Nutritional Strategies to Promote Bovine Oocyte Quality for In Vitro Embryo Production: Do They Really Work?
Abstract
Simple Summary
Abstract
1. Introduction
2. Impact of Dietary Intake of Bovine Oocyte Donors on In Vitro Embryo Production
3. Impact of Fatty Acid Supplementation of Bovine Oocyte Donors on In Vitro Embryo Production
4. Impact of Mineral and Vitamin Supplementation of Bovine Oocyte Donors on In Vitro Embryo Production
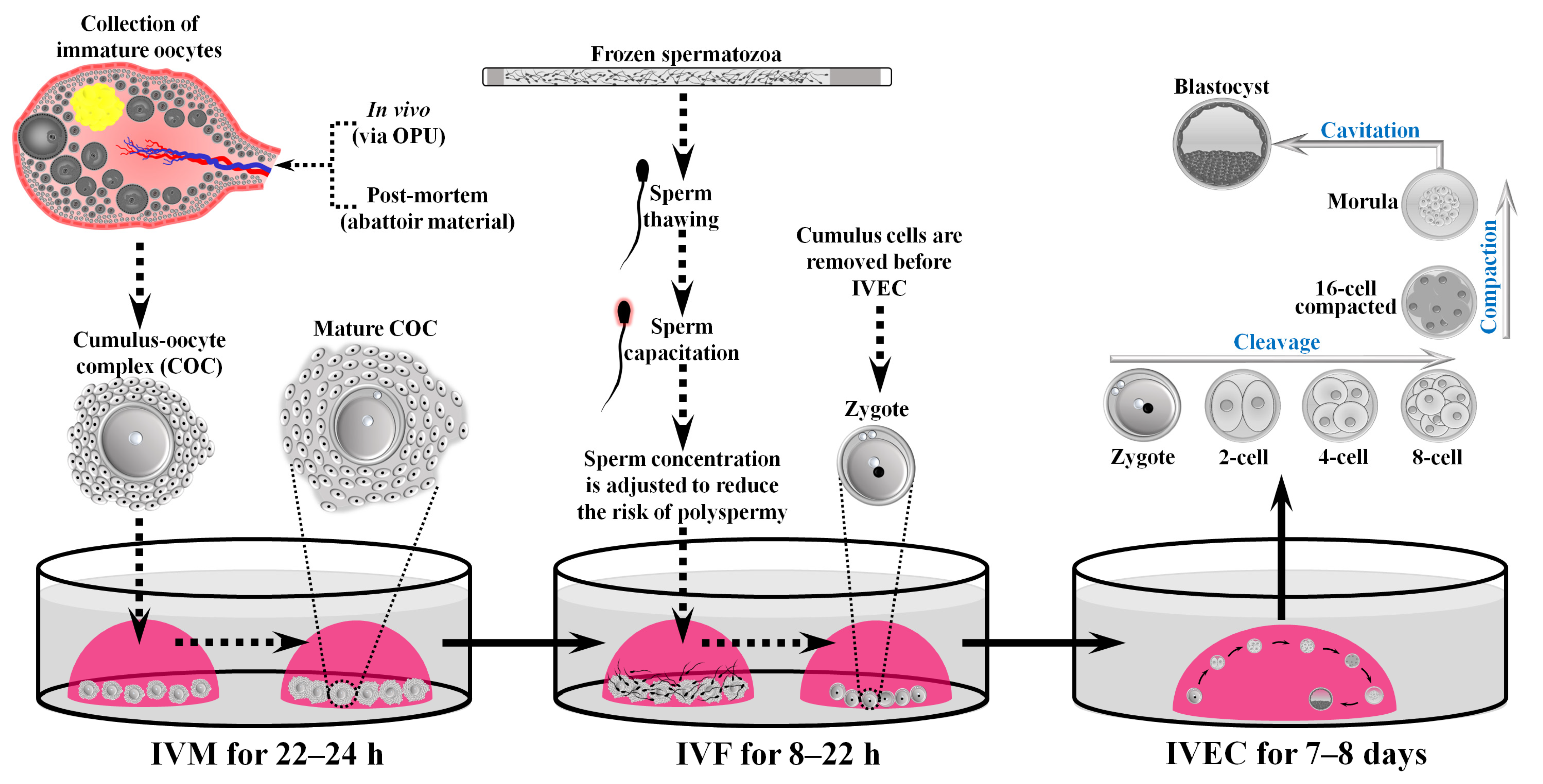
5. Experimental Considerations in Nutritional Research with Bovine OPU-IVEP Models
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerland, P.; Raftery, A.E.; Ševčíková, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Springmann, M.; Mason-D’Croz, D.; Robinson, S.; Garnett, T.; Godfray, H.C.J.; Gollin, D.; Rayner, M.; Ballon, P.; Scarborough, P. Global and regional health effects of future food production under climate change: A modelling study. Lancet 2016, 387, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Wulster-Radcliffe, M.C.; Aaron, D.K.; Davis, T.A. Importance of Animals in Agricultural Sustainability and Food Security. J. Nutr. 2015, 145, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.C.; White, R.R. Breeding animals to feed people: The many roles of animal reproduction in ensuring global food security. Theriogenology 2020, 150, 27–33. [Google Scholar] [CrossRef]
- Dahlen, C.; Larson, J.; Lamb, G.C. Impacts of reproductive technologies on beef production in the United States. In Current and Future Reproductive Technologies and World Food Production; Lamb, G., DiLorenzo, N., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 752, pp. 97–114. [Google Scholar] [CrossRef]
- Lagrange, V.; Whitsett, D.; Burris, C. Global market for dairy proteins. J. Food Sci. 2015, 80 (Suppl. 1), A16–A22. [Google Scholar] [CrossRef]
- Hansen, P.J. Current and future assisted reproductive technologies for mammalian farm animals. In Current and Future Reproductive Technologies and World Food Production; Lamb, G., DiLorenzo, N., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 752, pp. 1–22. [Google Scholar] [CrossRef]
- Stevenson, J.S. Impact of reproductive technologies on dairy food production in the dairy industry. In Current and Future Reproductive Technologies and World Food Production; Lamb, G., DiLorenzo, N., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 752, pp. 115–129. [Google Scholar] [CrossRef]
- Hernandez Gifford, J.A.; Gifford, C.A. Role of reproductive biotechnologies in enhancing food security and sustainability. Anim. Front. 2013, 3, 14–19. [Google Scholar] [CrossRef]
- Crowe, A.D.; Lonergan, P.; Butler, S.T. Invited review: Use of assisted reproduction techniques to accelerate genetic gain and increase value of beef production in dairy herds. J. Dairy Sci. 2021, 104, 12189–12206. [Google Scholar] [CrossRef]
- Mueller, M.L.; Van Eenennaam, A.L. Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agric. Biosci. 2022, 3, 13. [Google Scholar] [CrossRef]
- Viana, J.H. 2021 Statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2022, 40, 22–40. [Google Scholar]
- Goszczynski, D.E.; Cheng, H.; Demyda-Peyrás, S.; Medrano, J.F.; Wu, J.; Ross, P.J. In vitro breeding: Application of embryonic stem cells to animal production. Biol. Reprod. 2019, 100, 885–895. [Google Scholar] [CrossRef]
- Ferré, L.B.; Alvarez-Gallardo, H.; Romo, S.; Fresno, C.; Stroud, T.; Stroud, B.; Lindsey, B.; Kjelland, M.E. Transvaginal ultrasound-guided oocyte retrieval in cattle: State-of-the-art and its impact on the in vitro fertilization embryo production outcome. Reprod. Domest. Anim. 2023, 58, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.A.; Kues, W.A.; Niemann, H. Biomedical applications of ovarian transvaginal ultrasonography in cattle. Anim. Biotechnol. 2014, 25, 266–293. [Google Scholar] [CrossRef]
- Oropeza, A.; Hadeler, K.-G.; Niemann, H. Application of ultrasound-guided follicular aspiration (OPU) in prepubertal and adult cattle. J. Reprod. Dev. 2006, 52, S31–S38. [Google Scholar]
- Watanabe, Y.F.; de Souza, A.H.; Mingoti, R.D.; Ferreira, R.M.; Santana Batista, E.O.; Dayan, A.; Watanabe, O.; Meirelles, F.V.; Nogueira, M.F.G.; Ferraz, J.B.S.; et al. Number of oocytes retrieved per donor during OPU and its relationship with in vitro embryo production and field fertility following embryo transfer. Anim. Reprod. 2017, 14, 635–644. [Google Scholar] [CrossRef]
- Vieira, L.M.; Rodrigues, C.A.; Castro Netto, A.; Guerreiro, B.M.; Silveira, C.R.; Moreira, R.J.; Sa Filho, M.F.; Bó, G.A.; Mapletoft, R.J.; Baruselli, P.S. Superstimulation prior to the ovum pick-up to improve in vitro embryo production in lactating and non-lactating Holstein cows. Theriogenology 2014, 82, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Bayeux, B.M.; Joaquim, D.A.; Watanabe, Y.F.; Humblot, P. Antral follicle count, oocyte production and embryonic developmental competence of senescent Nellore (Bos indicus) cows. Theriogenology 2021, 174, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Baruselli, P.S.; Rodrigues, C.A.; Ferreira, R.M.; Sales, J.N.S.; Elliff, F.M.; Silva, L.G.; Viziack, M.P.; Factor, L.; D’Occhio, M.J. Impact of oocyte donor age and breed on in vitro embryo production in cattle, and relationship of dairy and beef embryo recipients on pregnancy and the subsequent performance of offspring: A review. Reprod. Fertil. Dev. 2021, 34, 36–51. [Google Scholar] [CrossRef]
