Diagnostic Potential for the Detection of Canine Visceral Leishmaniasis of an ELISA Assay Based on the Q5 Recombinant Protein: A Large-Scale and Comparative Evaluation Using Canine Sera with a Positive Diagnosis from the Dual-Path-Platform (DPP) Test
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
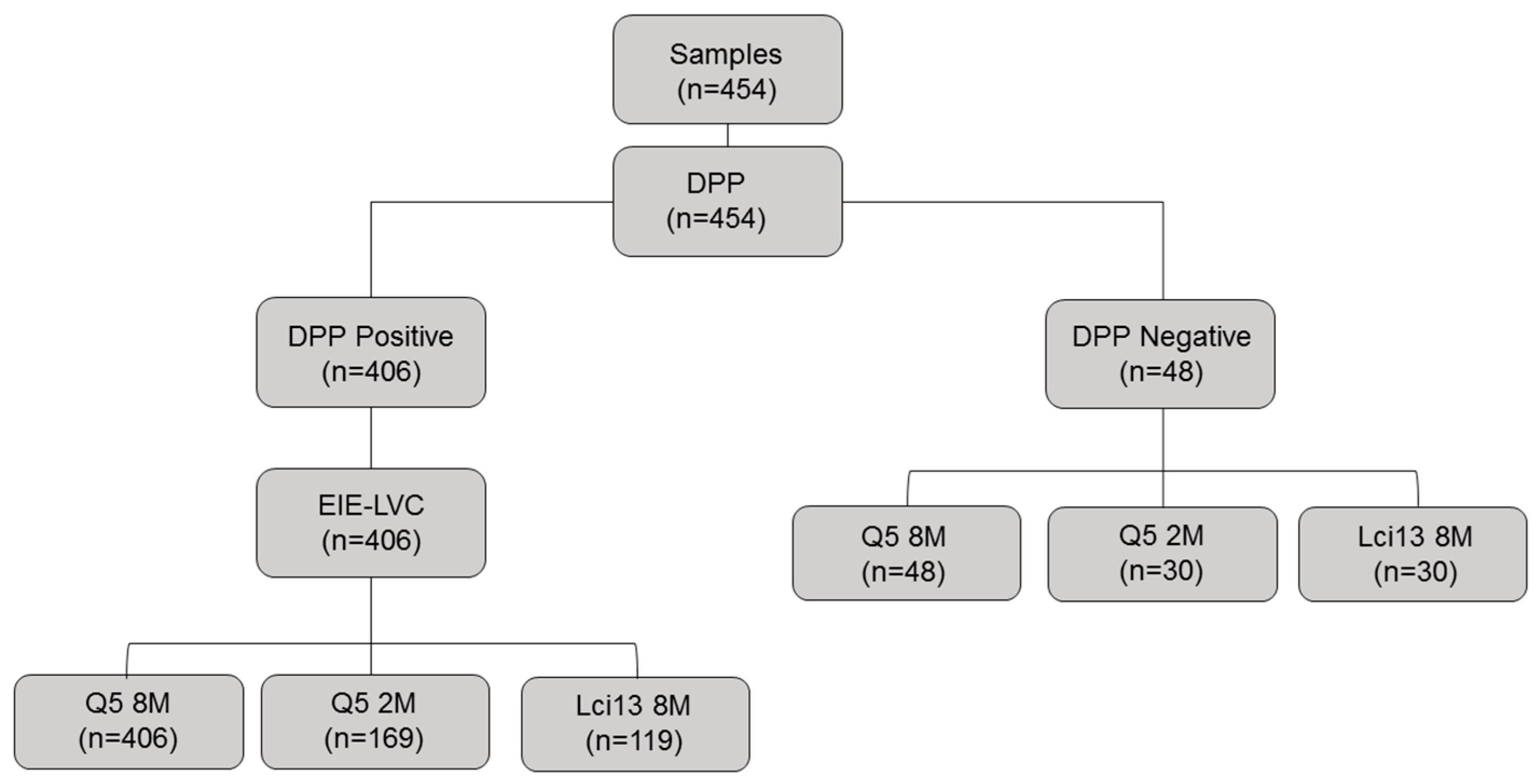
2.1. Canine Sera and Ethical Considerations
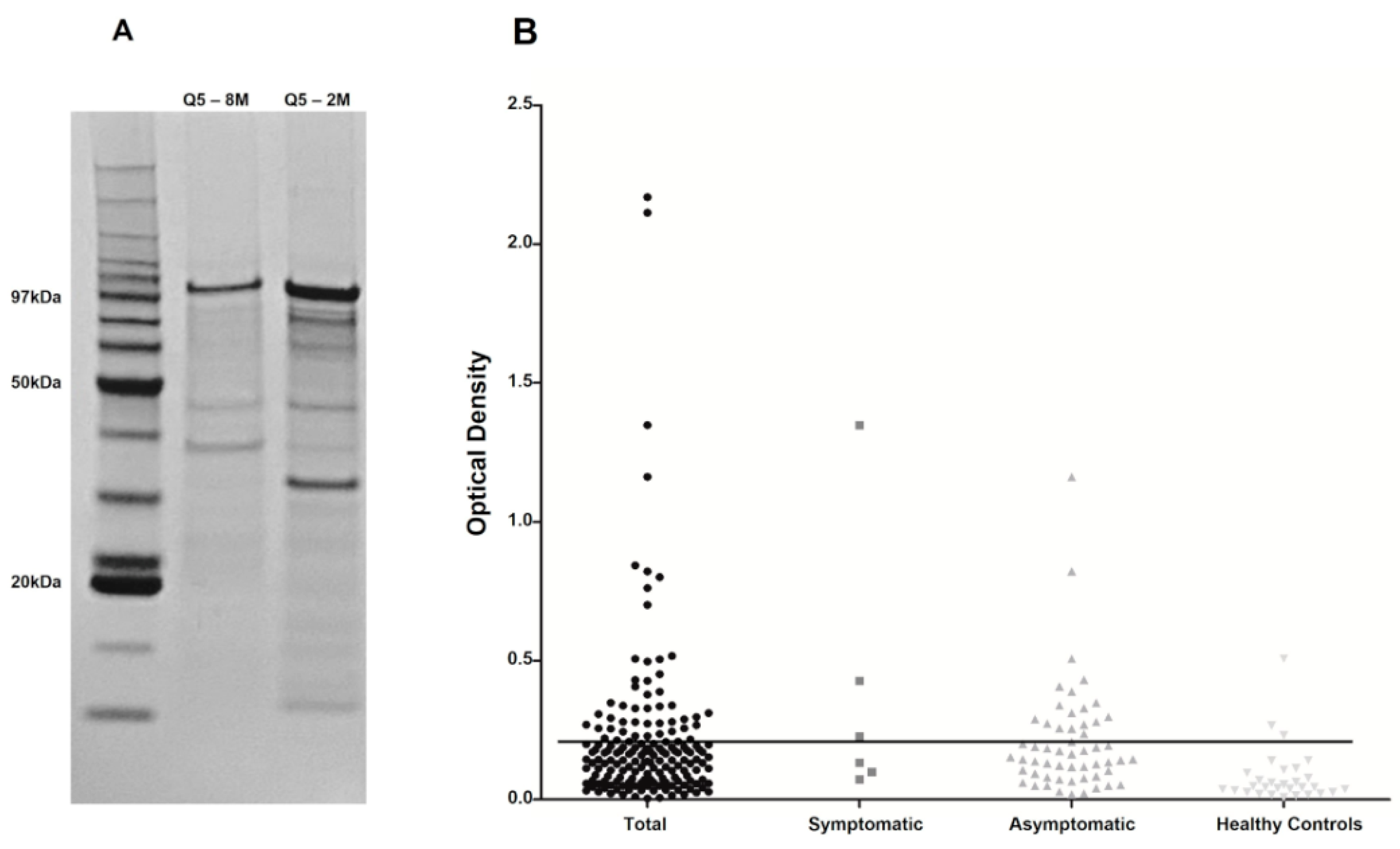
2.2. Protein Expression and Purification
2.3. Diagnostic Tests and ELISA
2.4. Data Collection
2.5. Statistical Analyses
3. Results
3.1. Environmental and Clinical Characteristics of the Study Population
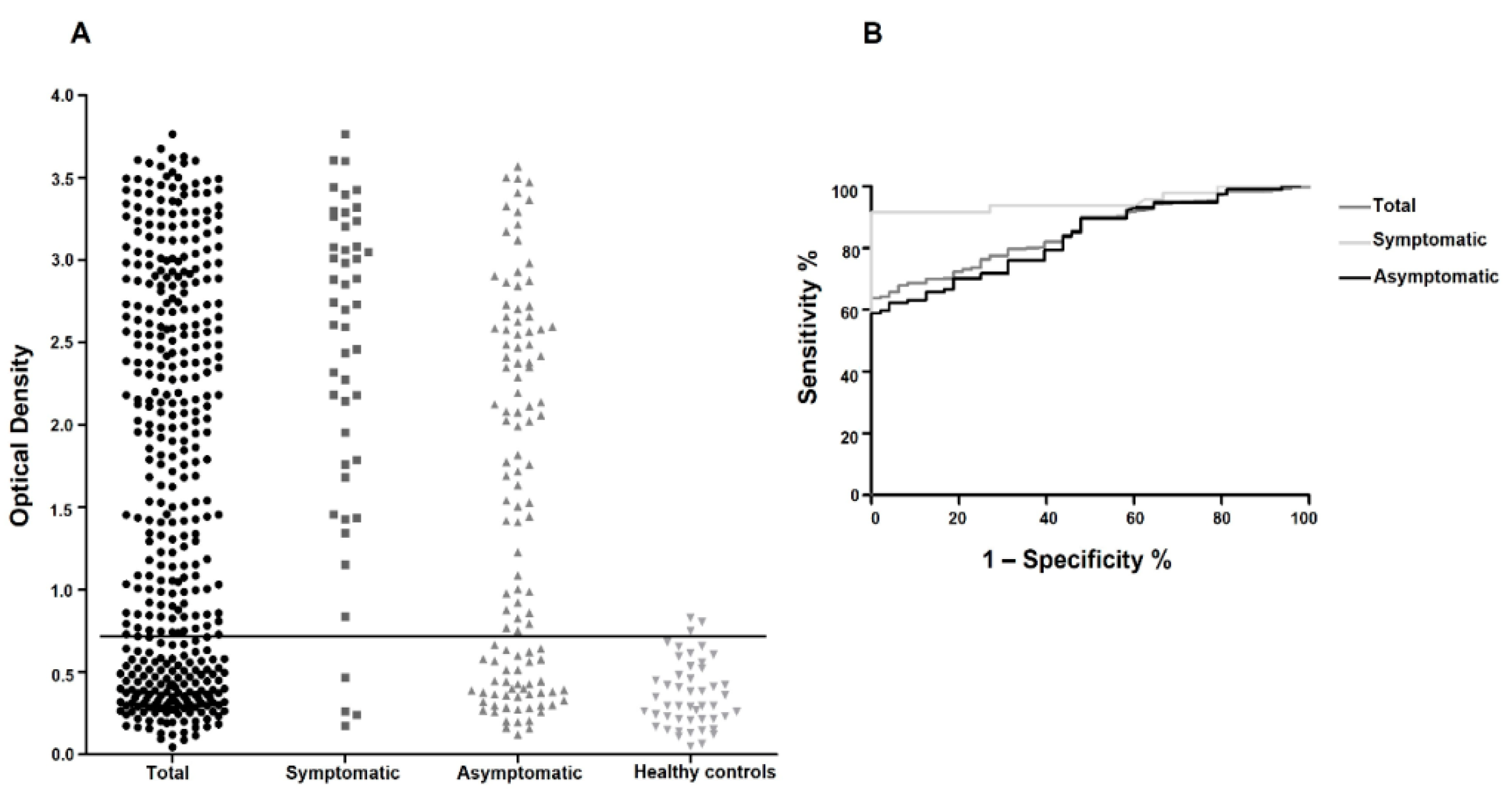
3.2. Large-Scale Evaluation of the Recombinant Q5 for the CVL Diagnosis
3.3. Comparison between EIE-LVC and Q5
3.4. Comparative Evaluation of Different Q5-Based ELISA Assays
3.5. Evaluation of the Lci13 Recombinant Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, I.D.; Lima, A.L.M.; Mendes-Aguiar, C.O.; Coutinho, J.F.V.; Wilson, M.E.; Pearson, R.D.; Queiroz, J.W.; Jeronimo, S.M.B. Changing demographics of visceral leishmaniasis in northeast Brazil: Lessons for the future. PLoS Neglected Trop. Dis. 2018, 12, e0006164. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.N.C.C.; Bermudi, P.M.M.; Rodas, L.A.C.; Nunes, C.M.; Hiramoto, R.M.; Tolezano, J.E.; Cipriano, R.S.; Cardoso, G.C.D.; Codeço, C.T.; Chiaravalloti Neto, F. Human visceral leishmaniasis and relationship with vector and canine control measures. Rev. Saude Publica 2018, 52, 92. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, M.; Moradi-Asl, E.; Rassi, Y. Geographic distribution and spatial analysis of Leishmania infantum infection in domestic and wild animal reservoir hosts of zoonotic visceral leishmaniasis in Iran: A systematic review. J. Vector Borne Dis. 2018, 55, 173–183. [Google Scholar]
