Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Selection of Animals
2.3. Seminal Plasma Preparation for Proteome Analysis
2.4. Extraction and Quantification of Soluble Proteins
2.5. SDS-PAGE
2.6. Sample Pooling
2.7. Gel Protein Digestion
2.8. LC-MS/MS
2.9. Protein Identification
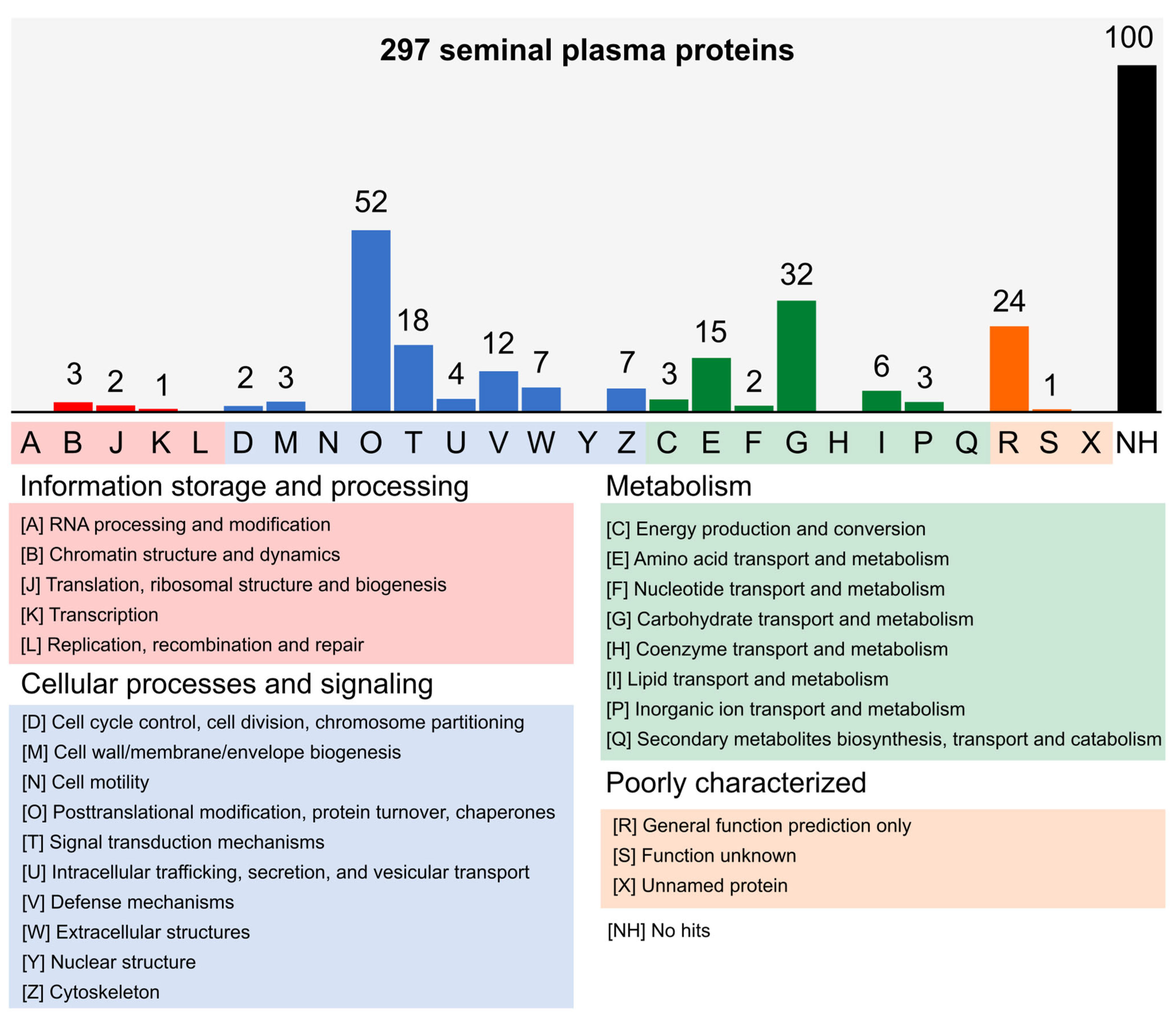
2.10. Functional Classification of Proteins
