Molecular Detection of Rickettsia hoogstraalii in Hyalomma anatolicum and Haemaphysalis sulcata: Updated Knowledge on the Epidemiology of Tick-Borne Rickettsia hoogstraalii
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
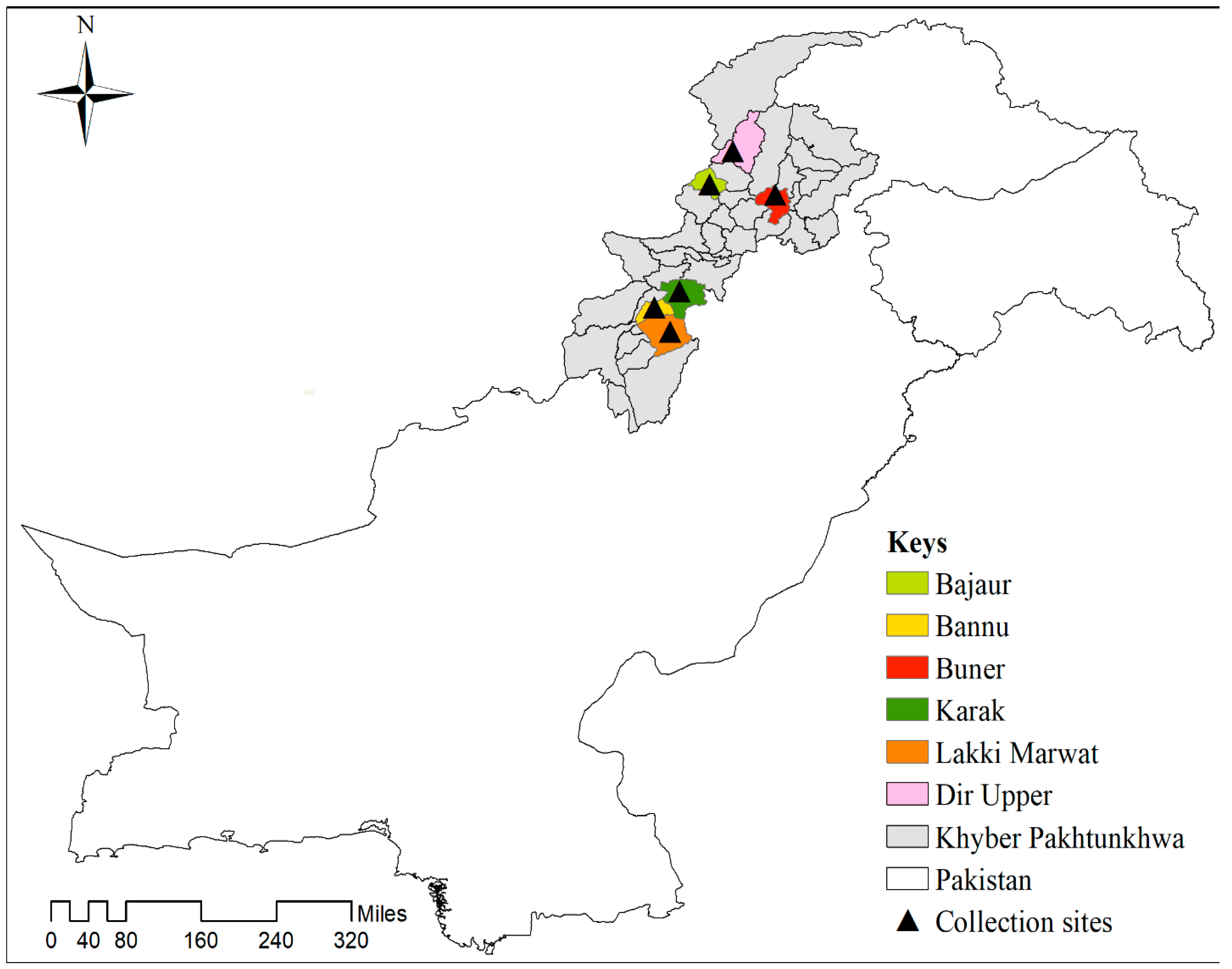
2.1. Study Area
2.2. Ticks Collection and Identification
2.3. DNA Extraction and PCR
2.4. Sequencing and Phylogenetic Analysis
2.5. Literature Search
3. Results
3.1. Ticks and Host Description
3.2. Detection of Rickettsial DNA in Ticks
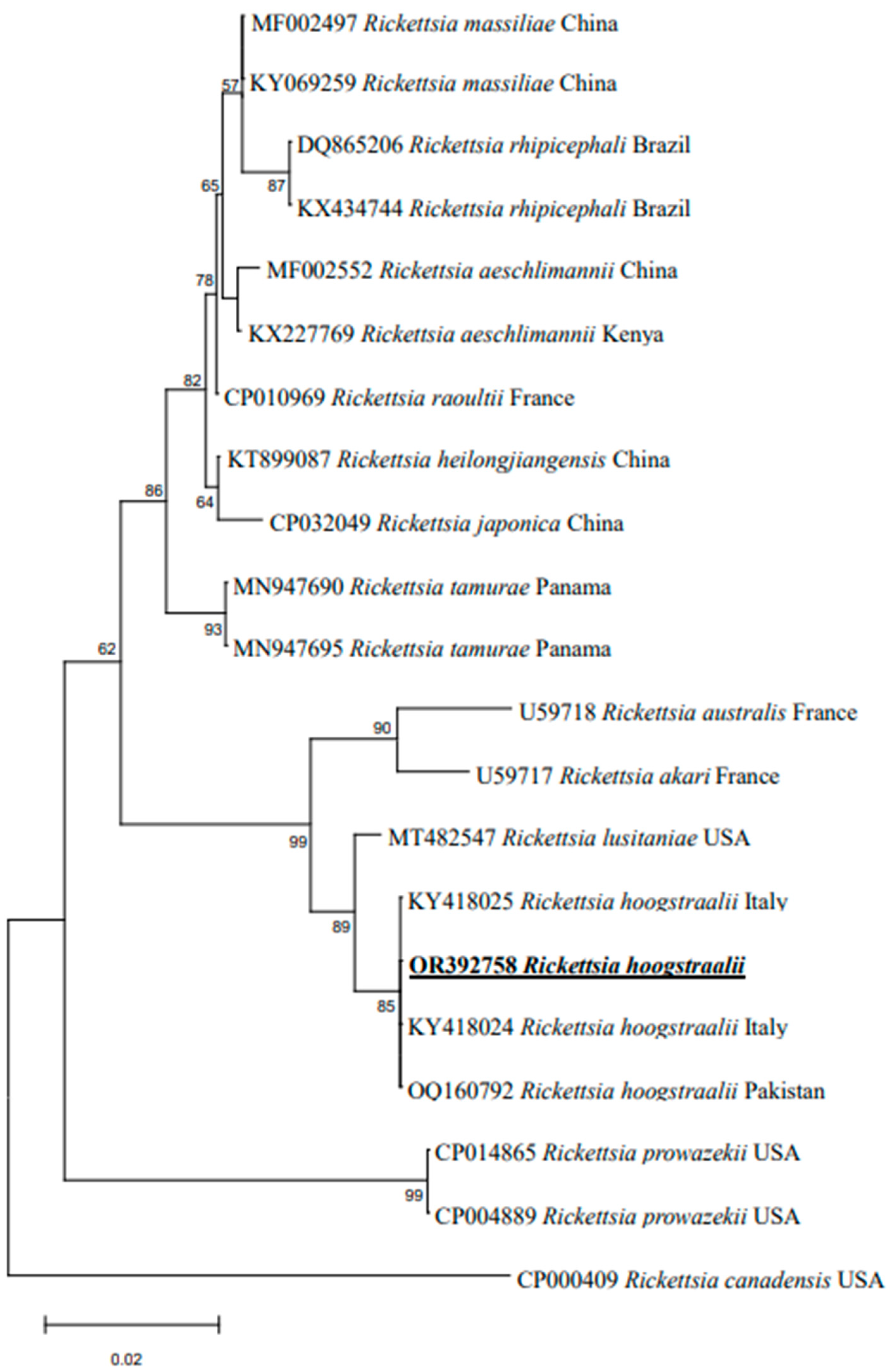
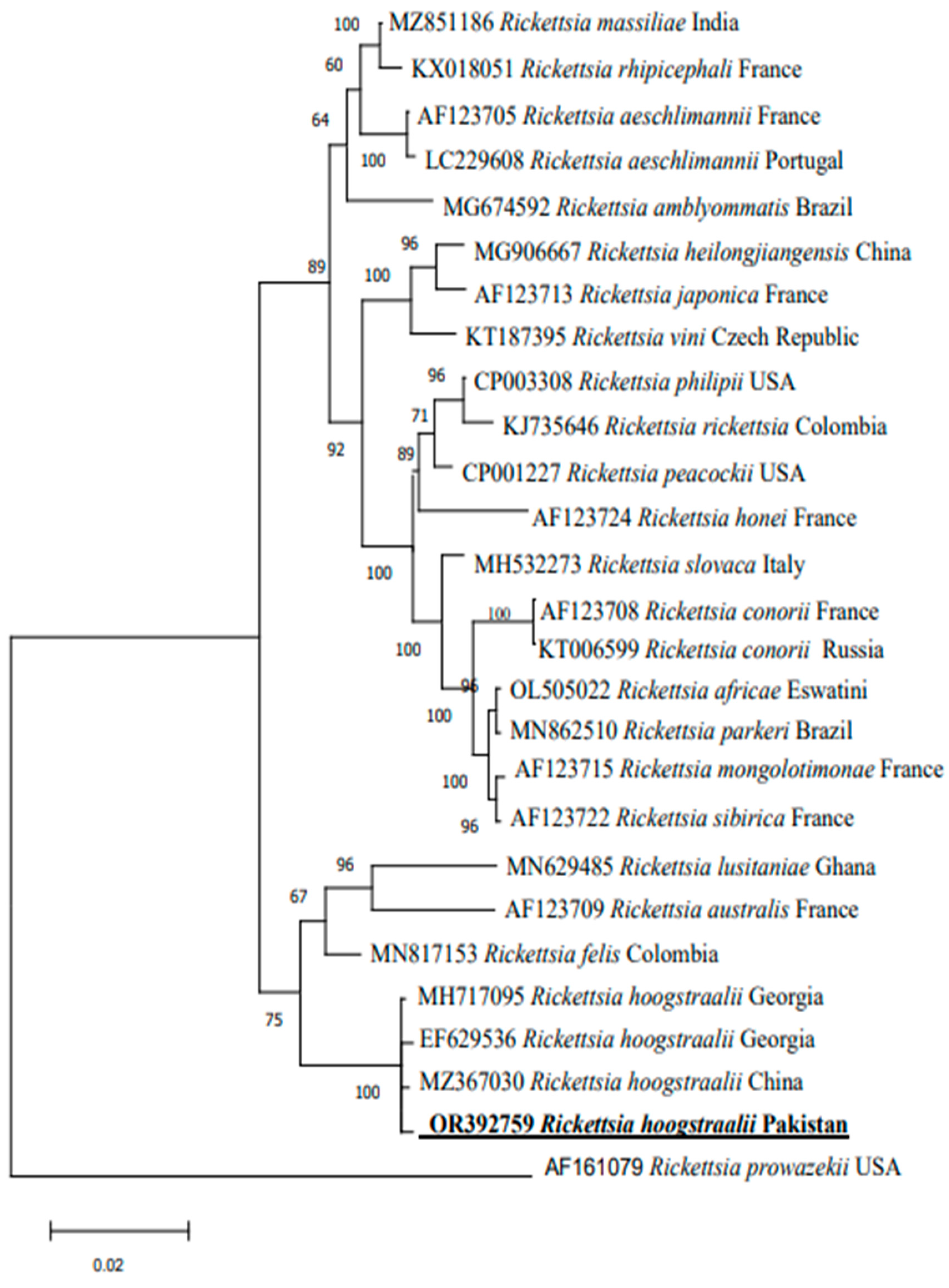
3.3. Sequences and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahid, H.; Zeb, I.; Tufail, M.; Khan, S.; Haroon, M.; Bilal, M.; Hussain, M.; Alouffi, A.S.; Muñoz-Leal, S.; et al. Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Parasit. Vectors 2021, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Nava, S.; Robbins, R.G. Geographic distribution of the hard ticks (Acari: Ixodida: Ixodidae) of the world by countries and territories. Zootaxa 2023, 52, 274–511. [Google Scholar] [CrossRef]
- Shih, C.M.; Yang, P.W.; Chao, L.L. Molecular Detection and genetic identification of Rickettsia infection in Ixodes granulatus ticks, an incriminated vector for geographical transmission in Taiwan. Microorganisms 2021, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Medecine et maladies infectieuses. Ticks. Tick. Borne. Dis. 2019, 49, 87–97. [Google Scholar]
- Yan, P.; Qiu, Z.; Zhang, T.; Li, Y.; Wang, W.; Li, M.; Yu, Z.; Liu, J. Microbial diversity in the tick Argas japonicus (Acari: Argasidae) with a focus on Rickettsia pathogens. Med. Vet. Entomol. 2019, 33, 327–335. [Google Scholar] [CrossRef]
- Ali, A.; Numan, M.; Khan, M.; Aiman, O.; Muñoz-Leal, S.; Chitimia-Dobler, L.; Labruna, M.B.; Nijhof, A.M. Ornithodoros (Pavlovskyella) ticks associated with a Rickettsia sp. in Pakistan. Parasit. Vectors 2022, 15, 138. [Google Scholar] [CrossRef]
- Jay, R.; Armstrong, P.A. Clinical characteristics of Rocky Mountain spotted fever in the United States: A literature review. Vector Borne Zoonotic Dis. 2020, 57, 114. [Google Scholar] [CrossRef]