- Marcondes, M.; Day, M.J. Current status and management of canine leishmaniasis in Latin America. Res. Vet. Sci. 2019, 123, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Lison, A.; Courtenay, O. Advances toward Diagnostic Tools for Managing Zoonotic Visceral Leishmaniasis. Trends Parasitol. 2018, 34, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Borja, L.S.; Coelho, L.B.; Jesus, M.S.; de Queiroz, A.T.L.; Celedon, P.A.F.; Zanchin, N.I.T.; Silva, E.D.; Ferreira, A.G.P.; Krieger, M.A.; Veras, P.S.T.; et al. High accuracy of an ELISA test based in a flagella antigen of Leishmania in serodiagnosis of canine visceral leishmaniasis with potential to improve the control measures in Brazil—A Phase II study. PLoS Neglected Trop. Dis. 2018, 12, e0006871. [Google Scholar] [CrossRef]
- Reis, L.E.; Coura-Vital, W.; Roatt, B.M.; Bouillet, L.; Ker, H.G.; Fortes de Brito, R.C.; Resende, D.d.M.; Carneiro, M.; Giunchetti, R.C.; Marques, M.J.; et al. Molecular diagnosis of canine visceral leishmaniasis: A comparative study of three methods using skin and spleen from dogs with natural Leishmania infantum infection. Vet. Parasitol. 2013, 197, 498–503. [Google Scholar] [CrossRef]
- Grimaldi, G.; Teva, A.; Ferreira, A.L.; dos Santos, C.B.; Pinto, I.; de-Azevedo, C.T.; Falqueto, A. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 54–59. [Google Scholar] [CrossRef]
- Pattabhi, S.; Whittle, J.; Mohamath, R.; El-Safi, S.; Moulton, G.G.; Guderian, J.A.; Colombara, D.; Abdoon, A.O.; Mukhtar, M.M.; Mondal, D.; et al. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Neglected Trop. Dis. 2010, 4, e822. [Google Scholar] [CrossRef]
- Lira, R.A.; Cavalcanti, M.P.; Nakazawa, M.; Ferreira, A.G.; Silva, E.D.; Abath, F.G.; Alves, L.C.; Souza, W.V.; Gomes, Y.M. Canine visceral leishmaniosis: A comparative analysis of the EIE-leishmaniose-visceral-canina-Bio-Manguinhos and the IFI-leishmaniose-visceral-canina-Bio-Manguinhos kits. Vet. Parasitol. 2006, 137, 11–16. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.L.N.; Riboldi, E.O.; Bello, G.L.; Ramos, R.R.; Barcellos, R.B.; Gehlen, M.; Halon, M.L.; Romão, P.R.T.; Dallegrave, E.; Rossetti, M.L.R. Canine visceral leishmaniasis diagnosis: A comparative performance of serological and molecular tests in symptomatic and asymptomatic dogs. Epidemiol. Infect. 