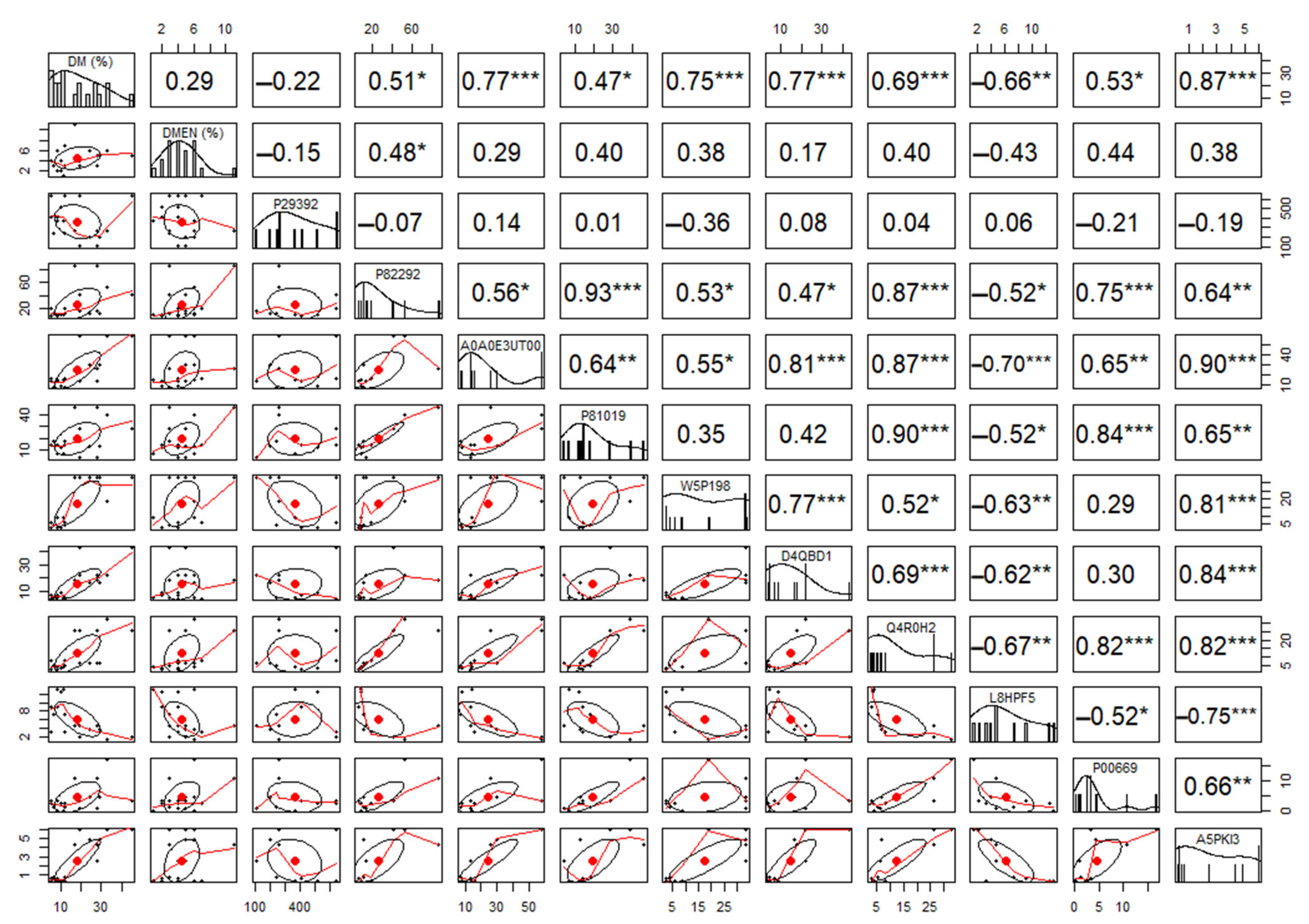
2.11. Quantitative Analysis of Identified Proteins
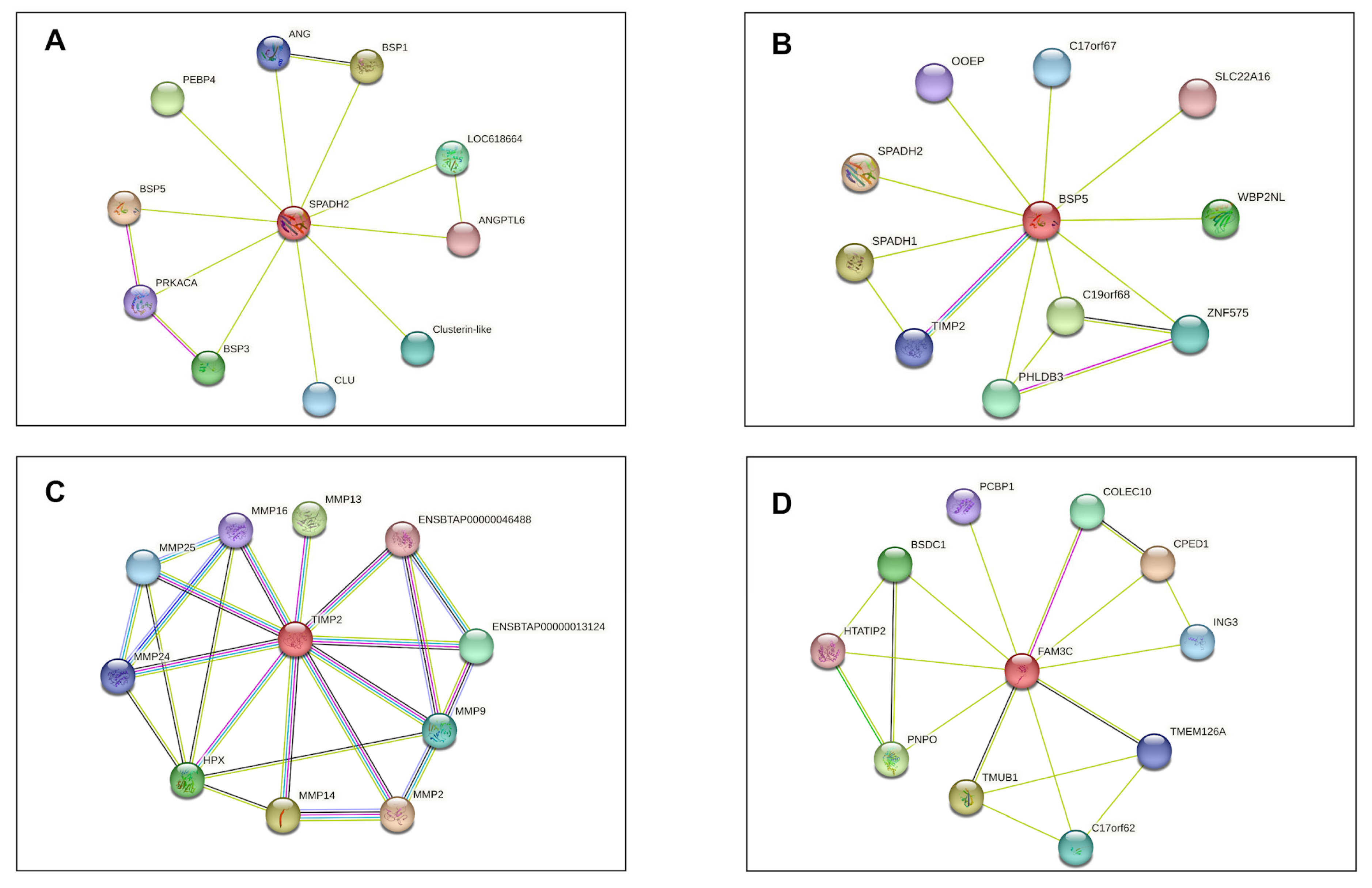
2.12. Analysis of Protein Interaction Networks
2.13. Phenotypic Data Analysis
3. Results
3.1. Spermogram of Bulls Classified as FIT and UNFIT for Reproduction
3.2. Proteomic Characterization of Seminal Plasma from Nellore Bulls