- Moonga, L.C.; Hayashida, K.; Mulunda, N.R.; Nakamura, Y.; Chipeta, J.; Moonga, H.B.; Namangala, B.; Sugimoto, C.; Mtonga, Z.; Mutengo, M.; et al. Molecular detection and characterization of Rickettsia asembonensis in human blood, Zambia. Emerg. Infect. Dis. 2021, 27, 2237. [Google Scholar] [CrossRef]
- Raoult, D.; Fournier, P.E.; Abboud, P.; Caron, F. First documented human Rickettsia aeschlimannii infection. Emerg. Infect. Dis. 2002, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Germanakis, A.; Chochlakis, D.; Angelakis, E.; Tselentis, Y.; Psaroulaki, A. Rickettsia aeschlimannii infection in a man, Greece. Emerg. Infect. Dis. 2013, 19, 1176. [Google Scholar] [CrossRef] [PubMed]
- Dieme, C.; Bechah, Y.; Socolovschi, C.; Audoly, G.; Berenger, J.M.; Faye, O.; Raoult, D.; Parola, P. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 8088–8093. [Google Scholar] [CrossRef] [PubMed]
- Moonga, L.C.; Hayashida, K.; Nakao, R.; Lisulo, M.; Kaneko, C.; Nakamura, I.; Eshita, Y.; Mweene, A.S.; Namangala, B.; Sugimoto, C.; et al. Molecular detection of Rickettsia felis in dogs, rodents and cat fleas in Zambia. Parasit. Vectors 2019, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Khan, M.; Alouffi, A.; Almutairi, M.M.; Numan, M.; Ullah, S.; Obaid, M.K.; Islam, Z.U.; Ahmed, H.; Tanaka, T.; et al. Phylogenetic Position of Haemaphysalis kashmirensis and Haemaphysalis cornupunctata, with Notes on Rickettsia spp. Genes 2023, 14, 360. [Google Scholar] [CrossRef]
- Abreu-Yanes, E.; Abreu-Acosta, N.; Foronda, P. Study of tick-borne zoonotic pathogens in questing and feeding ticks in Tenerife, Canary Islands, Spain. J. Vector Ecol. 2023, 48, 59–62. [Google Scholar] [CrossRef]
- Duh, D.; Punda-Polić, V.O.L.G.A.; Trilar, T.; Petrovec, M.; Bradarić, N.; Avšič-Županc, T.A. Molecular identification of Rickettsia felis like bacteria in Haemaphysalis sulcata ticks collected from domestic animals in southern Croatia. Ann. N. Y. Acad.Sci. 2006, 1078, 347–351. [Google Scholar] [CrossRef]
- Mattila, J.T.; Burkhardt, N.Y.; Hutcheson, H.J.; Munderloh, U.G.; Kurtti, T.J. Isolation of cell lines and a rickettsial endosymbiont from the soft tick Carios capensis (Acari: Argasidae: Ornithodorinae). J. Med. Entomol. 2007, 44, 1091–1101. [Google Scholar] [CrossRef]