2018, 146, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Krawczak, F.a.S.; Reis, I.A.; Silveira, J.A.; Avelar, D.M.; Marcelino, A.P.; Werneck, G.L.; Labruna, M.B.; Paz, G.F. Leishmania, Babesia and Ehrlichia in urban pet dogs: Co-infection or cross-reaction in serological methods? Rev. Soc. Bras. Med. Trop. 2015, 48, 64–68. [Google Scholar] [CrossRef]
- Travi, B.L.; Cordeiro-da-Silva, A.; Dantas-Torres, F.; Miró, G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Neglected Trop. Dis. 2018, 12, e0006082. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.M.; Nascimento, M.; Ferraz, J.C.; Pereira, M.M.; Rocha, P.O.; Thompson, G.M.; Cysne-Finkelstein, L.; Figueiredo, R.C.; de Melo Neto, O.P. Distinct mitochondrial HSP70 homologs conserved in various Leishmania species suggest novel biological functions. Mol. Biochem. Parasitol. 2008, 160, 157–162. [Google Scholar] [CrossRef]
- Magalhães, F.B.; Castro Neto, A.L.; Nascimento, M.B.; Santos, W.J.T.; Medeiros, Z.M.; Lima Neto, A.S.; Costa, D.L.; Costa, C.H.N.; Dos Santos, W.L.C.; Pontes de Carvalho, L.C.; et al. Evaluation of a new set of recombinant antigens for the serological diagnosis of human and canine visceral leishmaniasis. PLoS ONE 2017, 12, e0184867. [Google Scholar] [CrossRef]
- Santos, W.J.T.; Tavares, D.H.C.; Castro Neto, A.L.; Nascimento, M.B.; Dhalia, R.; Albuquerque, A.L.; Costa, C.H.N.; Magalhães, F.B.; Rezende, A.M.; de Melo Neto, O.P. Gene design, optimization of protein expression and preliminary evaluation of a new chimeric protein for the serological diagnosis of both human and canine visceral leishmaniasis. PLoS Neglected Trop. Dis. 2020, 14, e0008488. [Google Scholar] [CrossRef]
- de Paiva Cavalcanti, M.; Felinto de Brito, M.E.; de Souza, W.V.; de Miranda Gomes, Y.; Abath, F.G. The development of a real-time PCR assay for the quantification of Leishmania infantum DNA in canine blood. Vet. J. 2009, 182, 356–358. [Google Scholar] [CrossRef]