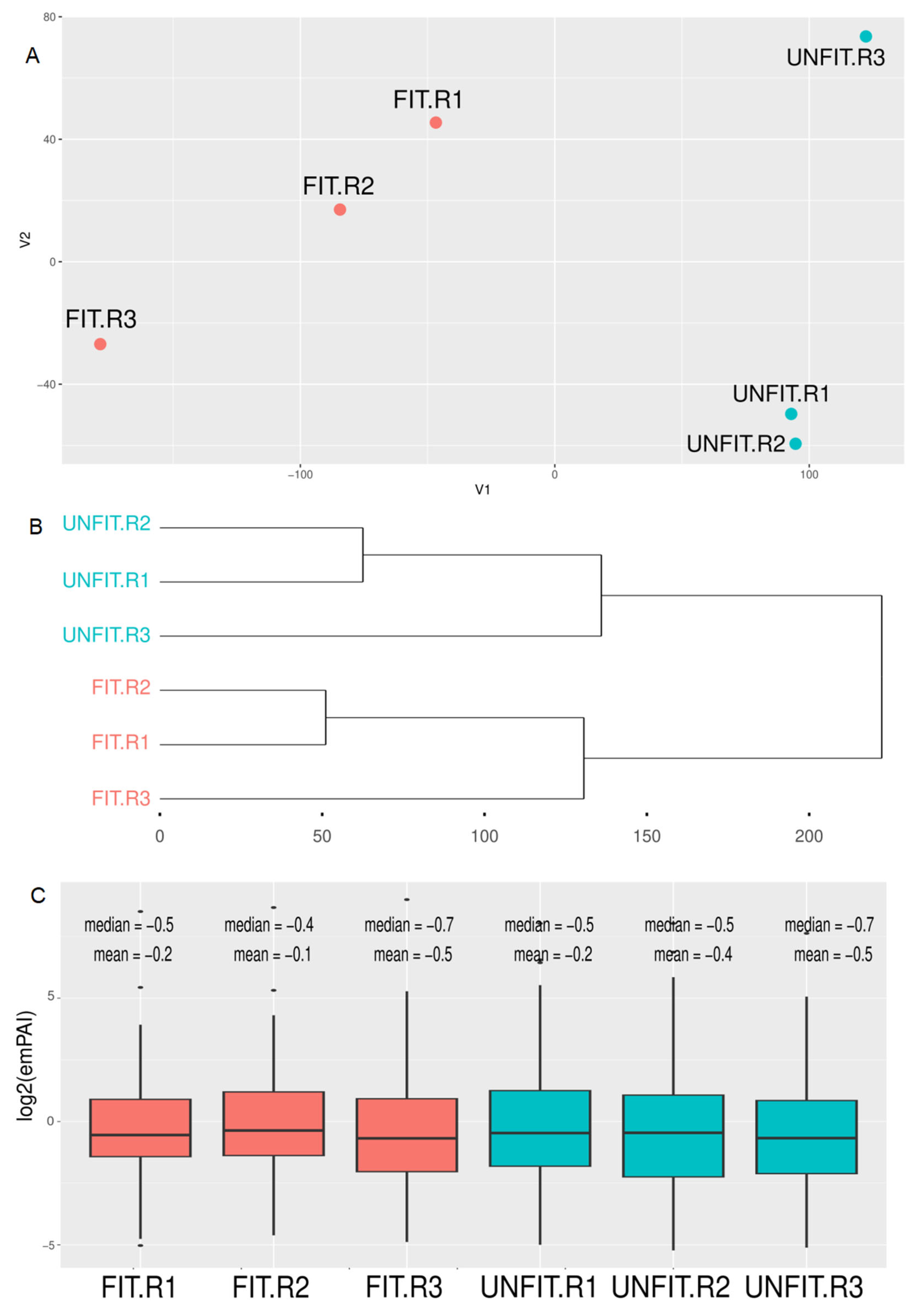
3.3. Quantitative Proteomics of Seminal Plasma from FIT and UNFIT Bulls for Reproduction
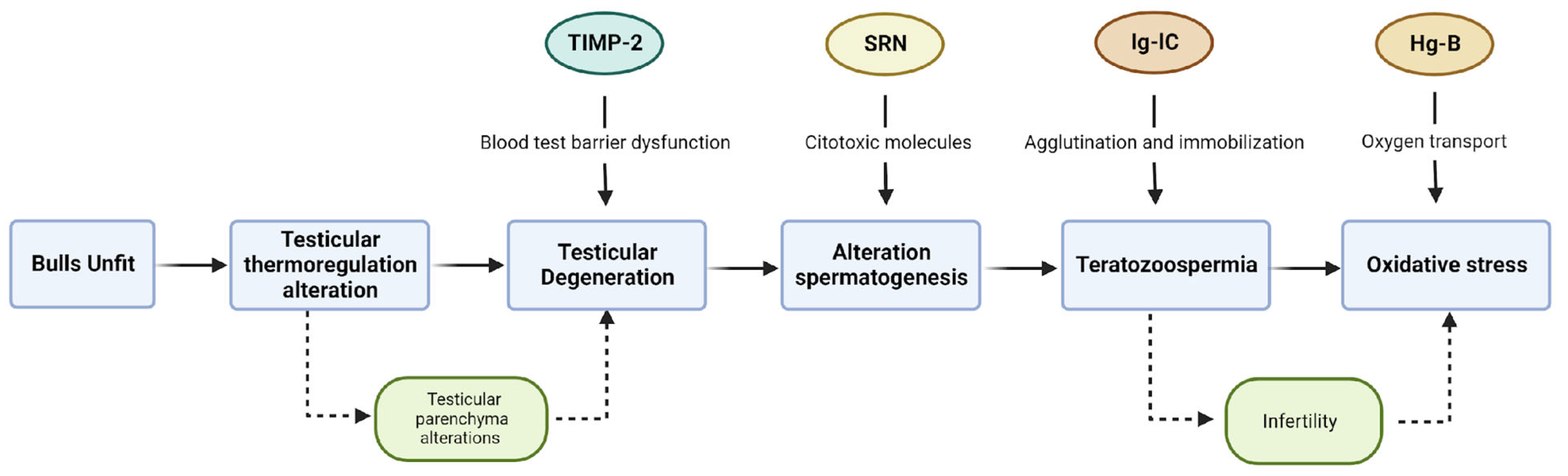
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beef Report 2022—ABIEC. Available online: https://www.abiec.com.br/ (accessed on 14 March 2023).