- Gimenes, L.U.; Ferraz, M.L.; Fantinato-Neto, P.; Chiaratti, M.R.; Mesquita, L.G.; Sa Filho, M.F.; Meirelles, F.V.; Trinca, L.A.; Rennó, F.P.; Watanabe, Y.F.; et al. The interval between the emergence of pharmacologically synchronized ovarian follicular waves and ovum pickup does not significantly affect in vitro embryo production in Bos indicus, Bos taurus, and Bubalus bubalis. Theriogenology 2015, 83, 385–393. [Google Scholar] [CrossRef]
- Sales, J.N.; Iguma, L.T.; Batista, R.I.; Quintão, C.C.; Gama, M.A.; Freitas, C.; Pereira, M.M.; Camargo, L.S.; Viana, J.H.; Souza, J.C.; et al. Effects of a high-energy diet on oocyte quality and in vitro embryo production in Bos indicus and Bos taurus cows. J. Dairy Sci. 2015, 98, 3086–3099. [Google Scholar] [CrossRef]
- de Lacerda, I.P.; Dode, M.A.N.; Lima, M.M.S.; Guerra, B.F.; Costa, E.S.; Moreira, G.R.; Carvalho, J.d.O. Cattle breed affects in vitro embryo production in a large-scale commercial program on dairy farms. Livest. Sci. 2020, 240, 104135. [Google Scholar] [CrossRef]
- Guimarães, A.S.B.; Rocha, L.F.; de Jesus, R.D.L.; Vasconcelos, G.L.; Anghinoni, G.; Santana, A.L.A.; Barbosa, L.P. In vitro performance of Zebu (Bos indicus) and Taurus (Bos taurus) donor cow embryos. Rev. Bras. Saúde Produção Anim. 2020, 21, e21200142020. [Google Scholar] [CrossRef]
- Palma, G.A.; Sinowatz, F. Male and female effects on the in vitro production of bovine embryos. Anat. Histol. Embryol. 2004, 33, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Morotti, F.; Sanches, B.V.; Pontes, J.H.; Basso, A.C.; Siqueira, E.R.; Lisboa, L.A.; Seneda, M.M. Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle. Theriogenology 2014, 81, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Baruselli, P.S.; MF, S.F.; Ferreira, R.M.; Sales, J.N.; Gimenes, L.U.; Vieira, L.M.; Mendanha, M.F.; Bó, G.A. Manipulation of follicle development to ensure optimal oocyte quality and conception rates in cattle. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 134–141. [Google Scholar] [CrossRef]
- Merton, J.S.; de Roos, A.P.; Mullaart, E.; de Ruigh, L.; Kaal, L.; Vos, P.L.; Dieleman, S.J. Factors affecting oocyte quality and quantity in commercial application of embryo technologies in the cattle breeding industry. Theriogenology 2003, 59, 651–674. [Google Scholar] [CrossRef]
- Sarwar, Z.; Sagheer, M.; Sosa, F.; Saad, M.; Hassan, M.; Husnain, A.; Arshad, U. Meta-analysis to determine effects of treatment with FSH when there is progestin-priming on in-vitro embryo production using ovum pick-up in Bos taurus cows. Anim. Reprod. Sci. 2020, 221, 106590. [Google Scholar] [CrossRef]
- Zangirolamo, A.F.; Morotti, F.; da Silva, N.C.; Sanches, T.K.; Seneda, M.M. Ovarian antral follicle populations and embryo production in cattle. Anim. Reprod. 2018, 15, 310–315. [Google Scholar] [CrossRef]
- Monteiro, F.M.; Batista, E.O.S.; Vieira, L.M.; Bayeux, B.M.; Accorsi, M.; Campanholi, S.P.; Dias, E.A.R.; Souza, A.H.; Baruselli, P.S. Beef donor cows with high number of retrieved COC produce more in vitro embryos compared with cows with low number of COC after repeated ovum pick-up sessions. Theriogenology 2017, 90, 54–58. [Google Scholar] [CrossRef]
- Barceló-Fimbres, M.; Campos-Chillón, L.F.; Mtango, N.R.; Altermatt, J.; Bonilla, L.; Koppang, R.; Verstegen, J.P. Improving in vitro maturation and pregnancy outcome in cattle using a novel oocyte shipping and maturation system not requiring a CO2 gas phase. Theriogenology 2015, 84, 109–117. [Google Scholar] [CrossRef]
- Sanches, B.V.; Zangirolamo, A.F.; Seneda, M.M. Intensive use of IVF by large-scale dairy programs. Anim. Reprod. 2019, 16, 394–401. [Google Scholar] [CrossRef]
- Boni, R. Ovum pick-up in cattle: A 25 yr retrospective analysis. Anim. Reprod. 2012, 9, 362–369. [Google Scholar]
- Blondin, P. Logistics of large scale commercial IVF embryo production. Reprod. Fertil. Dev. 2016, 29, 32–36. [Google Scholar] [CrossRef]
- Rose, B.I. Approaches to oocyte retrieval for advanced reproductive technology cycles planning to utilize in vitro maturation: A review of the many choices to be made. J. Assist. Reprod. Genet. 2014, 31, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, S.J.; Mackie, K.; Watt, R.G.; Webb, R.; Sinclair, K.D. Impact of nutrition on oocyte quality: Cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol. Reprod. 2005, 73, 918–926. [Google Scholar] [CrossRef]
- Freret, S.; Grimard, B.; Ponter, A.A.; Joly, C.; Ponsart, C.; Humblot, P. Reduction of body-weight gain enhances in vitro embryo production in overfed superovulated dairy heifers. Reproduction 2006, 131, 783–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grimard, B.; Marquant-Leguienne, B.; Remy, D.; Richard, C.; Nuttinck, F.; Humblot, P.; Ponter, A.A. Postpartum variations of plasma IGF and IGFBPs, oocyte production and quality in dairy cows: Relationships with parity and subsequent fertility. Reprod. Domest. Anim. 2013, 48, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Merton, J.S.; Knijn, H.M.; Flapper, H.; Dotinga, F.; Roelen, B.A.; Vos, P.L.; Mullaart, E. Cysteamine supplementation during in vitro maturation of slaughterhouse- and opu-derived bovine oocytes improves embryonic development without affecting cryotolerance, pregnancy rate, and calf characteristics. Theriogenology 2013, 80, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Moallem, U.; Shafran, A.; Zachut, M.; Dekel, I.; Portnick, Y.; Arieli, A. Dietary α-linolenic acid from flaxseed oil improved folliculogenesis and IVF performance in dairy cows, similar to eicosapentaenoic and docosahexaenoic acids from fish oil. Reproduction 2013, 146, 603–614. [Google Scholar] [CrossRef]