- Oliveira-Lima, J.W.; Faria Filho, O.F.; Vieira, J.B.; Gadelha, F.V.; Oliveira Filho, A.M. Peridomiciliary changes and implications for Triatoma brasiliensis control. Cad. Saude Publica 2000, 16 (Suppl. 2), 75–81. [Google Scholar] [CrossRef]
- Fraga, D.B.; Pacheco, L.V.; Borja, L.S.; Tuy, P.G.; Bastos, L.A.; Solcà, M.d.S.; Amorim, L.D.; Veras, P.S. The Rapid Test Based on Leishmania infantum Chimeric rK28 Protein Improves the Diagnosis of Canine Visceral Leishmaniasis by Reducing the Detection of False-Positive Dogs. PLoS Neglected Trop. Dis. 2016, 10, e0004333. [Google Scholar] [CrossRef]
- Figueiredo, F.B.; Vasconcelos, T.C.B.; Madeira, M.F.; Menezes, R.C.; Maia-Elkhoury, A.N.S.; Marcelino, A.P.; Werneck, G.L. Validation of the Dual-path Platform chromatographic immunoassay (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 2018, 113, e180260. [Google Scholar] [CrossRef]
- Fujimori, M.; de Almeida, A.D.B.P.; Barrouin-Melo, S.M.; Cortez, L.R.P.B.; Duthie, M.S.; Hiramoto, R.M.; de Pinho, F.A.; Reed, S.G.; Sousa, V.R.F.; Souza, N.F.; et al. Validation of ELISA with recombinant antigens in serological diagnosis of canine Leishmania infantum infection. Mem. Inst. Oswaldo Cruz 2021, 116, e200428. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.G.; Magalhães, F.B.; Teixeira, M.C.; Pereira, A.M.; Pinheiro, C.G.; Santos, L.R.; Nascimento, M.B.; Bedor, C.N.; Albuquerque, A.L.; dos-Santos, W.L.; et al. Characterization of novel Leishmania infantum recombinant proteins encoded by genes from five families with distinct capacities for serodiagnosis of canine and human visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2011, 85, 1025–1034. [Google Scholar] [CrossRef]