- Márquez, F.J. Spotted fever group Rickettsia in ticks from southeastern Spain natural parks. Exp. Appl. Acarol. 2008, 45, 185–194. [Google Scholar] [CrossRef]
- Portillo, A.; Santibáñez, P.; Santibáñez, S.; Pérez-Martínez, L.; Oteo, J.A. Detection of Rickettsia spp. in Haemaphysalis ticks collected in La Rioja, Spain. Vector Borne Zoonotic Dis. 2008, 8, 653–658. [Google Scholar] [CrossRef]
- Dietrich, M.; Lebarbenchon, C.; Jaeger, A.; Le Rouzic, C.; Bastien, M.; Lagadec, E.; McCoy, K.D.; Pascalis, H.; Le Corre, M.; Dellagi, K.; et al. Rickettsia spp. in seabird ticks from western Indian Ocean islands, 2011–2012. Emerg. Infect. Dis. 2014, 20, 838. [Google Scholar] [CrossRef] [PubMed]
- Duh, D.; Punda-Polic, V.; Avsic-Zupanc, T.; Bouyer, D.; Walker, D.H.; Popov, V.L.; Jelovsek, M.; Gracner, M.; Trilar, T.; Bradaric, N.; et al. Rickettsia hoogstraalii sp. nov., isolated from hard-and soft-bodied ticks. Int. J. Syst. Evol. Microbiol. 2010, 60, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Ioannou, I.; Sandalakis, V.; Dimitriou, T.; Kassinis, N.; Papadopoulos, B.; Tselentis, Y.; Psaroulaki, A. Spotted fever group Rickettsiae in ticks in Cyprus. Microb. Ecol. 2012, 63, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Pader, V.; Buniak, J.N.; Abdissa, A.; Adamu, H.; Tolosa, T.; Gashaw, A.; Cutler, R.R.; Cutler, S.J. Candidatus Rickettsia hoogstraalii in Ethiopian Argas persicus ticks. Ticks. Tick. Borne. Dis. 2012, 3, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Orkun, Ö.; Karaer, Z.; Çakmak, A.; Nalbantoğlu, S. Spotted fever group rickettsiae in ticks in Turkey. Ticks. Tick. Borne. Dis. 2014, 5, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.; Bursali, A.; Keskin, A.; Tekin, S. Molecular detection of spotted fever group rickettsiae in ticks removed from humans in Turkey. Ticks. Tick. Borne. Dis. 2016, 7, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Leulmi, H.; Masala, G.; Piredda, M.; Foxi, C.; Parola, P. Detection of Rickettsia hoogstraalii, Rickettsia helvetica, Rickettsia massiliae, Rickettsia slovaca and Rickettsia aeschlimannii in ticks from Sardinia, Italy. Ticks. Tick. Borne. Dis. 2017, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; Ceballos, L.A.; Ragagli, C.; Martello, E.; De Sousa, R.; Stella, M.C.; Mannelli, A. Importance of common wall lizards in the transmission dynamics of tick-borne pathogens in the northern Apennine Mountains, Italy. Microb. Ecol. 2017, 74, 961–968. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Angelou, A.; Giannelli, A.; Annoscia, G.; Ravagnan, S.; Dantas-Torres, F.; Capelli, G.; Halos, L.; Beugnet, F.; Papadopoulos, E.; et al. Ticks and associated pathogens in dogs from Greece. Parasit. Vectors 2017, 10, 301. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Chaligiannis, Ι.; Cabezas-Cruz, A.; Papa, A.; Sotiraki, S.; de la Fuente, J.; G Fernández de Mera, I. Molecular identification of spotted fever group Rickettsia in ticks collected from dogs and small ruminants in Greece. Exp. Appl. Acarol. 2019, 78, 421–430. [Google Scholar] [CrossRef]
- Kooshki, H.; Goudarzi, G.; Faghihi, F.; Telmadarraiy, Z.; Edalat, H.; Hosseini-Chegeni, A. The first record of Rickettsia hoogstraalii (Rickettsiales: Rickettsiaceae) from Argas persicus (Acari: Argasidae) in Iran. Syst. Appl. Acarol. 2020, 25, 1611–1617. [Google Scholar]
- Latas, P.; Auckland, L.D.; Teel, P.D.; Hamer, S.A. Argas (Persicargas) giganteus soft tick infection with Rickettsia hoogstraali and relapsing fever Borrelia on wild avian species of the desert southwest, USA. J. Wildl. Dis. 2020, 56, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Orkun, Ö.; Emir, H. Identification of tick-borne pathogens in ticks collected from wild animals in Turkey. Parasitol. Res. 2020, 119, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Sukhiashvili, R.; Zhgenti, E.; Khmaladze, E.; Burjanadze, I.; Imnadze, P.; Jiang, J.; John, H.S.; Farris, C.M.; Gallagher, T.; Obiso, R.J.; et al. Identification and distribution of nine tick-borne spotted fever group rickettsiae in the country of Georgia. Ticks. Tick. Borne. Dis. 2020, 11, 101470. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.K.; Mans, B.J.; Durden, L.A.; Miller, M.M.; Gratton, E.M.; Laverty, T.M. Rickettsia hoogstraalii and a Rickettsiella from the Bat Tick Argas transgariepinus, in Namibia. J. Parasitol. 2020, 106, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Simuunza, M.; Kajihara, M.; Chambaro, H.; Harima, H.; Eto, Y.; Simulundu, E.; Squarre, D.; Torii, S.; Takada, A.; et al. Screening of tick-borne pathogens in argasid ticks in Zambia: Expansion of the geographic distribution of Rickettsia lusitaniae and Rickettsia hoogstraalii and detection of putative novel Anaplasma species. Ticks Tick-Borne Dis. 2021, 12, 101720. [Google Scholar] [CrossRef]