- Galli, C.; Duchi, R.; Colleoni, S.; Lagutina, I.; Lazzari, G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: From the research laboratory to clinical practice. Theriogenology 2014, 81, 138–151. [Google Scholar] [CrossRef]
- Guerreiro, B.M.; Batista, E.O.; Vieira, L.M.; Sa Filho, M.F.; Rodrigues, C.A.; Castro Netto, A.; Silveira, C.R.; Bayeux, B.M.; Dias, E.A.; Monteiro, F.M.; et al. Plasma anti-mullerian hormone: An endocrine marker for in vitro embryo production from Bos taurus and Bos indicus donors. Domest. Anim. Endocrinol. 2014, 49, 96–104. [Google Scholar] [CrossRef]
- Gamarra, G.; Ponsart, C.; Lacaze, S.; Le Guienne, B.; Humblot, P.; Deloche, M.-C.; Monniaux, D.; Ponter, A.A. Dietary propylene glycol and in vitro embryo production after ovum pick-up in heifers with different anti-Müllerian hormone profiles. Reprod. Fertil. Dev. 2015, 27, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Vernunft, A.; Schwerhoff, M.; Viergutz, T.; Diederich, M.; Kuwer, A. Anti-Muellerian hormone levels in plasma of Holstein-Friesian heifers as a predictive parameter for ovum pick-up and embryo production outcomes. J. Reprod. Dev. 2015, 61, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Rodrigues, C.A.; Netto, A.C.; Guerreiro, B.M.; Silveira, C.R.; Freitas, B.G.; Bragança, L.G.; Marques, K.N.; Sa Filho, M.F.; Bó, G.A.; et al. Efficacy of a single intramuscular injection of porcine FSH in hyaluronan prior to ovum pick-up in Holstein cattle. Theriogenology 2016, 85, 877–886. [Google Scholar] [CrossRef]
- Tutt, D.A.R.; Silvestri, G.; Serrano-Albal, M.; Simmons, R.J.; Kwong, W.Y.; Guven-Ates, G.; Canedo-Ribeiro, C.; Labrecque, R.; Blondin, P.; Handyside, A.H.; et al. Analysis of bovine blastocysts indicates ovarian stimulation does not induce chromosome errors, nor discordance between inner-cell mass and trophectoderm lineages. Theriogenology 2021, 161, 108–119. [Google Scholar] [CrossRef]
- Soares, A.C.S.; Marques, K.N.G.; Bragança, L.G.M.; Lodde, V.; Luciano, A.M.; Buratini, J. Synchronization of germinal vesicle maturity improves efficacy of in vitro embryo production in Holstein cows. Theriogenology 2020, 154, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Añez, J.C.; Lucas-Hahn, A.; Hadeler, K.-G.; Aldag, P.; Niemann, H. Melatonin enhances in vitro developmental competence of cumulus-oocyte complexes collected by ovum pick-up in prepubertal and adult dairy cattle. Theriogenology 2021, 161, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hayden, C.B.; Sala, R.V.; Absalón-Medina, V.A.; Motta, J.C.L.; Pereira, D.; Moreno, J.F.; García-Guerra, A. Synchronization of follicle wave emergence before ovarian superstimulation with FSH and ovum pick-up improves in vitro embryo production in pregnant heifers. Theriogenology 2022, 188, 71–78. [Google Scholar] [CrossRef]
- Sanei, M.; Kowsar, R.; Heidaran Ali Abadi, M.; Sadeghi, N.; Boroumand Jazi, M. The relationship between bovine blastocyst formation in vitro and follicular fluid amino acids. Theriogenology 2023, 206, 197–204. [Google Scholar] [CrossRef]
- Simmons, R.; Tutt, D.A.; Guven-Ates, G.; Kwong, W.Y.; Labrecque, R.; Randi, F.; Sinclair, K.D. Enhanced progesterone support during stimulated cycles of transvaginal follicular aspiration improves bovine in vitro embryo production. Theriogenology 2023, 199, 77–85. [Google Scholar] [CrossRef]
- Sprícigo, J.F.W.; Diógenes, M.N.; Leme, L.O.; Guimarães, A.L.; Muterlle, C.V.; Silva, B.D.M.; Solà-Oriol, D.; Pivato, I.; Silva, L.P.; Dode, M.A.N. Effects of Different Maturation Systems on Bovine Oocyte Quality, Plasma Membrane Phospholipid Composition and Resistance to Vitrification and Warming. PLoS ONE 2015, 10, e0130164. [Google Scholar] [CrossRef]
- Ghanem, N.; Jin, J.I.; Kim, S.S.; Choi, B.H.; Lee, K.L.; Ha, A.N.; Song, S.H.; Kong, I.K. The Anti-Müllerian Hormone Profile is Linked with the In Vitro Embryo Production Capacity and Embryo Viability after Transfer but Cannot Predict Pregnancy Outcome. Reprod. Domest. Anim. 2016, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Itahashi, T.; Numabe, T. Improved embryo development in Japanese black cattle by in vitro fertilization using ovum pick-up plus intracytoplasmic sperm injection with dithiothreitol. J. Reprod. Dev. 2016, 62, 11–16. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.M.G.; Silva-Santos, K.C.; Barreiros, T.R.R.; Morotti, F.; Sanches, B.V.; de Moraes, F.L.Z.; Blaschi, W.; Seneda, M.M. High numbers of antral follicles are positively associated with in vitro embryo production but not the conception rate for FTAI in Nelore cattle. Anim. Reprod. Sci. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Sakagami, N.; Konda, K.; Hashimura, S.; Kawate, N.; Inaba, T.; Tamada, H. Production of Japanese Black calves by the transfer of embryos developed from in vitro-fertilized oocytes derived by ovum pick up and matured in culture with the mitogen-activated protein kinase kinase inhibitor U0126. J. Vet. Med. Sci. 2019, 81, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.M.; Morotti, F.; Cavalieri, F.L.B.; Lunardelli, P.A.; Santos, A.O.; Membrive, C.M.B.; Castilho, C.; Puelker, R.Z.; Silva, J.O.F.; Zangirolamo, A.F.; et al. Synchronization of stage of follicle development before OPU improves embryo production in cows with large antral follicle counts. Anim. Reprod. Sci. 2020, 221, 106601. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Cortés, E.; Jannaman, E.A.; Block, J.; Amaral, T.F.; Hansen, P.J. Programming of postnatal phenotype caused by exposure of cultured embryos from Brahman cattle to colony-stimulating factor 2 and serum. J. Anim. Sci. 2021, 99, skab180. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Cortés, E.; Ortiz, W.; Rabaglino, M.B.; Block, J.; Rae, O.; Jannaman, E.A.; Xiao, Y.; Hansen, P.J. Choline acts during preimplantation development of the bovine embryo to program postnatal growth and alter muscle DNA methylation. FASEB J. 2021, 35, e21926. [Google Scholar] [CrossRef]
- Saleem, M.; Yousuf, M.R.; Ghafoor, A.; Riaz, A. Effect of three schemes of ovum pick-up on the follicular dynamics, gene expression, and in-vitro developmental competence of oocytes in Sahiwal cattle. Reprod. Domest. Anim. 2022, 57, 1230–1243. [Google Scholar] [CrossRef]
- Saleem, M.; Yousuf, M.R.; Ghafoor, A.; Riaz, A. Influence of endometritis on the follicular dynamics, recovery, quality, gene expression, nuclear maturation and in-vitro developmental competence of oocytes in Sahiwal cattle. Reprod. Domest. Anim. 2023, 58, 207–218. [Google Scholar] [CrossRef]
- Tomita, K.; Ishii, T.; Endo, N.; Tanaka, T. Effects of short-term dietary supplementation on the number of ovarian follicles, quantity and quality of oocytes, and in vitro embryo production in Japanese Black cows. J. Reprod. Dev. 2023, 69, 65–71. [Google Scholar] [CrossRef]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 2019, 125, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Rodney, R.M.; Celi, P.; Scott, W.; Breinhild, K.; Santos, J.E.P.; Lean, I.J. Effects of nutrition on the fertility of lactating dairy cattle. J. Dairy Sci. 2018, 101, 5115–5133. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.H.; Sadri, H.; Sauerwein, H. Invited review: Assessment of body condition score and body fat reserves in relation to insulin sensitivity and metabolic phenotyping in dairy cows. J. Dairy Sci. 2023, 106, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef]
- Chrenek, P.; Kubovičová, E.; Olexíková, L.; Makarevich, A.V.; Toporcerová, S.; Ostró, A. Effect of body condition and season on yield and quality of in vitro produced bovine embryos. Zygote 2015, 23, 893–899. [Google Scholar] [CrossRef]
- Kakar, M.A.; Maddocks, S.; Lorimer, M.F.; Kleemann, D.O.; Rudiger, S.R.; Hartwich, K.M.; Walker, S.K. The effect of peri-conception nutrition on embryo quality in the superovulated ewe. Theriogenology 2005, 64, 1090–1103. [Google Scholar] [CrossRef]
- Nolan, R.; O’Callaghan, D.; Duby, R.T.; Lonergan, P.; Boland, M.P. The influence of short-term nutrient changes on follicle growth and embryo production following superovulation in beef heifers. Theriogenology 1998, 50, 1263–1274. [Google Scholar] [CrossRef]
- Velazquez, M.A. Impact of maternal malnutrition during the periconceptional period on mammalian preimplantation embryo development. Domest. Anim. Endocrinol. 2015, 51, 27–45. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V.; Kastelic, J.P.; Ramsey, K. Metabolic biomarkers, body condition, uterine inflammation and response to superovulation in lactating Holstein cows. Theriogenology 2020, 146, 71–79. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Souza, A.H.; Amundson, M.C.; Hackbart, K.S.; Fuenzalida, M.J.; Herlihy, M.M.; Ayres, H.; Dresch, A.R.; Vieira, L.M.; Guenther, J.N.; et al. Relationships between fertility and postpartum changes in body condition and body weight in lactating dairy cows. J. Dairy Sci. 2014, 97, 3666–3683. [Google Scholar] [CrossRef]
- Ruiz, L.L.; Alvarez, N.; Nuñez, I.; Montes, I.; Solano, R.; Fuentes, D.; Pedroso, R.; Palma, G.A.; Brem, G. Effect of body condition on the developmental competence of IVM/IVF bovine oocytes. Theriogenology 1996, 45, 292. [Google Scholar] [CrossRef]
- Snijders, S.E.; Dillon, P.; O’Callaghan, D.; Boland, M.P. Effect of genetic merit, milk yield, body condition and lactation number on in vitro oocyte development in dairy cows. Theriogenology 2000, 53, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; McEvoy, T.G.; Baxter, G.; Robinson, J.J.; Hogg, C.O.; Woad, K.J.; Webb, R.; Sinclair, K.D. Effect of dietary energy and protein on bovine follicular dynamics and embryo production in vitro: Associations with the ovarian insulin-like growth factor system. Biol. Reprod. 2001, 64, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.A. The role of nutritional supplementation on the outcome of superovulation in cattle. Anim. Reprod. Sci. 2011, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, A.V.; Stádník, L.; Kubovičová, E.; Hegedüšová, Z.; Holásek, R.; Louda, F.; Beran, J.; Nejdlová, M. Quality of preimplantation embryos recovered in vivo from dairy cows in relation to their body condition. Zygote 2016, 24, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Shamsuddin, M.; Bhuiyan, M.M.; Akbar, M.A.; Kamaruddin, K.M. Effect of feeding and body condition score on multiple ovulation and embryo production in zebu cows. Reprod. Domest. Anim. 2002, 37, 37–41. [Google Scholar] [CrossRef]