- Santos, A.S.; Villela, S.D.; Leonel, F.D.P.; Verardo, L.L.; Regina Paschoaloto, J.; Paulino, P.V.; Maria De Almeida Matos, É.; De Almeida Martins, P.G.M.; Dallago, G.M.; Costa, P.M. Performance and Economic Analysis of Nellore Cattle Finished in Feedlot during Dry and Rainy Seasons. Livest. Sci. 2022, 260, 104903. [Google Scholar] [CrossRef]
- de Carvalho Porto Barbosa, M.; Fioravanti, M.C.S.; Peripolli, V.; do Egito, A.A.; Juliano, R.S.; Ramos, A.F.; Cardoso, D.; Laudares, K.M.; Feijó, G.L.D.; Prado, C.S.; et al. Performance, Carcass, and Meat Traits of Locally Adapted Brazilian Cattle Breeds under Feedlot Conditions. Trop. Anim. Health Prod. 2023, 55, 243. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.V.D.; Souza, J.C.D.; Ferraz Filho, P.B.; Silva, L.O.C.D.; Arruda, R.M.D.S.; Machado, C.H.C.; Pereira, M.A. Adaptability and Phenotypic Stability for Productive and Reproductive Traits in Nelore Cattle. Cienc. Rural 2019, 49, e20180327. [Google Scholar] [CrossRef]
- Mueller, M.L.; Van Eenennaam, A.L. Synergistic Power of Genomic Selection, Assisted Reproductive Technologies, and Gene Editing to Drive Genetic Improvement of Cattle. CABI Agric. Biosci. 2022, 3, 13. [Google Scholar] [CrossRef]
- Baruselli, P.S.; Ferreira, R.M.; Sá Filho, M.F.; Bó, G.A. Review: Using Artificial Insemination v. Natural Service in Beef Herds. Animal 2018, 12, s45–s52. [Google Scholar] [CrossRef]
- Ugur, M.R.; Guerreiro, D.D.; Moura, A.A.; Memili, E. Identification of Biomarkers for Bull Fertility Using Functional Genomics. Anim. Reprod. 2022, 19, e20220004. [Google Scholar] [CrossRef]
- Harighi, M.F.; Wahid, H.; Khumran, A.M.; Baiee, F. Breeding Soundness Examination (BSE): A Decision-Making Tool That Requires a Particular Guideline for Male Goats. Trop. Anim. Health Prod. 2022, 54, 174. [Google Scholar] [CrossRef]
- Elango, K.; Karuthadurai, T.; Kumaresan, A.; Sinha, M.K.; Ebenezer Samuel King, J.P.; Nag, P.; Sharma, A.; Raval, K.; Paul, N.; Talluri, T.R. High-Throughput Proteomic Characterization of Seminal Plasma from Bulls with Contrasting Semen Quality. 3 Biotech 2023, 13, 60. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Rego, J.P.A.; Crisp, J.M.; Moura, A.A.; Nouwens, A.S.; Li, Y.; Venus, B.; Burns, B.M.; McGowan, M.R. Seminal Plasma Proteins and Their Relationship with Percentage of Morphologically Normal Sperm in 2-Year-Old Brahman (Bos Indicus) Bulls. Anim. Reprod. Sci. 2015, 162, 20–30. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the Seminal Plasma Proteome and Its Role in Male Fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef]
- Agarwal, A.; Panner Selvam, M.K.; Baskaran, S. Proteomic Analyses of Human Sperm Cells: Understanding the Role of Proteins and Molecular Pathways Affecting Male Reproductive Health. Int. J. Mol. Sci. 2020, 21, 1621. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Filho, I.C.; Pasini, M.; Moura, A.A. Spermatozoa and Seminal Plasma Proteomics: Too Many Molecules, Too Few Markers. The Case of Bovine and Porcine Semen. Anim. Reprod. Sci. 2022, 247, 107075. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, K.P.; Mol, P.; Kannegundla, U.; Thota, L.N.; Gopalakrishnan, L.; Rana, E.; Azharuddin, N.; Mangalaparthi, K.K.; Kumar, M.; Dey, G.; et al. Deep Proteome Profiling of Semen of Indian Indigenous Malnad Gidda (Bos indicus) Cattle. J. Proteome Res. 2020, 19, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Ashwitha, A.; Ramesha, K.P.; Ramesh, P.; Kootimole, C.N.; Devadasan, M.J.; Ammankallu, S.; Jeyakumar, S.; Kumaresan, A.; Veerappa, V.G.; Das, D.N.; et al. Quantitative Proteomics Profiling of Spermatozoa and Seminal Plasma Reveals Proteins Associated with Semen Quality in Bos Indicus Bulls. J. Proteom. 2023, 273, 104794. [Google Scholar] [CrossRef]
- Somashekar, L.; Selvaraju, S.; Parthipan, S.; Patil, S.K.; Binsila, B.K.; Venkataswamy, M.M.; Karthik Bhat, S.; Ravindra, J.P. Comparative Sperm Protein Profiling in Bulls Differing in Fertility and Identification of Phosphatidylethanolamine-Binding Protein 4, a Potential Fertility Marker. Andrology 2017, 5, 1032–1051. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Arangasamy, A.; Kastelic, J.P. Sperm and Seminal Plasma Proteomics of High- versus Low-Fertility Holstein Bulls. Theriogenology 2019, 126, 41–48. [Google Scholar] [CrossRef]
- Colégio Brasileiro de Reprodução Animal. Manual para Exame Andrológico e Avaliação de Sêmen Animal, 3rd ed.; CBRA: Belo Horizonte, Brazil, 2013; pp. 15–30. [Google Scholar]