- Pascucci, I.; Antognini, E.; Canonico, C.; Montalbano, M.G.; Necci, A.; di Donato, A.; Moriconi, M.; Morandi, B.; Morganti, G.; Crotti, S.; et al. One Health Approach to Rickettsiosis: A Five-Year Study on Spotted Fever Group Rickettsiae in Ticks Collected from Humans. Microorganisms 2021, 10, 35. [Google Scholar] [CrossRef]
- Ivan, T.; Matei, I.A.; Novac, C.Ș.; Kalmár, Z.; Borșan, S.D.; Panait, L.C.; Gherman, C.M.; Ionică, A.M.; Papuc, I.; Mihalca, A.D. Spotted Fever Group Rickettsia spp. diversity in ticks and the first report of Rickettsia hoogstraalii in Romania. Vet. J. 2022, 9, 343. [Google Scholar] [CrossRef]
- Pan, Y.S.; Cui, X.M.; Du, L.F.; Xia, L.Y.; Du, C.H.; Bell-Sakyi, L.; Zhang, M.Z.; Zhu, D.Y.; Dong, Y.; Wei, W.; et al. Coinfection of Two Rickettsia Species in a Single Tick Species Provides New Insight into Rickettsia-Rickettsia and Rickettsia-Vector Interactions. Microbiol. Spectr. 2022, 10, e0232322. [Google Scholar] [CrossRef]
- Duan, D.Y.; Liu, Y.K.; Liu, L.; Liu, G.H.; Cheng, T.Y. Microbiome analysis of the midguts of different developmental stages of Argas persicus in China. Ticks. Tick. Borne. Dis 2022, 10, 1868. [Google Scholar] [CrossRef]
- Hornok, S.; Kontschán, J.; Takács, N.; Chaber, A.L.; Halajian, A.; Szekeres, S.; Sándor, A.D.; Plantard, O. Rickettsiaceae in two reptile-associated tick species, Amblyomma exornatum and Africaniella transversale: First evidence of Occidentia massiliensis in hard ticks (Acari: Ixodidae). Ticks. Tick. Borne. Dis. 2022, 13, 101830. [Google Scholar] [CrossRef] [PubMed]
- Orkun, Ö. Comprehensive screening of tick-borne microorganisms indicates that a great variety of pathogens are circulating between hard ticks (Ixodoidea: Ixodidae) and domestic ruminants in natural foci of Anatolia. Ticks. Tick. Borne. Dis. 2022, 13, 102027. [Google Scholar] [CrossRef] [PubMed]
- Pakistan Economic Survey 2022–2023. Available online: https://www.finance.gov.pk/survey (accessed on 1 March 2023).