- Liu, T.; Liu, D.; Song, X.; Qu, J.; Zheng, X.; Li, J.; Yang, R.; Yang, S.; Zhang, X.; Wang, H.; et al. Lipid Metabolism Was Associated With Oocyte in vitro Maturation in Women With Polycystic Ovarian Syndrome Undergoing Unstimulated Natural Cycle. Front. Cell Dev. Biol. 2021, 9, 719173. [Google Scholar] [CrossRef]
- Rooke, J.A.; Ainslie, A.; Watt, R.G.; Alink, F.M.; McEvoy, T.G.; Sinclair, K.D.; Garnsworthy, P.C.; Webb, R. Feeding frequency has diet-dependent effects on plasma hormone concentrations but does not affect oocyte quality in dairy heifers fed fibre- or starch-based diets. Animal 2008, 2, 1361–1370. [Google Scholar] [CrossRef]
- Rooke, J.A.; Ainslie, A.; Watt, R.G.; Alink, F.M.; McEvoy, T.G.; Sinclair, K.D.; Garnsworthy, P.C.; Webb, R. Dietary carbohydrates and amino acids influence oocyte quality in dairy heifers. Reprod. Fertil. Dev. 2009, 21, 419–427. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef]
- Batista, E.O.; Macedo, G.G.; Sala, R.V.; Ortolan, M.D.; MF, S.F.; Del Valle, T.A.; Jesus, E.F.; Lopes, R.N.; Rennó, F.P.; Baruselli, P.S. Plasma antimullerian hormone as a predictor of ovarian antral follicular population in Bos indicus (Nelore) and Bos taurus (Holstein) heifers. Reprod. Domest. Anim. 2014, 49, 448–452. [Google Scholar] [CrossRef]
- Sartori, R.; Bastos, M.R.; Baruselli, P.S.; Gimenes, L.U.; Ereno, R.L.; Barros, C.M. Physiological differences and implications to reproductive management of Bos taurus and Bos indicus cattle in a tropical environment. Soc. Reprod. Fertil. Suppl. 2010, 67, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Moschini, G.A.d.L.; Gaitkoski, D.; de Almeida, A.B.M.; Hidalgo, M.M.T.; Martins, M.I.M.; Blaschi, W.; Barreiros, T.R.R. Comparison between in vitro embryo production in Bos indicus and Bos taurus cows. Res. Soc. Dev. 2021, 10, e38810716712. [Google Scholar] [CrossRef]
- Narváez Bedoya, H.J. Effect of the genetic group of cows of the Gyr and Holstein breeds on the in vitro production technique of bovine embryos. Cienc. Technol. Agropecu. 2020, 21, 1697. [Google Scholar]
- Borowczyk, E.; Caton, J.S.; Redmer, D.A.; Bilski, J.J.; Weigl, R.M.; Vonnahme, K.A.; Borowicz, P.P.; Kirsch, J.D.; Kraft, K.C.; Reynolds, L.P.; et al. Effects of plane of nutrition on in vitro fertilization and early embryonic development in sheep. J. Anim. Sci. 2006, 84, 1593–1599. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Borowczyk, E.; Bilski, J.J.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Vonnahme, K.A. Overfeeding and underfeeding have detrimental effects on oocyte quality measured by in vitro fertilization and early embryonic development in sheep. Domest. Anim. Endocrinol. 2012, 43, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.M.; Lonergan, P.; Boland, M.P.; O’Callaghan, D. Influence of nutrition on the effectiveness of superovulation programmes in ewes: Effect on oocyte quality and post-fertilization development. Reproduction 2003, 125, 543–553. [Google Scholar] [CrossRef]
- Bender, R.W.; Hackbart, K.S.; Dresch, A.R.; Carvalho, P.D.; Vieira, L.M.; Crump, P.M.; Guenther, J.N.; Fricke, P.M.; Shaver, R.D.; Combs, D.K.; et al. Effects of acute feed restriction combined with targeted use of increasing luteinizing hormone content of follicle-stimulating hormone preparations on ovarian superstimulation, fertilization, and embryo quality in lactating dairy cows. J. Dairy Sci. 2014, 97, 764–778. [Google Scholar] [CrossRef]
- Abecia, J.A.; Forcada, F.; Palacín, I.; Sánchez-Prieto, L.; Sosa, C.; Fernández-Foren, A.; Meikle, A. Undernutrition affects embryo quality of superovulated ewes. Zygote 2015, 23, 116–124. [Google Scholar] [CrossRef]
- Gamarra, G.; Ponsart, C.; Lacaze, S.; Nuttinck, F.; Cordova, A.; Mermillod, P.; Guienne, B.M.-L.; Monniaux, D.; Humblot, P.; Ponter, A.A. Oral propylene glycol modifies follicular fluid and gene expression profiles in cumulus-oocyte complexes and embryos in feed-restricted heifers. Reprod. Fertil. Dev. 2018, 30, 417–429. [Google Scholar] [CrossRef]
- Rezende, R.G.; Mingoti, R.D.; Bayeux, B.M.; Elliff, F.M.; Carneiro, T.; Zanatta, G.M.; Barreto, A.E.; Bergamo, L.Z.; Colli, M.H.A.; Watanabe, Y.F.; et al. In vitro production of embryos from Holstein females treated with propylene glycol. Anim. Reprod. 2019, 16, 593. [Google Scholar]
- Rezende, R.G.; Mingoti, R.D.; Ferreira, R.M.; Colli, M.H.A.; Elliff, F.M.; Watanabe, Y.F.; Carneiro, T.D.O.; Belli, R.S.; Barreto, A.E.N.P.; Baruselli, P.S. Propilenoglycol treatment increases blastocyst production rate on Holstein cows on lactation PEAK. Anim. Reprod. 2017, 14, 764. [Google Scholar]
- Rizos, D.; Kenny, D.A.; Griffin, W.; Quinn, K.M.; Duffy, P.; Mulligan, F.J.; Roche, J.F.; Boland, M.P.; Lonergan, P. The effect of feeding propylene glycol to dairy cows during the early postpartum period on follicular dynamics and on metabolic parameters related to fertility. Theriogenology 2008, 69, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Hopster, H.; van der Werf, J.T.N.; Erkens, J.H.F.; Blokhuis, H.J. Effects of repeated jugular puncture on plasma cortisol concentrations in loose-housed dairy cows. J. Anim. Sci. 1999, 77, 708–714. [Google Scholar] [CrossRef] [PubMed]