- Cañavate, C.; Bern, C.; Chicharro, C.; Blackstock, A.J.; Alvar, J.; Herrero, M.; Aparicio, P.; Cruz, I.; Argaw, D.; Nieto, J.; et al. Evaluation of two rK39 dipstick tests, direct agglutination test, and indirect fluorescent antibody test for diagnosis of visceral leishmaniasis in a new epidemic site in highland Ethiopia. Am. J. Trop. Med. Hyg. 2011, 84, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Campino, L. Biomarkers Associated with Leishmania infantum Exposure, Infection, and Disease in Dogs. Front. Cell. Infect. Microbiol. 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]

| N = 406 | Characteristics | N (%) |
|---|---|---|
| Origin of the animal | Domiciled | 244 (60.1) |
| Semi-domiciled | 4 (1.0) | |
| Communitarian | 63 (15.5) | |
| Errant | 6 (1.5) | |
| NI | 89 (21.9) | |
| Sex | Female | 176 (43.3) |
| Male | 214 (52.7) | |
| NI | 16 (3.9) | |
| Age | Up to 12 months | 21 (5.2) |
| One year old or older | 274 (67.5) | |
| NI | 111 (27.3) | |
| Human contact | Yes | 280 (69.0) |
| No | 2 (0.5) | |
| NI | 124 (30.5) | |
| Type of shelter * | Intra-residence | 100 (24.6) |
| Extra-residence | 51 (12.6) | |
| Peri-residence | 70 (17.2) | |
| NI | 185 (45.6) | |
| Symptoms | Asymptomatic | 117 (28.8) |
| Symptomatic | 48 (11.8) | |
| NI | 241 (59.4) | |
| Clinical characteristics | Generalized dermatitis | 35 (72.91) |
| Onychogryphosis | 23 (47.91) | |
| Weight loss | 22 (45.83) | |
| Hair loss | 13 (27.08) | |
| Keratoconjunctivitis | 7 (14.58) |
| ELISA | Sensitivity % (n = 406) | Symptomatic (n = 48) | Asymptomatic (n = 117) | Specificity % (n = 48) | Accuracy % | Kappa |
|---|---|---|---|---|---|---|
| Q5-8M | 68 (63–72) | 92 (80–98) | 62 (53–71) | 94 (83–99) | 71 (66–75) | 0.48 (0.38–0.60) |
| EIE-LVC | 67 (61–72) | 92 (80–98) | 58 (50–68) | Not assayed | Not assayed | 0.48 (0.40–0.60) |
| Q5 (8 M) Positive | Q5 (8 M) Negative | Q5 (8 M) Positive | Q5 (8 M) Negative | |
|---|---|---|---|---|
| Q5 (2 M) Positive | Q5 (2 M) Positive | Q5 (2 M) Negative | Q5 (2 M) Negative | |
| EIE-LVC positive | 4 | 7 | 4 | 21 |
| EIE-LVC negative | 19 | 13 | 13 | 88 |