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A.; et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, 0005681. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Alouffi, A.; Almutairi, M.M.; Islam, N.; Rehman, G.; Ul Islam, Z.; Ahmed, H.; Júnior, I.D.S.V.; Labruna, M.B.; Tanaka, T.; et al. First Report of Rickettsia conorii in Hyalomma kumari Ticks. Animals 2023, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Selmi, R.; Ben Said, M.; Ben Yahia, H.; Abdelaali, H.; Messadi, L. Molecular epidemiology and phylogeny of spotted fever group Rickettsia in camels (Camelus dromedarius) and their infesting ticks from Tunisia. Transbound. Emerg. Dis. 2020, 67, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Muñoz-Leal, S.; Khan, M.Q.; Alouffi, A.S.; Labruna, M.B.; Ali, A. Life cycle and genetic identification of Argas persicus infesting domestic fowl in Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2021, 8, 664731. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, M.; Alouffi, A.; Almutairi, M.M.; Ullah, S.; Numan, M.; Islam, N.; Khan, Z.; Aiman, O.; Zaman Safi, S. Spatio-temporal patterns of ticks and molecular survey of Anaplasma marginale, with notes on their phylogeny. Microorganisms 2022, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Islam, N.; Khan, A.; Islam, Z.U.; Muñoz-Leal, S.; Labruna, M.B.; Ali, A. New records of Amblyomma gervaisi from Pakistan, with detection of a reptile-associated Borrelia sp. Ticks. Tick. Borne. Dis. 2022, 13, 102047. [Google Scholar] [CrossRef]
- Khan, Z.; Shehla, S.; Alouffi, A.; Kashif Obaid, M.; Zeb Khan, A.; Almutairi, M.M.; Numan, M.; Aiman, O.; Alam, S.; Ullah, S.; et al. Molecular survey and genetic characterization of Anaplasma marginale in ticks collected from livestock hosts in Pakistan. Animals 2022, 12, 1708. [Google Scholar] [CrossRef]
- Numan, M.; Islam, N.; Adnan, M.; Zaman Safi, S.; Chitimia-Dobler, L.; Labruna, M.B.; Ali, A. First genetic report of Ixodes kashmiricus and associated Rickettsia sp. Parasit. Vectors 2022, 15, 378. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.A.; Zahid, H.; Yaseen, P.M.; Qayash Khan, M.; Nawab, J.; Ur Rehman, Z.; Ateeq, M.; Khan, S.; Ibrahim, M. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shehla, S.; Zahid, H.; Ullah, F.; Zeb, I.; Ahmed, H.; da Silva Vaz, I., Jr.; Tanaka, T. Molecular survey and spatial distribution of Rickettsia spp. in ticks infesting free-ranging wild animals in Pakistan (2017–2021). Pathogens 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Numan, M.; Ullah, S.; Khan, M.; Kamran, K. Genetic characterization of Haemaphysalis (Rhipistoma) indica and Haemaphysalis (Segalia) montgomeryi ticks (Ixodoidea: Ixodidae). Ticks. Tick. Borne. Dis. 2023, 14, 102105. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, G.; Dhanda, V. The Indian Hyalomma Ticks (Ixodoidea: Ixodidae). The Indian Hyalomma Ticks (Ixodoidea: Ixodidae); I.C.A.R.: New Delhi, India, 1987. [Google Scholar]
- Walker, A. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World; Jane B. Walker, James E. Keirans and Ivan G. Horak. Trop. Anim. Health Prod. 2000, 32, 417. [Google Scholar] [CrossRef]
- Sambrook, J. A Laboratory Manual. Molecular Cloning; Cold Spring Harbor Laboratory: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. Clin. Microbiol. Infect. 2004, 42, 90–98. [Google Scholar]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. Clin. Microbiol. Infect. 1996, 34, 2058–2065. [Google Scholar] [CrossRef]
- Roux, V.; Raoult, D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J.Syst. Evol. Microbiol. 2000, 50, 1449–1455. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C.J.G.B.B. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and tick-borne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F.H.; Devillers, E.; Cosson, J.F.; Gasqui, P.; Van, V.T.; Mavingui, P.; et al. Co-infection of ticks: The rule rather than the exception. PLoS. Negl. Trop. Dis. 2016, 10, 0004539. [Google Scholar] [CrossRef]