- da Costa, N.N.; Brito, K.N.; Santana, P.D.; da Silva Cordeiro, M.; Silva, T.V.; Santos, A.X.; do Carmo Ramos, P.; Santos, S.D.; King, W.A.; dos Santos Miranda, M.; et al. Effect of cortisol on bovine oocyte maturation and embryo development in vitro. Theriogenology 2016, 85, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Wang, J.-Z.; Li, J.-J.; Wei, D.-L.; Sui, H.-S.; Zhang, Z.-H.; Zhou, P.; Tan, J.-H. Maternal restraint stress diminishes the developmental potential of oocytes. Biol. Reprod. 2011, 84, 672–681. [Google Scholar] [CrossRef]
- Mahdy, A.K.H. Effect of cortisol on bovine oocytes maturation and further embryonic development after in vitro fertilization. Biomed. J. Sci. Tech. Res. 2018, 10, 8029–8034. [Google Scholar]
- González, R.; Ruiz-León, Y.; Gomendio, M.; Roldan, E.R. The effect of glucocorticoids on ERK-1/2 phosphorylation during maturation of lamb oocytes and their subsequent fertilization and cleavage ability in vitro. Reprod. Toxicol. 2010, 29, 198–205. [Google Scholar] [CrossRef]
- Yuan, H.-J.; Han, X.; He, N.; Wang, G.-L.; Gong, S.; Lin, J.; Gao, M.; Tan, J.-H. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. Sci. Rep. 2016, 6, 24036. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- Zhai, Q.-Y.; Wang, J.-J.; Tian, Y.; Liu, X.; Song, Z. Review of psychological stress on oocyte and early embryonic development in female mice. Reprod. Biol. Endocrinol. 2020, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C. Stress, strain, and pregnancy outcome in postpartum cows. Anim. Reprod. 2019, 16, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Kaewlamun, W.; Grimard, B.; Duvaux-Ponter, C.; Ponter, A.A. Kick-starting ovarian cyclicity by using dietary glucogenic precursors in post-partum dairy cows: A review. Int. J. Vet. Sci. Med. 2020, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020, 11, 110. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated Fatty Acids in Male and Female Reproduction1. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Zeng, X.; Li, S.; Liu, L.; Cai, S.; Ye, Q.; Xue, B.; Wang, X.; Zhang, S.; Chen, F.; Cai, C.; et al. Role of functional fatty acids in modulation of reproductive potential in livestock. J. Anim. Sci. Biotechnol. 2023, 14, 24. [Google Scholar] [CrossRef]
- Rodney, R.M.; Celi, P.; Scott, W.; Breinhild, K.; Lean, I.J. Effects of dietary fat on fertility of dairy cattle: A meta-analysis and meta-regression. J. Dairy Sci. 2015, 98, 5601–5620. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. A 100-Year Review: Fat feeding of dairy cows. J. Dairy Sci. 2017, 100, 10061–10077. [Google Scholar] [CrossRef]
- Aardema, H.; van Tol, H.T.A.; Vos, P. An overview on how cumulus cells interact with the oocyte in a condition with elevated NEFA levels in dairy cows. Anim. Reprod. Sci. 2019, 207, 131–137. [Google Scholar] [CrossRef]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Rizos, D.; Sturmey, R.; Bossaert, P.; Gutierrez-Adan, A.; Van Hoeck, V.; Valckx, S.; Bols, P.E.J. Intrafollicular conditions as a major link between maternal metabolism and oocyte quality: A focus on dairy cow fertility. Reprod. Fertil. Dev. 2011, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Dekel, I.; Lehrer, H.; Arieli, A.; Arav, A.; Livshitz, L.; Yakoby, S.; Moallem, U. Effects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. J. Dairy Sci. 2010, 93, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Bilby, T.R.; Block, J.; do Amaral, B.C.; Sa Filho, O.; Silvestre, F.T.; Hansen, P.J.; Staples, C.R.; Thatcher, W.W. Effects of dietary un-saturated fatty acids on oocyte quality and follicular development in lactating dairy cows in summer. J. Dairy Sci. 2006, 89, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Fouladi-Nashta, A.A.; Gutierrez, C.G.; Gong, J.G.; Garnsworthy, P.C.; Webb, R. Impact of dietary fatty acids on oocyte quality and development in lactating dairy cows. Biol. Reprod. 2007, 77, 9–17. [Google Scholar] [CrossRef]
- Fouladi-Nashta, A.A.; Wonnacott, K.E.; Gutierrez, C.G.; Gong, J.G.; Sinclair, K.D.; Garnsworthy, P.C.; Webb, R. Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction 2009, 138, 771–781. [Google Scholar] [CrossRef]
- Kraisoon, A.; Navanukraw, C.; Inthamonee, W.; Bunma, T. Embryonic development, luteal size and blood flow area, and concentrations of PGF2α metabolite in dairy cows fed a diet enriched in polysaturated or polyunsaturated fatty acid. Anim. Reprod. Sci. 2018, 195, 291–301. [Google Scholar] [CrossRef]
- Gardinal, R.; Calomeni, G.D.; Zanferari, F.; Vendramini, T.H.A.; Takiya, C.S.; Del Valle, T.A.; Renno, F.P. Different durations of whole raw soybean supplementation during the prepartum period: Milk fatty acid profile and oocyte and embryo quality of early-lactating Holstein cows. J. Dairy Sci. 2018, 101, 675–689. [Google Scholar] [CrossRef]
- Freret, S.; Oseikria, M.; Le Bourhis, D.; Desmarchais, A.; Briant, E.; Desnoes, O.; Dupont, M.; Le Berre, L.; Ghazouani, O.; Bertevello, P.S.; et al. Effects of a n-3 polyunsaturated fatty acid-enriched diet on embryo production in dairy cows. Reproduction 2019, 158, 71–83. [Google Scholar] [CrossRef]
- Sharma, A.; Baddela, V.S.; Roettgen, V.; Vernunft, A.; Viergutz, T.; Dannenberger, D.; Hammon, H.M.; Schoen, J.; Vanselow, J. Effects of Dietary Fatty Acids on Bovine Oocyte Competence and Granulosa Cells. Front. Endocrinol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Plante-Dubé, M.; Picard, C.; Gilbert, I.; Robert, C.; Fievez, V.; Vlaeminck, B.; Belleannée, C.; Gervais, R.; Chouinard, P.Y. Effects of a dietary supplement enriched in palmitoleic acid on fatty acid composition of follicular fluid, granulosa cell metabolism, and oocyte developmental capacity in early lactation dairy cows. J. Dairy Sci. 2021, 104, 3693–3706. [Google Scholar] [CrossRef]