- Siqueira, J.; Oba, E.; Pinho, R.; Quintino, H.; Eler, J.; Miranda Neto, T.; Guimarães, S.; Guimarães, J. Heritability Estimate and Genetic Correlations of Reproductive Features in Nellore Bulls, Offspring of Super Precocious, Precocious and Normal Cows Under Extensive Farming Conditions: Heritability Estimate and Genetic Correlations. Reprod. Domest. Anim. 2012, 47, 313–318. [Google Scholar] [CrossRef]
- Okano, D.S.; Penitente-Filho, J.M.; Gomez León, V.E.; Maitan, P.P.; Silveira, C.O.; Waddington, B.; Díaz-Miranda, E.A.; Da Costa, E.P.; Guimarães, S.E.F.; Guimarães, J.D. In Vitro Evaluation of Cryopreserved Bovine Sperm and Its Relation to Field Fertility in Fixed-time Artificial Insemination. Reprod. Dom. Anim. 2019, 54, 604–612. [Google Scholar] [CrossRef]
- Hancock, J.L. The morphology of boar spermatozoa. J. R. Microsc. Soc. 1956, 76, 84–97. [Google Scholar] [CrossRef]
- Blom, E. The ultrastructure of some characteristic sperm defects and a proposal for a new classification of the bull spirogram (author’s transl). Nord. Vet. Med. 1973, 25, 383–391. [Google Scholar]
- Magalhães, M.J.; Martins, L.F.; Senra, R.L.; Santos, T.F.D.; Okano, D.S.; Pereira, P.R.G.; Faria-Campos, A.; Campos, S.V.A.; Guimarães, J.D.; Baracat-Pereira, M.C. Differential Abundances of Four Forms of Binder of SPerm 1 in the Seminal Plasma of Bos Taurus Indicus Bulls with Different Patterns of Semen Freezability. Theriogenology 2016, 86, 766–777.e2. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Resjö, S.; Brus, M.; Ali, A.; Meijer, H.J.G.; Sandin, M.; Govers, F.; Levander, F.; Grenville-Briggs, L.; Andreasson, E. Proteomic Analysis of Phytophthora Infestans Reveals the Importance of Cell Wall Proteins in Pathogenicity. Mol. Cell Proteom. 2017, 16, 1958–1971. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-Gel Digestion for Mass Spectrometric Characterization of Proteins and Proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Proteomic Analysis of Whey Proteins in the Colostrum and Mature Milk of Xinong Saanen Goats. J. Dairy Sci. 2020, 103, 1164–1174. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG Database: An Updated Version Includes Eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef]
- Pini, T.; Farmer, K.; Druart, X.; Teixeira-Gomes, A.P.; Tsikis, G.; Labas, V.; Leahy, T.; De Graaf, S.P. Binder of Sperm Proteins Protect Ram Spermatozoa from Freeze-Thaw Damage. Cryobiology 2018, 82, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Gregori, J.; Villarreal, L.; Sánchez, A.; Baselga, J.; Villanueva, J. An Effect Size Filter Improves the Reproducibility in Spectral Counting-Based Comparative Proteomics. J. Proteom. 2013, 95, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING Database in 2011: Functional Interaction Networks of Proteins, Globally Integrated and Scored. Nucleic Acids Res. 2011, 39, D561–D568. [Google Scholar] [CrossRef]
- SAEG. Sistema Para Análises Estatísticas, Versão 9.1: Viçosa: UFV/Fundação Arthur Bernardes, 2007. Available online: http://arquivo.ufv.br/saeg/ (accessed on 27 March 2018).
- Szczykutowicz, J.; Kałuża, A.; Kaźmierowska-Niemczuk, M.; Ferens-Sieczkowska, M. The Potential Role of Seminal Plasma in the Fertilization Outcomes. Biomed. Res. Int. 2019, 2019, 5397804. [Google Scholar] [CrossRef] [PubMed]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The Enigmatic Seminal Plasma: A Proteomics Insight from Ejaculation to Fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Samanta, L.; Swain, N.; Ayaz, A.; Venugopal, V.; Agarwal, A. Post-Translational Modifications in Sperm Proteome: The Chemistry of Proteome Diversifications in the Pathophysiology of Male Factor Infertility. Biochim. Biophys. Acta 2016, 1860, 1450–1465. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.B.; De Oliveira, R.V.; Van Tilburg, M.F.; Barbosa, E.A.; Nascimento, N.V.; Velho, A.L.M.C.S.; Moreno, F.B.; Moreira, R.A.; Monteiro-Moreira, A.C.O.; Carvalho, G.M.C.; et al. Proteomic Analysis of Seminal Plasma from Locally-Adapted “Curraleiro Pé-Duro Bulls” (Bos taurus): Identifying Biomarkers Involved in Sperm Physiology in Endangered Animals for Conservation of Biodiversity. Anim. Reprod. Sci. 2017, 183, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Özbek, M.; Hitit, M.; Kaya, A.; Jousan, F.D.; Memili, E. Sperm Functional Genome Associated with Bull Fertility. Front. Vet. Sci. 2021, 8, 610888. [Google Scholar] [CrossRef]
- Luongo, C.; González-Brusi, L.; Cots-Rodríguez, P.; Izquierdo-Rico, M.J.; Avilés, M.; García-Vázquez, F.A. Sperm Proteome after Interaction with Reproductive Fluids in Porcine: From the Ejaculation to the Fertilization Site. Int. J. Mol. Sci. 2020, 21, 6060. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal Plasma Proteins as Markers of Sperm Fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef]