- Kamran, K.; Ali, A.; Villagra, C.A.; Bazai, Z.A.; Iqbal, A.; Sajid, M.S. Hyalomma anatolicum resistance against ivermectin and fipronil is associated with indiscriminate use of acaricides in southwestern Balochistan, Pakistan. Parasitol. Res. 2021, 120, 15–25. [Google Scholar]
- Shehla, S.; Ullah, F.; Alouffi, A.; Almutairi, M.M.; Khan, Z.; Tanaka, T.; Labruna, M.B.; Tsai, K.-H.; Ali, A. Association of SFG Rickettsia massiliae and Candidatus Rickettsia shennongii with Different Hard Ticks Infesting Livestock Hosts. Pathogens 2023, 12, 1080. [Google Scholar] [CrossRef]
- Fournier, P.E.; Roux, V.; Raoult, D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Evol. Microbiol. 1998, 48, 839–849. [Google Scholar]
- Thu, M.J.; Qiu, Y.; Matsuno, K.; Kajihara, M.; Mori-Kajihara, A.; Omori, R.; Monma, N.; Chiba, K.; Seto, J.; Gokuden, M.; et al. Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci. Rep. 2019, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Primers Sequence 5′-3′ | Amplicons | Reference |
|---|---|---|---|---|
| Rickettsia gltA | CS-78 | GCAAGTATCGGTGAGGATGTAAT | 401 bp | [58] |
| CS-323 | GCTTCCTTAAAATTCAATAAATCAGGAT | |||
| Rickettsia ompA | Rrl9O.70 | ATGGCGAATATTTCTCCAAAA | 631 bp | [59] |
| Rr190.701n | GTTCCGTTAATGGCAGCATCT | |||
| Rickettsia ompB | 120-M59 | CCGCAGGGTTGGTAACTGC | 862 bp | [60] |
| 120–807 | CCTTTTAGATTACCGCCTAA |
| Country/Year | Tick Specie/Source | Detected in Host | Serologically/Molecularly (PCR) | Reference |
|---|---|---|---|---|
| Croatia 2006 | Ha. sulcata | Sheep | PCR | [17] |
| Goats | ||||
| Georgia, USA 2007 “Candidatus R. hoogstraalii” | Carios capensis | Brown pelicans | PCR | [18] |
| Southeastern Spain 2008 | Ha. punctata | Vegetation | PCR | [19] |
| Ha. sulcata | ||||
| La Rioja, Spain 2008 | Ha. punctata | Sheep | PCR | [20] |
| Ha. sulcata | Cow | |||
| Western India 2011–2012 | C. capensis | Seabird | PCR | [21] |
| Croatia 2010 | Ha. sulcata | Sheep | PCR/TEM | [22] |
| Cyprus 2011 | Ha. punctata | Mouflons (Wild sheep) | PCR | [23] |
| Ethiopia 2012 | Ar. persicus | Cracks and crevices of livestock areas | PCR | [24] |
| Turkey 2014 | Ha. parva | Humans | PCR | [25] |
| Turkey 2016 | Ha. parva | Humans | PCR | [26] |
| Italy 2016 | Ha. punctata | Mouflons | PCR | [27] |
| Ha. sulcata | ||||
| Italy 2017 | I. ricinus | Lizards | PCR | [28] |
| Ha. sulcata | ||||
| Greece 2017 | Ha. parva | Dog | PCR | [29] |
| Greece 2019 | Ha. sulcata | Goats | PCR | [30] |
| Ha. parva, and Ha. sulcata | Sheep | |||
| Iran 2020 | Ar. persicus | Aviary | PCR | [31] |
| DESERT SOUTHWEST, USA 2020 | Ar. persicus | Birds | PCR | [32] |
| Turkey 2020 | Ha. parva | Wild animals include wolves, fox, hare, and lynx, | PCR | [33] |
| Georgia 2020 | D. marginatus | Domestic animals | PCR | [34] |
| Ha. sulcata | ||||
| Namibia 2020 | Argus transgariepinus | Bat | PCR | [35] |
| Zambia 2021 | Ar. walkerae | Chicken coop | PCR | [36] |
| Italy 2021 | I. ricinus | Domestic animals | PCR | [37] |
| Romania 2022 | Rh. rossicus | Dog | PCR | [38] |
| China 2022 | Ha. montgomeryi | Goats | PCR | [39] |
| China 2022 | Ar. persicus | From Cracks in hen house | PCR | [40] |
| Africa 2022 | Africaniella transversale | Python regius | PCR | [41] |
| Anatolia 2022 | Ha. parva | Cattle, Sheep, and Goats | PCR | [42] |
| Ha. sulcata | ||||
| Pakistan 2023 | Ha. montgomeryi | Goats | PCR | [15] |
| Sheep | ||||
| Spain 2023 | Ha. formosensis | Vegetation | PCR | [16] |
| District | Examined Host | Ticks Species | Infested/Examined (%) | Number of Ticks (%) (F, M, N) | Ticks Subjected to PCR (F, M, N) | Rickettsia hoogstraalii Detected via Both gltA, and ompB |
|---|---|---|---|---|---|---|
| Buner | Goats | Rh. turanicus | 8/12 (66.6) | 25 (17.1), (7,5,13) | 2, 1, 4 | - |
| Ha. sulcata | 18 (11.3), (8,3,7) | 2, 2, 3 | 1 N | |||
| Sheep | Ha. bispinosa | 6/10 (60) | 15 (10.2), (7,3,5) | 4, 0, 3 | - | |
| Ha. sulcata | 12 (7.6), (4,3,5) | 2, 1, 4 | 1 N | |||