- Höffmann, K.; Hanstedt, A.; Onnen-Lübben, E.; Stinshoff, H.; Wilkening-Krass, S.; Bollwein, H.; Wrenzycki, C. Dietary CLA supplementation affects developmental competence of oocytes in lactating dairy cows. Reprod. Domest. Anim. 2008, 43, 101. [Google Scholar]
- Bailey, C.L. Effects of Dietary Conjugated Linoleic Acid Supplementation on Bovine Oocyte Lipid Metabolism, Lipid Composition and Embryo Cryotolerance. Doctoral Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2014. [Google Scholar]
- Perkel, K.J.; Tscherner, A.; Merrill, C.; Lamarre, J.; Madan, P. The ART of selecting the best embryo: A review of early embryonic mortality and bovine embryo viability assessment methods. Mol. Reprod. Dev. 2015, 82, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.C.; Passalia, F.; Matos, F.D.; Maserati, M.P., Jr.; Alves, M.F.; Almeida, T.G.; Cardoso, B.L.; Basso, A.C.; Nogueira, M.F. Methods for assessing the quality of mammalian embryos: How far we are from the gold standard? JBRA Assist. Reprod. 2016, 20, 150–158. [Google Scholar] [CrossRef]
- Janati Idrissi, S.; Le Bourhis, D.; Lefevre, A.; Emond, P.; Le Berre, L.; Desnoës, O.; Joly, T.; Buff, S.; Freret, S.; Schibler, L.; et al. Effects of the donor factors and freezing protocols on the bovine embryonic lipid profile. Biol. Reprod. 2021, 106, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.C.N.; Rigolon, L.P.; Cavalieri, F.L.B.; Seko, M.B.; Albuquerque, K.; Zancheta, C.G. Viability of vitrified embryos obtained from the in vitro fertilization of oocytes from cows supplemented with canola. Arq. Bras. Med. Veterinária Zootec. 2014, 66, 145–151. [Google Scholar] [CrossRef]
- Ponter, A.A.; Guyader-Joly, C.; Nuttinck, F.; Grimard, B.; Humblot, P. Oocyte and embryo production and quality after OPU-IVF in dairy heifers given diets varying in their n-6/n-3 fatty acid ratio. Theriogenology 2012, 78, 632–645. [Google Scholar] [CrossRef]
- Nogueira, E.; Loureiro, J.M.; Rodrigues, G.L.; Mingoti, G.Z. Effect of the energetic and lipid supplementation with soy oil or protect fat in dynamics follicular and production of embryos in vitro of Nellore heifers. Acta Sci. Vet. 2008, 36, s531. [Google Scholar]
- González-Serrano, A.F.; Ferreira, C.R.; Pirro, V.; Lucas-Hahn, A.; Heinzmann, J.; Hadeler, K.-G.; Baulain, U.; Aldag, P.; Meyer, U.; Piechotta, M.; et al. Effects of long-term dietary supplementation with conjugated linoleic acid on bovine oocyte lipid profile. Reprod. Fertil. Dev. 2015, 28, 1326–1339. [Google Scholar] [CrossRef]
- Adamiak, S.J.; Powell, K.; Rooke, J.A.; Webb, R.; Sinclair, K.D. Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction 2006, 131, 247–258. [Google Scholar] [CrossRef]
- Leroy, J.L.; Sturmey, R.G.; Van Hoeck, V.; De Bie, J.; McKeegan, P.J.; Bols, P.E. Dietary fat supplementation and the consequences for oocyte and embryo quality: Hype or significant benefit for dairy cow reproduction? Reprod. Domest. Anim. 2014, 49, 353–361. [Google Scholar] [CrossRef]
- Hidalgo, C.; Díez, C.; Duque, P.; Prendes, J.M.; Rodríguez, A.; Goyache, F.; Fernández, I.; Facal, N.; Ikeda, S.; Alonso-Montes, C.; et al. Oocytes recovered from cows treated with retinol become unviable as blastocysts produced in vitro. Reproduction 2005, 129, 411–421. [Google Scholar] [CrossRef]
- Evangelista, J.J.F.; Souza, C.E.A.; Moraes, M.E.A.; Moura, A.A.A. Treatment with vitamins A and E improves oocyte quality and in vitro embryo development in Bos indicus cows. Reprod. Fertil. Dev. 2010, 23, 175. [Google Scholar] [CrossRef]
- Gomes da Silva, G.; da Silva Dias, M.S.; Takiya, C.S.; Nunes, A.T.; Del Valle, T.A.; Grigoletto, N.T.S.; Batista, C.F.; Santos, K.R.; Della Libera, A.; Rennó, L.N.; et al. Feeding reduced levels of trace minerals in proteinate form and selenium-yeast to transition cows: Performance, trace minerals, and antioxidant status, peripheral neutrophil activity, and oocyte quality. J. Dairy Sci. 2023, 106, 3023–3042. [Google Scholar] [CrossRef] [PubMed]
- Dantas, F.G.; Reese, S.T.; Filho, R.V.O.; Carvalho, R.S.; Franco, G.A.; Abbott, C.R.; Payton, R.R.; Edwards, J.L.; Russell, J.R.; Smith, J.K.; et al. Effect of complexed trace minerals on cumulus-oocyte complex recovery and in vitro embryo production in beef cattle. J. Anim. Sci. 2019, 97, 1478–1490. [Google Scholar] [CrossRef]
- Yaakub, H.; O’Callaghan, D.; Boland, M.P. Effect of roughage type and concentrate supplementation on follicle numbers and in vitro fertilisation and development of oocytes recovered from beef heifers. Anim. Reprod. Sci. 1999, 55, 1–12. [Google Scholar] [CrossRef]
- Demetrio, D.; Demetrio, C.; Oliveira, M.; Reis, R.; Santos, R. From oocyte to calf: Practical aspects of bovine in vitro embryo production. Clin. Theriogenology 2022, 14, 193. [Google Scholar]
- Matoba, S.; O’Hara, L.; Carter, F.; Kelly, A.K.; Fair, T.; Rizos, D.; Lonergan, P. The association between metabolic parameters and oocyte quality early and late postpartum in Holstein dairy cows. J. Dairy Sci. 2012, 95, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, E.; Gibbons, J.; Rushen, J.; de Passillé, A.M. Development and implementation of a training program to ensure high repeatability of body condition scoring of dairy cows. J. Dairy Sci. 2013, 96, 4725–4737. [Google Scholar] [CrossRef]