| Total | 23 | 20 | 17 | 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Paz, L.F.; da Silva, A.; da Silva, H.R.F.; Cavalcanti, M.P.; de Lima, V.M.F.; da Cunha Beltrão, M.R.O.; Silva, M.B.A.; de Melo Neto, O.P.; Medeiros, Z.M.; Santos, W.J.T.d. Diagnostic Potential for the Detection of Canine Visceral Leishmaniasis of an ELISA Assay Based on the Q5 Recombinant Protein: A Large-Scale and Comparative Evaluation Using Canine Sera with a Positive Diagnosis from the Dual-Path-Platform (DPP) Test. Vet. Sci. 2023, 10, 608. https://doi.org/10.3390/vetsci10100608
de Araújo Paz LF, da Silva A, da Silva HRF, Cavalcanti MP, de Lima VMF, da Cunha Beltrão MRO, Silva MBA, de Melo Neto OP, Medeiros ZM, Santos WJTd. Diagnostic Potential for the Detection of Canine Visceral Leishmaniasis of an ELISA Assay Based on the Q5 Recombinant Protein: A Large-Scale and Comparative Evaluation Using Canine Sera with a Positive Diagnosis from the Dual-Path-Platform (DPP) Test. Veterinary Sciences. 2023; 10(10):608. https://doi.org/10.3390/vetsci10100608
Chicago/Turabian Stylede Araújo Paz, Larissa Ferreira, Adalúcia da Silva, Hemilly Rayanne Ferreira da Silva, Milena Paiva Cavalcanti, Valeria Marçal Felix de Lima, Maria Rosário Oliveira da Cunha Beltrão, Maria Beatriz Araújo Silva, Osvaldo Pompílio de Melo Neto, Zulma Maria Medeiros, and Wagner José Tenório dos Santos. 2023. "Diagnostic Potential for the Detection of Canine Visceral Leishmaniasis of an ELISA Assay Based on the Q5 Recombinant Protein: A Large-Scale and Comparative Evaluation Using Canine Sera with a Positive Diagnosis from the Dual-Path-Platform (DPP) Test" Veterinary Sciences 10, no. 10: 608. https://doi.org/10.3390/vetsci10100608
APA Stylede Araújo Paz, L. F., da Silva, A., da Silva, H. R. F., Cavalcanti, M. P., de Lima, V. M. F., da Cunha Beltrão, M. R. O., Silva, M. B. A., de Melo Neto, O. P., Medeiros, Z. M., & Santos, W. J. T. d. (2023). Diagnostic Potential for the Detection of Canine Visceral Leishmaniasis of an ELISA Assay Based on the Q5 Recombinant Protein: A Large-Scale and Comparative Evaluation Using Canine Sera with a Positive Diagnosis from the Dual-Path-Platform (DPP) Test. Veterinary Sciences, 10(10), 608. https://doi.org/10.3390/vetsci10100608