| Ha. montgomeryi | 10 (6.8), (3,2,5) | 3, 1, 3 | - | |||
| Cattle | Rh. microplus | 10/14 (71.4) | 66 (41.7), (21,14,31) | 4, 0, 3 | - | |
| Total | 24/36 (66.6) | 146 (17.7), (50, 30, 66) | 17, 5, 20 | 2 N | ||
| Bajaur | Goats | Rh. turanicus | 6/10 (60) | 18 (11.3), (5,4,9) | 3, 0, 4 | - |
| Ha. sulcata | 13 (8.2), (3,2,8) | 3, 1, 3 | 1 N | |||
| Ha. montgomeryi | 9 (5.6), (3,2,4) | 3, 1, 3 | - | |||
| Sheep | Ha. bispinosa | 5/8 (62.5) | 10 (6.5), (4,2,4) | 2, 1, 4 | - | |
| Ha. sulcata | 15 (9.3), (5,2,8) | 4, 0, 3 | 2F | |||
| Cattle | Rh. microplus | 13/15(86.6) | 50 (31.6), (19,10,21) | 2, 0, 5 | - | |
| Hy. anatolicum | 43 (26.0), (16,9,18) | 2, 1, 4 | - | |||
| Total | 25/33 (75.7) | 158 (19.9), (55, 31, 72) | 19, 4, 26 | 2 F, 1 N | ||
| Lakki Marwat | Sheep | Ha. sulcata | 6/12 (50) | 18 (13.5), (5,4,9) | 3, 1, 3 | - |
| Ha. montgomeryi | 13(9.7), (5,2,6) | 2, 1, 4 | - | |||
| Cattle | Rh. microplus | 12/14 (85.7) | 60 (45.1), (20,17,23) | 2, 1, 4 | - | |
| Hy. anatolicum | 42 (13.5), (15,10,17) | 3, 0, 4 | 1 N | |||
| Total | 18/26 (75) | 133 (16.1), (45, 33, 55) | 10, 3, 5 | 1 N | ||
| Bannu | Goats | Rh. turanicus | 5/8 (62.5) | 19 (15.0), (7,4,8) | 3, 1, 3 | - |
| Ha. montgomeryi | 10 (7.9), (4,2,4) | 3, 0, 4 | - | |||
| Cattle | Rh. microplus | 12/15 (80) | 47 (44.3), (19,8,20) | 2, 0, 5 | - | |
| Hy. anatolicum | 50 (39.6), (18,11,21) | 1, 1, 5 | 1F, 1 N | |||
| Total | 17/23 (74) | 126 (15.3), (48, 25, 53) | 9, 2, 17 | 1 F, 1 N | ||
| Dir-Upper | Goats | Rh. turanicus | 6/8 (75) | 30 (25.8), (11,4,15) | 2, 2, 3 | - |
| Ha. sulcata | 18 (15.5), (6,2,10) | 4, 1, 2 | 1 N | |||
| Sheep | Ha. sulcata | 6/10 (60) | 10 (8.6), (4,2,4) | 3, 1, 3 | ||
| Ha. montgomeryi | 12 (10.3), (4,3,5) | 2, 2, 3 | - | |||
| Cattle | Hy. anatolicum | 8/10 (80) | 46 (27.7), (14,14,18) | 3, 0, 4 | - | |
| Total | 20/28 (71.4) | 116 (14.1), (39, 25, 52) | 14, 6, 15 | 1 N | ||
| Karak | Goats | Rh. turanicus | 14/16 (87.5) | 30 (20.8), (12,5,13) | 2, 1, 4 | - |
| Ha. montogomeryi | 12 (8.3), (4,2,6) | 3, 1, 3 | - | |||
| Cattle | Rh. microplus | 16/21 (76.1) | 60 (41.6), (22,13,25) | 3, 0, 4 | - | |
| Hy. anatolicun | 42 (23.2), (16,9,17) | 2, 2, 3 | - | |||
| Total | 30/37 (81.0) | 144 (17.5), (54, 29, 61) | 10, 4, 14 | - | ||
| Overall Total | 134/183 (73.2) | 823 (297, 173, 353) | 210 (79, 24, 107) | 9 (3 F, 6 N) (4.3%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aneela, A.; Almutairi, M.M.; Alouffi, A.; Ahmed, H.; Tanaka, T.; da Silva Vaz, I., Junior; Chang, S.-C.; Chen, C.-C.; Ali, A. Molecular Detection of Rickettsia hoogstraalii in Hyalomma anatolicum and Haemaphysalis sulcata: Updated Knowledge on the Epidemiology of Tick-Borne Rickettsia hoogstraalii. Vet. Sci. 2023, 10, 605. https://doi.org/10.3390/vetsci10100605
Aneela A, Almutairi MM, Alouffi A, Ahmed H, Tanaka T, da Silva Vaz I Junior, Chang S-C, Chen C-C, Ali A. Molecular Detection of Rickettsia hoogstraalii in Hyalomma anatolicum and Haemaphysalis sulcata: Updated Knowledge on the Epidemiology of Tick-Borne Rickettsia hoogstraalii. Veterinary Sciences. 2023; 10(10):605. https://doi.org/10.3390/vetsci10100605
Chicago/Turabian StyleAneela, Aneela, Mashal M. Almutairi, Abdulaziz Alouffi, Haroon Ahmed, Tetsuya Tanaka, Itabajara da Silva Vaz, Junior, Shun-Chung Chang, Chien-Chin Chen, and Abid Ali. 2023. "Molecular Detection of Rickettsia hoogstraalii in Hyalomma anatolicum and Haemaphysalis sulcata: Updated Knowledge on the Epidemiology of Tick-Borne Rickettsia hoogstraalii" Veterinary Sciences 10, no. 10: 605. https://doi.org/10.3390/vetsci10100605
APA StyleAneela, A., Almutairi, M. M., Alouffi, A., Ahmed, H., Tanaka, T., da Silva Vaz, I., Junior, Chang, S.-C., Chen, C.-C., & Ali, A. (2023). Molecular Detection of Rickettsia hoogstraalii in Hyalomma anatolicum and Haemaphysalis sulcata: Updated Knowledge on the Epidemiology of Tick-Borne Rickettsia hoogstraalii. Veterinary Sciences, 10(10), 605. https://doi.org/10.3390/vetsci10100605