- Codognoto, V.M.; Yamada, P.H.; Schmith, R.A.; De Ruediger, F.R.; Scott, C.; De Faria Lainetti, P.; Brochine, S.; De Paula Freitas-Dell’Aqua, C.; De Souza, F.F.; Oba, E. Functional Insights into the Role of Seminal Plasma Proteins on Sperm Motility of Buffalo. Anim. Reprod. Sci. 2018, 195, 251–258. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, D.; Singh, I.; Yadav, P.S. Seminal Plasma Proteome: Promising Biomarkers for Bull Fertility. Agric. Res. 2012, 1, 78–86. [Google Scholar] [CrossRef]
- Töpfer-Petersen, E.; Romero, A.; Varela, P.F.; Ekhlasi-Hundrieser, M.; Dostàlovà, Z.; Sanz, L.; Calvete, J.J. Spermadhesins: A New Protein Family. Facts, Hypotheses and Perspectives. Andrologia 2009, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jobim, M.I.M.; Oberst, E.R.; Salbego, C.G.; Souza, D.O.; Wald, V.B.; Tramontina, F.; Mattos, R.C. Two-Dimensional Polyacrylamide Gel Electrophoresis of Bovine Seminal Plasma Proteins and Their Relation with Semen Freezability. Theriogenology 2004, 61, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Westfalewicz, B.; Słowińska, M.; Judycka, S.; Ciereszko, A.; Dietrich, M.A. Comparative Proteomic Analysis of Young and Adult Bull (Bos taurus) Cryopreserved Semen. Animals 2021, 11, 2013. [Google Scholar] [CrossRef]
- Kovac, J.R.; Pastuszak, A.W.; Lamb, D.J. The Use of Genomics, Proteomics, and Metabolomics in Identifying Biomarkers of Male Infertility. Fertil. Steril. 2013, 99, 998–1007. [Google Scholar] [CrossRef]
- Killian, G.J.; Chapman, D.A.; Rogowski, L.A. Fertility-Associated Proteins in Holstein Bull Seminal Plasma1. Biol. Reprod. 1993, 49, 1202–1207. [Google Scholar] [CrossRef]
- Moura, A.A. Identification of Proteins in the Accessory Sex Gland Fluid Associated with Fertility Indexes of Dairy Bulls: A Proteomic Approach. J. Androl. 2006, 27, 201–211. [Google Scholar] [CrossRef]
- Divyashree, B.C.; Roy, S.C. Species-Specific and Differential Expression of BSP-5 and Other BSP Variants in Normozoospermic and Asthenozoospermic Buffalo (Bubalus bubalis) and Cattle (Bos taurus) Seminal Plasma. Theriogenology 2018, 106, 279–286. [Google Scholar] [CrossRef]
- Viana, A.G.A.; Martins, A.M.A.; Pontes, A.H.; Fontes, W.; Castro, M.S.; Ricart, C.A.O.; Sousa, M.V.; Kaya, A.; Topper, E.; Memili, E.; et al. Proteomic Landscape of Seminal Plasma Associated with Dairy Bull Fertility. Sci. Rep. 2018, 8, 16323. [Google Scholar] [CrossRef]
- Gomes, F.P.; Park, R.; Viana, A.G.; Fernandez-Costa, C.; Topper, E.; Kaya, A.; Memili, E.; Yates, J.R.; Moura, A.A. Protein Signatures of Seminal Plasma from Bulls with Contrasting Frozen-Thawed Sperm Viability. Sci. Rep. 2020, 10, 14661. [Google Scholar] [CrossRef] [PubMed]
- Plante, G.; Prud’homme, B.; Fan, J.; Lafleur, M.; Manjunath, P. Evolution and Function of Mammalian Binder of Sperm Proteins. Cell Tissue Res. 2016, 363, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Aslam, M.K.; Sharma, V.K.; Pandey, S.; Kumaresan, A.; Srinivasan, A.; Datta, T.K.; Mohanty, T.K.; Yadav, S. Identification of Biomarker Candidates for Fertility in Spermatozoa of Crossbred Bulls through Comparative Proteomics. Theriogenology 2018, 119, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rego, J.P.A.; Moura, A.A.; Nouwens, A.S.; McGowan, M.R.; Boe-Hansen, G.B. Seminal Plasma Protein Profiles of Ejaculates Obtained by Internal Artificial Vagina and Electroejaculation in Brahman Bulls. Anim. Reprod. Sci. 2015, 160, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Westfalewicz, B.; Dietrich, M.; Słowińska, M.; Judycka, S.; Ciereszko, A. Seasonal Changes in the Proteome of Cryopreserved Bull Semen Supernatant. Theriogenology 2019, 126, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Gotte, G.; Vottariello, F.; Libonati, M. Thermal Aggregation of Ribonuclease A: A contribution to the understanding of the role of 3D domain swapping in protein aggregation. J. Biol. Chem. 2003, 278, 10763–10769. [Google Scholar] [CrossRef]
- Kurzawski, M.; Kaczmarek, M.; Kłysz, M.; Malinowski, D.; Kazienko, A.; Kurzawa, R.; Droździk, M. MMP2, MMP9 and TIMP2 Polymorphisms Affect Sperm Parameters but Not Fertility in Polish Males. Andrologia 2017, 49, e12654. [Google Scholar] [CrossRef]
- Belardin, L.B.; Antoniassi, M.P.; Camargo, M.; Intasqui, P.; Fraietta, R.; Bertolla, R.P. Semen Levels of Matrix Metalloproteinase (MMP) and Tissue Inhibitor of Metalloproteinases (TIMP) Protein Families Members in Men with High and Low Sperm DNA Fragmentation. Sci. Rep. 2019, 9, 903. [Google Scholar] [CrossRef]
