14alpha-demethylase as a Potential Target for Antitrypanosomal Therapy: Enzyme Inhibition and Parasite Cell Growth. Chem. Biol. 2007, 14, 1283–1293. [Google Scholar] [CrossRef]
- Urbina, J.A.; Payares, G.; Sanoja, C.; Molina, J.; Lira, R.; Brener, Z.; Romanha, A.J. Parasitological Cure of Acute and Chronic Experimental Chagas disease Using the Long-Acting Experimental Triazole TAK-187. Activity Against Drug-Resistant Trypanosoma cruzi Strains. Int. J. Antimicrob. Agents 2003, 1, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A.; Payares, G.; Contreras, L.M.; Liendo, A.; Sanoja, C.; Molina, J.; Piras, M.; Piras, R.; Perez, N.; Wincker, P.; et al. Antiproliferative Effects and Mechanism of Action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: In Vitro and In Vivo Studies. Antimicrob. Agents Chemother. 1998, 42, 1771–1777. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Hargrove, T.Y.; Kleshchenko, Y.; Nes, W.D.; Villalta, F.; Waterman, M.R. CYP51: A Major Drug Target in the Cytochrome P450 Superfamily. Lipids 2008, 43, 1117–1125. [Google Scholar] [CrossRef]
- Keurulainen, L.; Siiskonen, A.; Nasereddin, A.; Kopelyanskiy, D.; Sacerdoti-Sierra, N.; Leino, T.O.; Kiuru, P. Synthesis and Biological Evaluation of 2-arylbenzimidazoles Targeting Leishmania donovani. Bioorg. Med. Chem. Lett. 2015, 25, 1933–1937. [Google Scholar] [CrossRef]
- Zeyrek, F.Y.; Gürses, G.; Uluca, N.; Doni, N.Y.; Toprak, S.; Yesilova, Y.; Çulha, G. Is the Agent of Cutaneous Leishmaniasis in Sanliurfa changing? First Cases of Leishmania majör. Turk. Parazitol. Derg. 2014, 38, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Acar Cevik, U.; Saglik, B.N.; Levent, S.; Osmaniye, D.; Kaya Cavuşoglu, B.; Ozkay, Y.; Kaplancikli, Z.A. Synthesis and AChE-inhibitory Activity of New Benzimidazole Derivatives. Molecules 2019, 24, 861. [Google Scholar] [CrossRef]
- Can, N.Ö.; Acar Çevik, U.; Sağlık, B.N.; Levent, S.; Korkut, B.; Özkay, Y.; Koparal, A.S. Synthesis, Molecular Docking Studies and antifungal activity evaluation of New Benzimidazole-Triazole as Potential Lanosterol 14α-Demethylase Inhibitors. J. Chem. 2017, 2017, 9387102. [Google Scholar] [CrossRef]
- Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Özbilgin, A.; Çavuş, İ.; Yıldırım, A.; Kaya, T.; Ertabaklar, H. Evaluation of In vitro and In vivo Drug Efficacy Over Leishmania tropica: A Pilot Study. Turk. Parazitol. Derg. 2018, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Espinosa, O.A.; Montenegro, H.; Cubilla, L.; Capson, T.L.; Ortega-Barrıa, E.; Romero, L.I. Hydrosoluble formazan XTT: Its application to natural products drug discovery for Leishmania. J. Microbiol. Methods 2003, 55, 813–816. [Google Scholar] [CrossRef]
- Özbilgin, A.; Zeyrek, F.Y.; Güray, M.Z.; Çulha, G.; Akyar, I.; Harman, M.; Gündüz, C. Determination of antimony resistance mechanism of Leishmania tropica causing cutaneous leishmaniasis in Turkey. Mikrobiyoloji Bul. 2020, 54, 444–462. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; Lepesheva, G.I. Structural Analyses of Candida Albicans Sterol 14α-Demethylase Complexed with Azole Drugs Address the Molecular Basis of Azole-Mediated Inhibition of Fungal Sterol Biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef] [PubMed]
- Maestro, 10.6; Schrödinger LLC: New York, NY, USA, 2020.
- Schrödinger. LigPrep, Version 3.8; Schrödinger LLC: New York, NY, USA, 2020.
- Schrödinger. Glide, Version 7.1; Schrödinger LLC: New York, NY, USA, 2020.
- Liu, X.; Shi, D.; Zhou, S.; Liu, H.; Liu, H.; Yao, X. Molecular Dynamics Simulations and Novel Drug Discovery. Expert Opin. Drug Discov. 2018, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- M.D.I. Tools. Schrödinger Release 2018-3: Prime, 2018; Schrödinger LLC: New York, NY, USA, 2020. [Google Scholar]
- Saral, A.; Sudha, P.; Muthu, S.; Sevvanthi, S.; Sangeetha, P.; Selvakumari, S. Vibrational Spectroscopy, Quantum Computational and Molecular Docking Studies on 2-Chloroquinoline-3-Carboxaldehyde. Heliyon 2021, 7, e07529. [Google Scholar] [CrossRef]
- Schrödinger Release. 1: Desmond Molecular Dynamics System, Version 3.7; DE Shaw Research: New York, NY, USA, 2014.
| Compound | Concentration (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1000 | 500 | 250 | 125 | 62.50 | 31.25 | 15.63 | 7.81 | 3.91 | 1.95 | 0.98 | 0.49 | IC50 | |
| 5a | 72 | 78 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | N.c |
| 5b | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | N.c |
| 5c | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | N.c |
| 5d | 30 | 41 | 61 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 239.03 |
| 5e | 82 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | N.c |
| 5f | 20 | 35 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 460.37 |
| 5g | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | N.c |
| 5h | 29 | 46 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 468.01 |
| Drug Control (Amphotericin B) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | <0.49 |
| Parasite Control | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | N.c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eser, M.; Çavuş, İ. In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives. Vet. Sci. 2023, 10, 648. https://doi.org/10.3390/vetsci10110648
Eser M, Çavuş İ. In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives. Veterinary Sciences. 2023; 10(11):648. https://doi.org/10.3390/vetsci10110648
Chicago/Turabian StyleEser, Mustafa, and İbrahim Çavuş. 2023. "In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives" Veterinary Sciences 10, no. 11: 648. https://doi.org/10.3390/vetsci10110648
APA StyleEser, M., & Çavuş, İ. (2023). In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives. Veterinary Sciences, 10(11), 648. https://doi.org/10.3390/vetsci10110648