- Bell, M.J.; Maak, M.; Sorley, M.; Proud, R. Comparison of methods for monitoring the body condition of dairy cows. Front. Sustain. Food Syst. 2018, 2, 80. [Google Scholar] [CrossRef]
- Pinedo, P.; Manríquez, D.; Azocar, J.; Klug, B.R.; De Vries, A. Dynamics of automatically generated body condition scores during early lactation and pregnancy at first artificial insemination of Holstein cows. J. Dairy Sci. 2022, 105, 4547–4564. [Google Scholar] [CrossRef]
- Consentini, C.E.C.; Souza, A.H.; Sartori, R.; Carvalho, P.D.; Shaver, R.; Wiltbank, M.C. Relationships among total mixed ration nutritional components and reproductive performance in high-producing dairy herds. JDS Commun. 2023, 4, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Blache, D.; Martin, G.B.; Maloney, S.K. Towards ethically improved animal experimentation in the study of animal reproduction. Reprod. Domest. Anim. 2008, 43 (Suppl. 2), 8–14. [Google Scholar] [CrossRef] [PubMed]
| Donor Type 1 (n) | No. of OPUs 2 | OVS 3 | Embryo Rate 4 (Day) 5 | Embryos per OPU 6 | Country | Ref. |
|---|---|---|---|---|---|---|
| Dairy-C (n = 32) | NR | N | 8–14% (D8) | NR | France | [39] |
| Dairy-C/H (NR) | 1285 | N | 23–34% (D7) | 1.06–1.73 | Netherlands | [40] |
| Dairy-C (n = 15) | 20 | N | 8–15% (D7–8) | 1.8–2.28 | Israel | [41] |
| Dairy-C/H (NR) | 2817 | NR | 17–19% (NR) | 1.43–2.49 | Italy | [42] |
| Dairy-C/H (n = 59) | NR | N | 19–20% (D7) | 1.2–3.0 | Brazil | [43] |
| Dairy-C (n = 30) | 60 | Y/N b | 10–52% (D7) | 1.0–4.4 | Brazil | [18] |
| Dairy-C/H (n = 26) | 134 | Y | 33–62% (D7) | 2.9–7.3 | USA | [32] |
| Dairy-H (n = 16) | 32 | Y | 36–50% (D7) c | NR | France | [44] |
| Dairy-H (n = 9) | 54 | N | 10–16% (D7) c | NR | Brazil | [21] |
| Dairy-H (n = 64) | 202 | NO | 17% (D7) | 1.1–1.4 | Germany | [45] |
| Dairy-H (n = 90) | NR | Y/N b | 25–30% (D7) | 2.4–4.7 | Brazil | [46] |
| Dairy-H (n = 9) | 81 | Y/N b | 46–71% (D8) c | 5.6 d | UK | [47] |
| Dairy-C (n = 35) | 35 | Y | 21–38% (D7) | 4.1–5.6 | Brazil | [48] |
| Dairy-C (n = 15) | 240 | N | 26–42% (D8) | NR | Germany | [49] |
| Dairy-H (n = 64) a | 64 | Y | 33–48% (D7) | NR | Brazil | [50] |
| Dairy-H (n = 41) | 41 | Y | 0–38% (D7) | NR | Iran | [51] |
| Dairy-H (n = 20) | 110 | Y | 36–65% (D8) c | NR | UK | [52] |
| Beef-H (n = 34) | NR | N | 27–33% (D7) | 2.2–7.0 | Brazil | [43] |
| Beef-C (n = 6) | 32 | Y | 43–47% (D7) | 3.3–3.8 | USA | [32] |
| Beef-H (n = 9) | 54 | N | 28% (D7) | NR | Brazil | [21] |
| Beef-H (n = 43) | NR | Y/N b | 51–62% (D8) | NR | Brazil | [53] |
| Beef-C (n = 19) | 152 | N | 32–47% (D7) | 1.5–4.3 d | S. Korea | [54] |
| Beef-C (n = 11) | 55 | N | 2.7–50% (D8) | 0.4–6.4 d | Japan | [55] |
| Beef-C (n = 66) | NR | N | 13–41% (D7) | 0.6–18.4 | Brazil | [56] |
| Beef-C (n = 36) | 432 | N | 33–34% (D7) | 5.4 | Brazil | [31] |
| Beef-C (n = 2) | 16 | Y | 22–39% (D8) | NR | Japan | [57] |
| Beef-C (n = 32) | 224 | N | 28–49% (D7) | 1.7–10.3 e | Brazil | [58] |
| Beef-C (n = 20) | 31 | N | 7–33% (D7) c | NR | USA | [59] |
| Beef-C (n = 18) | 32 | N | 12–14% (D7) c | NR | USA | [60] |
| Beef-C (n = 18) | 180 | N | 18–29% (D7) | NR | Pakistan | [61] |
| Beef-C (n = 12) | 104 | N | 12–16% (D7) c | NR | Pakistan | [62] |
| Beef-C (n = 6) | 18 | N | 36–41% (D7–8) | NR | Japan | [63] |
| Diet Treatments | Time of Treatment 1 | COC Quality | Blastocyst (B) Production | Embryo Quality | Ref. | ||
|---|---|---|---|---|---|---|---|
| B/IVF | B/Cleaved | ||||||
| −21 to +107 | ↔ oocyte apoptosis | ↔ | 8.4% 6.9% 2.0% 5.2% | 13.1% 9.2% 3.0% 9.1% | ↔ cell number ↔ apoptosis | [116] |
| +40 to +60 | ↔ morphology grade | ↑ | 19.4% 27.4% | 29.1% 38.0% | ↑ cell number | [117] |
| +46 to +125 | ↔ morphology grade | ↔ | 20.0% 19.0% 19.0% | 29.0% 30.0% 32.0% | ↔ cell number ↔ apoptosis | [118] |
| −24 to +100 | ↑↓ FA composition | ↔ | 8.8% 15.2% 13.4% | NR NR NR | NR | [41] |
| −28 to +111 | ↑ morphology grade | ↔ | 17.6% 20.7% 18.6% | NR NR NR | NR | [119] |
| −90 to 0 | ↔ morphology grade | ↔ | 10–17% | NR | NR | [120] |
| +77 to +141 | ↑↓ oocyte lipidome | ↔ | NR NR | 49.6% 42.3% | ↑ Morphological quality | [121] |
| −63 to +56 | NR | ↔ | NR NR NR NR | 23.5% 17.4% 26.1% 15.2% | ↔ cell number | [122] |
| −20 to +67 | ↔ GC gene expression | ↔ | 34% * 39% * | NR NR | ↔ lipid accumulation | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velazquez, M.A. Nutritional Strategies to Promote Bovine Oocyte Quality for In Vitro Embryo Production: Do They Really Work? Vet. Sci. 2023, 10, 604. https://doi.org/10.3390/vetsci10100604
Velazquez MA. Nutritional Strategies to Promote Bovine Oocyte Quality for In Vitro Embryo Production: Do They Really Work? Veterinary Sciences. 2023; 10(10):604. https://doi.org/10.3390/vetsci10100604
Chicago/Turabian StyleVelazquez, Miguel A. 2023. "Nutritional Strategies to Promote Bovine Oocyte Quality for In Vitro Embryo Production: Do They Really Work?" Veterinary Sciences 10, no. 10: 604. https://doi.org/10.3390/vetsci10100604
APA StyleVelazquez, M. A. (2023). Nutritional Strategies to Promote Bovine Oocyte Quality for In Vitro Embryo Production: Do They Really Work? Veterinary Sciences, 10(10), 604. https://doi.org/10.3390/vetsci10100604