- Baumgart, E.; Lenk, S.V.; Loening, S.A.; Jung, K. Quantitative Differences in Matrix Metalloproteinase (MMP)-2, but Not in MMP-9, Tissue Inhibitor of Metalloproteinase (TIMP)-1 or TIMP-2, in Seminal Plasma of Normozoospermic and Azoospermic Patients. Hum. Reprod. 2002, 17, 2919–2923. [Google Scholar] [CrossRef]
- Rego, J.P.A.; Crisp, J.M.; Moura, A.A.; Nouwens, A.S.; Li, Y.; Venus, B.; Corbet, N.J.; Corbet, D.H.; Burns, B.M.; Boe-Hansen, G.B.; et al. Seminal Plasma Proteome of Electroejaculated Bos Indicus Bulls. Anim. Reprod. Sci. 2014, 148, 1–17. [Google Scholar] [CrossRef]
- Pereira, G.R.; De Lazari, F.L.; Dalberto, P.F.; Bizarro, C.V.; Sontag, E.R.; Koetz Junior, C.; Menegassi, S.R.O.; Barcellos, J.O.J.; Bustamante-Filho, I.C. Effect of Scrotal Insulation on Sperm Quality and Seminal Plasma Proteome of Brangus Bulls. Theriogenology 2020, 144, 194–203. [Google Scholar] [CrossRef]
- Newton, L.D.; Kastelic, J.P.; Wong, B.; Van Der Hoorn, F.; Thundathil, J. Elevated Testicular Temperature Modulates Expression Patterns of Sperm Proteins in Holstein Bulls. Mol. Reprod. Dev. 2009, 76, 109–118. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Rêgo, J.P.A.; Satake, N.; Venus, B.; Sadowski, P.; Nouwens, A.; Li, Y.; McGowan, M. Effects of Increased Scrotal Temperature on Semen Quality and Seminal Plasma Proteins in Brahman Bulls. Mol. Reprod. Dev. 2020, 87, 574–597. [Google Scholar] [CrossRef]
- de Arruda, R.P.; Celeghini, E.C.C.; Garcia, A.R.; dos Santos, G.D.C.; Leite, T.G.; Oliveira, L.Z.; Lançoni, R.; Rodrigues, M.d.P. Morfologia Espermática de Touros: Interpretação e Impacto Na Fertilidade. Rev. Bras. Reprod. Anim. 2015, 39, 44–60. [Google Scholar]
- Pham, S.; Schultz, J.S. Testicular Thermoregulation with Respect to Spermatogenesis and Contraception. J. Therm. Biol. 2021, 99, 102954. [Google Scholar] [CrossRef]
- Setchell, B. The Scrotum and Thermoregulation. In The Mammalian Testis; Cornell University Press: Ithaca, NY, USA, 1978. [Google Scholar]
- Avital-Cohen, N.; Heiblum, R.; Argov, N.; Rosenstrauch, A.; Chaiseha, Y.; Mobarkey, N.; Rozenboim, I. The Effect of Active Immunization against Vasoactive Intestinal Peptide (VIP) and Inhibin on Reproductive Performance of Aging White Leghorn Roosters. Poult. Sci. 2012, 91, 161–174. [Google Scholar] [CrossRef]
- Pinho, M.S.; Afonso, F.; Rodrigues, G.; Gulbenkian, S.; Mata, L.R. Neuropeptides in the Seminal Vesicles: Locations, Binding Sites and Functional Implications. Histol. Histopathol. 1997, 12, 503–512. [Google Scholar] [PubMed]
- Raut, S.; Deshpande, S.; Balasinor, N. Unveiling the Role of Prolactin and Its Receptor in Male Reproduction. Horm. Metab. Res. 2019, 51, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Dabbous, Z.; Atkin, S.L. Hyperprolactinaemia in Male Infertility: Clinical Case Scenarios. Arab. J. Urol. 2018, 16, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Avital-Cohen, N.; Heiblum, R.; Argov, N.; Rosenstrauch, A.; Chaiseha, Y.; Mobarkey, N.; Rozenboim, I. The Effect of Active Immunization against Vasoactive Intestinal Peptide and Inhibin on Reproductive Performance of Young White Leghorn Roosters. Poult. Sci. 2011, 90, 2321–2331. [Google Scholar] [CrossRef]
- Bendre, A.; Büki, K.G.; Määttä, J.A. Fam3c Modulates Osteogenic Differentiation by Down-Regulating Runx2. Differentiation 2017, 93, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, W.; Wang, J.; Meng, Y.; Guan, Y.; Yang, J. FAM3 Gene Family: A Promising Therapeutical Target for NAFLD and Type 2 Diabetes. Metabolism 2018, 81, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.J.B.; Arruda-Alencar, J.M.; Martins, J.A.M.; Viana, A.G.A.; Viana Neto, A.M.; Rêgo, J.P.A.; Oliveira, R.V.; Lobo, M.; Moreira, A.C.O.; Moreira, R.A.; et al. Major Seminal Plasma Proteome of Rabbits and Associations with Sperm Quality. Theriogenology 2019, 128, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.; Lipiński, P.; Ogórek, M.; Starzyński, R.; Grzmil, P.; Bednarz, A.; Lenartowicz, M. Molecular Regulation of Copper Homeostasis in the Male Gonad during the Process of Spermatogenesis. Int. J. Mol. Sci. 2020, 21, 9053. [Google Scholar] [CrossRef]
- Ghiasvand, T.; Goodarzi, M.T.; Shafiee, G.; Zamani, A.; Karimi, J.; Ghorbani, M.; Amiri, I. Association between Seminal Plasma Neopterin and Oxidative Stress in Male Infertility: A Case-Control Study. Int. J. Reprod. Biomed. 2018, 16, 93–100. [Google Scholar] [CrossRef]
- González-Cadavid, V.; Martins, J.A.M.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.L.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Moura, A.A. Seminal Plasma Proteins of Adult Boars and Correlations with Sperm Parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, N.K. Emerging Role of Novel Seminal Plasma Bio-Markers in Male Infertility: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 170–179. [Google Scholar] [CrossRef]
- Shibahara, H.; Chen, Y.; Honda, H.; Wakimoto, Y.; Fukui, A.; Hasegawa, A. Sex Difference in Anti-Sperm Antibodies. Reprod Med. Biol. 2022, 21, e12477. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, X.; Pu, Q.; Huang, T.; Xie, Q.; Wang, Y.; Li, J.; Wang, Y.; Gu, H.; Huang, T.; et al. Immunoglobulin G Expression in Human Sperm and Possible Functional Significance. Sci. Rep. 2016, 6, 20166. [Google Scholar] [CrossRef]

| Variable | FIT Bulls | UNFIT Bulls | Mean | CV | V. Min | V. Max |
|---|---|---|---|---|---|---|
| PE | 34.91 ± 1.22 | 34.51 ± 1.52 | 34.61 ± 1.39 | 3.99 | 31 | 36.6 |
| Vol | 3.40 ± 0.69 | 4.70 ± 1.33 | 4.05 ± 1.23 | 26.35 | 2 | 6 |
| Asp | 2.70 ± 0.67 | 2.60 ± 0.51 | 2.65 ± 0.58 | 22.68 | 2 | 4 |
| MM | 1.65 ± 1.49 | 0.70 ± 0.82 | 1.17 ± 1.27 | 102.53 | 0 | 4 |
| Mot | 70.00 ± 11.05 | 61.00 ± 23.66 | 65.00 ± 18.56 | 28.2 | 0 | 90 |
| Vig | 3.15 ± 0.34 | 2.80 ± 1.01 | 2.97 ± 0.75 | - | 0 | 4 |
| Morp | 12.30 ± 3.27 b | 32.90 ± 9.48 a | 22.60 ± 12.62 | 31.37 | 9 | 50 |
| UniProtKb ID | Protein | Avg. FIT | % | Avg. UNFIT | % |
|---|---|---|---|---|---|
| P29392 | Spermadhesin-1 | 428.58 | 47.7% | 240.36 | 25.7% |
| Q8HZY1 | Serine protease inhibitor clade E member 2 | 34.75 | 3.9% | 36.74 | 3.9% |
| P02784 | Seminal plasma protein PDC-109 | 20.32 | 2.3% | 76.44 | 8.2% |
| A0A4W2BP54 | Jacalin-type lectin domain-containing protein | 14.70 | 1.6% | 22.09 | 2.4% |
| G3MWX7 | Lipoclin_cytosolic_FA-bd_dom | 13.69 | 1.5% | 18.87 | 2.0% |
| P17697 | Clusterin | 13.23 | 1.5% | 6.43 | 0.7% |
| P80311 | Peptidyl-prolyl cis-trans isomerase B | 12.12 | 1.3% | 18.63 | 2.0% |
| A0A0E3UT00 | Metalloproteinase inhibitor 2 | 11.79 | 1.3% | 37.80 | 4.0% |
| L8HPF5 | Ig gamma-1 chain C region | 11.54 | 1.3% | 3.31 | 0.4% |
| P81019 | Seminal plasma protein BSP-30 kDa | 11.05 | 1.2% | 32.77 | 3.5% |
| P79345 | NPC intracellular cholesterol transporter 2 | 10.87 | 1.2% | 20.18 | 2.2% |
| F1MCF5 | Glutathione peroxidase | 10.69 | 1.2% | 9.20 | 1.0% |
| D4QBC5 | Hemoglobin beta | 9.02 | 1.0% | 4.86 | 0.5% |
| P82292 | Spermadhesin Z13 | 7.80 | 0.9% | 50.14 | 5.4% |
| F1MGQ1 | Deoxyribonuclease | 7.15 | 0.8% | 17.13 | 1.8% |
| D4QBD1 | Hemoglobin beta | 6.93 | 0.8% | 18.93 | 2.0% |
| W5P198 | Vasoactive intestinal peptide | 4.46 | 0.5% | 28.53 | 3.0% |
| Q4R0H2 | Spermadhesin 2 | 3.46 | 0.4% | 21.71 | 2.3% |
| P00669 | Seminal ribonuclease | 1.95 | 0.2% | 10.68 | 1.1% |
| Other proteins | 29.5% | 27.9% |
| GoTerm | Functional Annotation Clusters | Number of Protein | Enrichment Score |
|---|---|---|---|
| BP | Phospholipid efflux | 4 | 3.12 |
| Sperm capacitation | 4 | ||
| Positive regulation of sperm capacitation | 3 | ||
| MF | ATP binding | 16 | 4.42 |
| Unfolded protein binding | 11 | ||
| ATPase activity | 10 | ||
| Protein binding involved in protein folding | 8 | ||
| CC | chaperonin-containing T-complex | 4 | 2.04 |
| Cell body | 4 | ||
| Microtubule | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-López, C.J.; Barros, E.; Vidigal, P.M.P.; Silva Okano, D.; Duarte Rodrigues, J.N.; Lopes Gomes, L.; Montes-Vergara, J.C.; Petro Hernandez, V.G.; Baracat-Pereira, M.C.; Guimarães, S.E.F.; et al. Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters. Vet. Sci. 2023, 10, 610. https://doi.org/10.3390/vetsci10100610
Ramírez-López CJ, Barros E, Vidigal PMP, Silva Okano D, Duarte Rodrigues JN, Lopes Gomes L, Montes-Vergara JC, Petro Hernandez VG, Baracat-Pereira MC, Guimarães SEF, et al. Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters. Veterinary Sciences. 2023; 10(10):610. https://doi.org/10.3390/vetsci10100610
Chicago/Turabian StyleRamírez-López, Camilo José, Edvaldo Barros, Pedro Marcus Pereira Vidigal, Denise Silva Okano, Juliana Nascimento Duarte Rodrigues, Lidiany Lopes Gomes, José Carlos Montes-Vergara, Victor Gerardo Petro Hernandez, Maria Cristina Baracat-Pereira, Simone Eliza Facioni Guimarães, and et al. 2023. "Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters" Veterinary Sciences 10, no. 10: 610. https://doi.org/10.3390/vetsci10100610
APA StyleRamírez-López, C. J., Barros, E., Vidigal, P. M. P., Silva Okano, D., Duarte Rodrigues, J. N., Lopes Gomes, L., Montes-Vergara, J. C., Petro Hernandez, V. G., Baracat-Pereira, M. C., Guimarães, S. E. F., & Guimarães, J. D. (2023). Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters. Veterinary Sciences, 10(10), 610. https://doi.org/10.3390/vetsci10100610








