Malformation of the Cortical Development Associated with Severe Clusters of Epileptic Seizures
Simple Summary
Abstract
1. Introduction
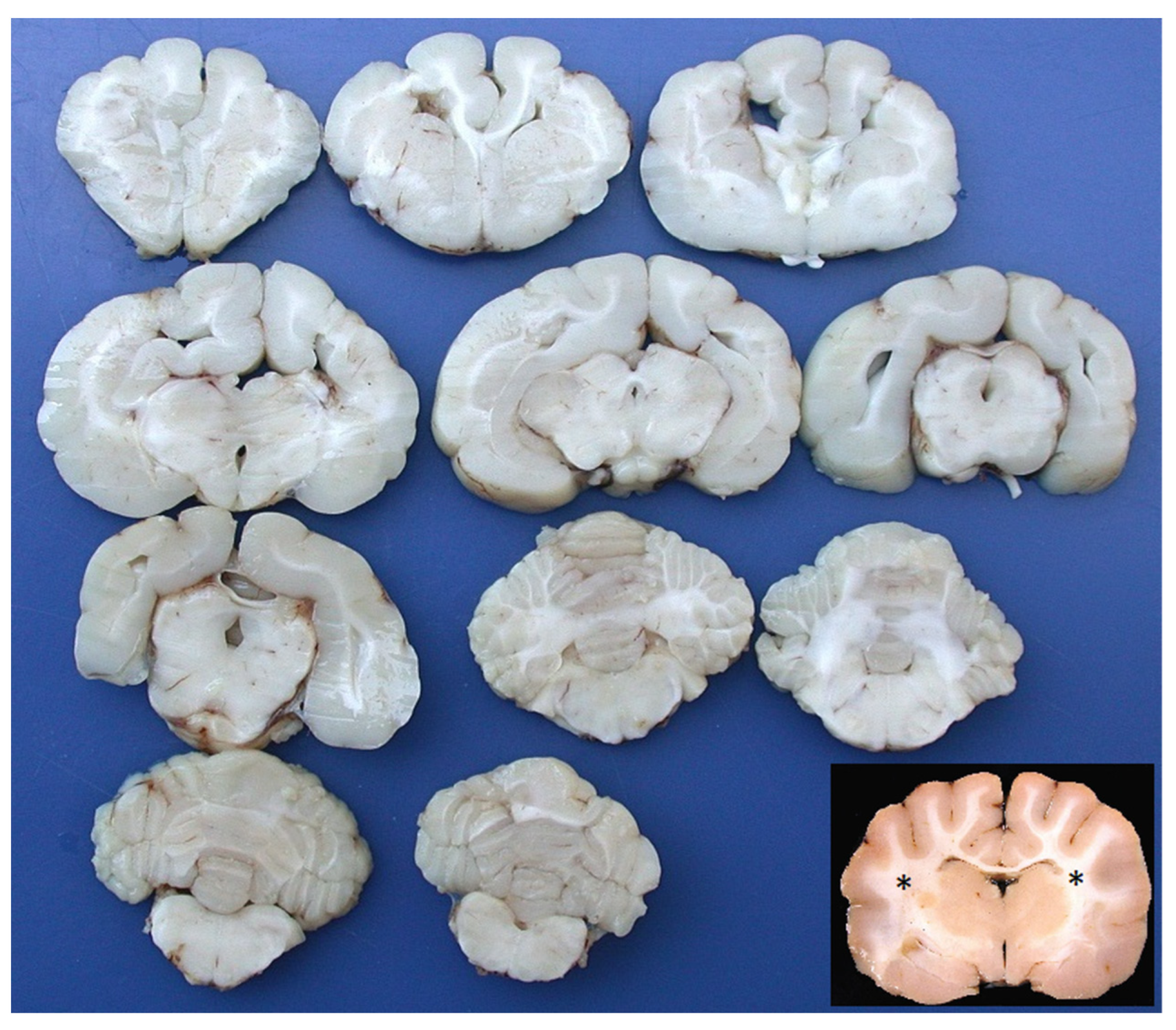
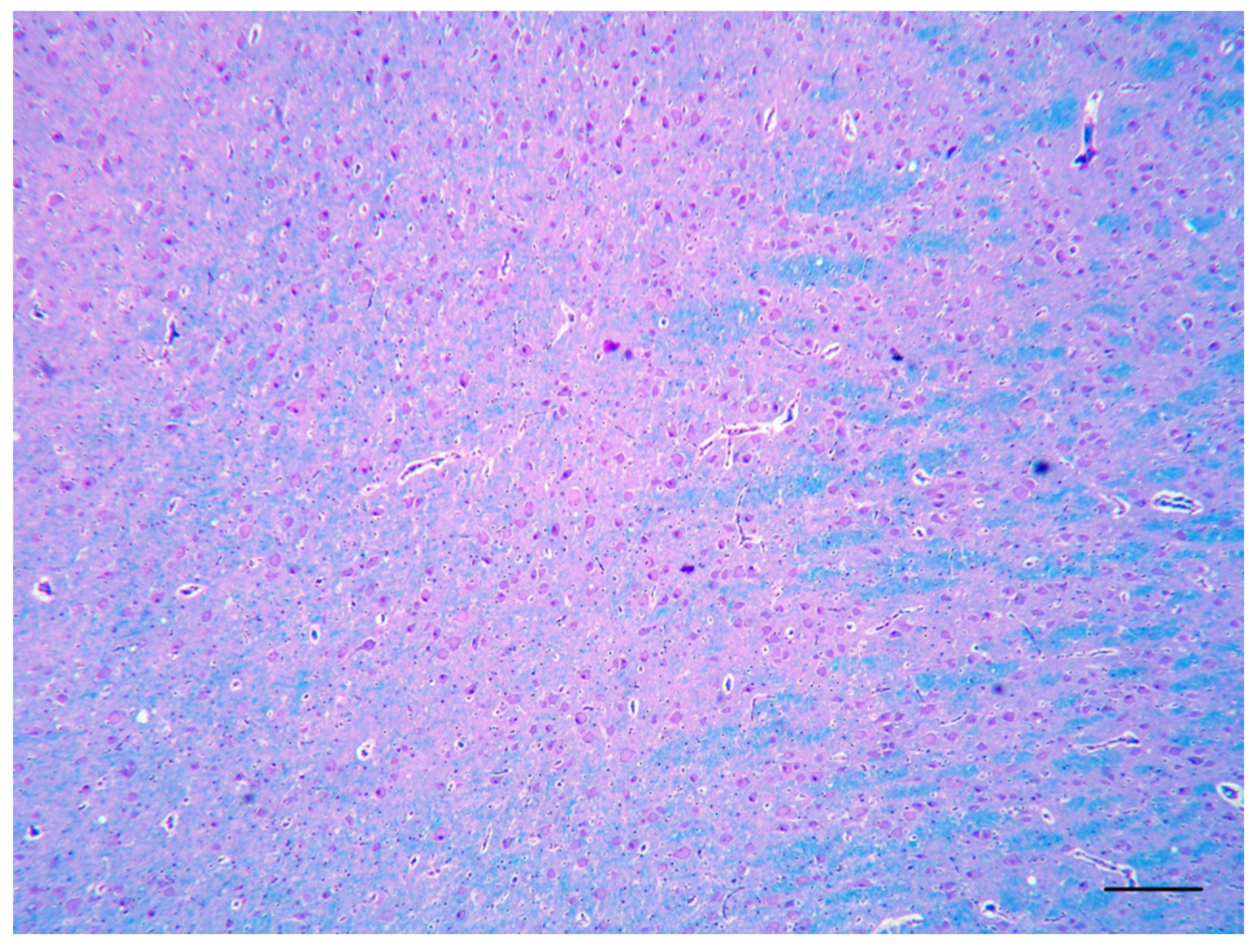
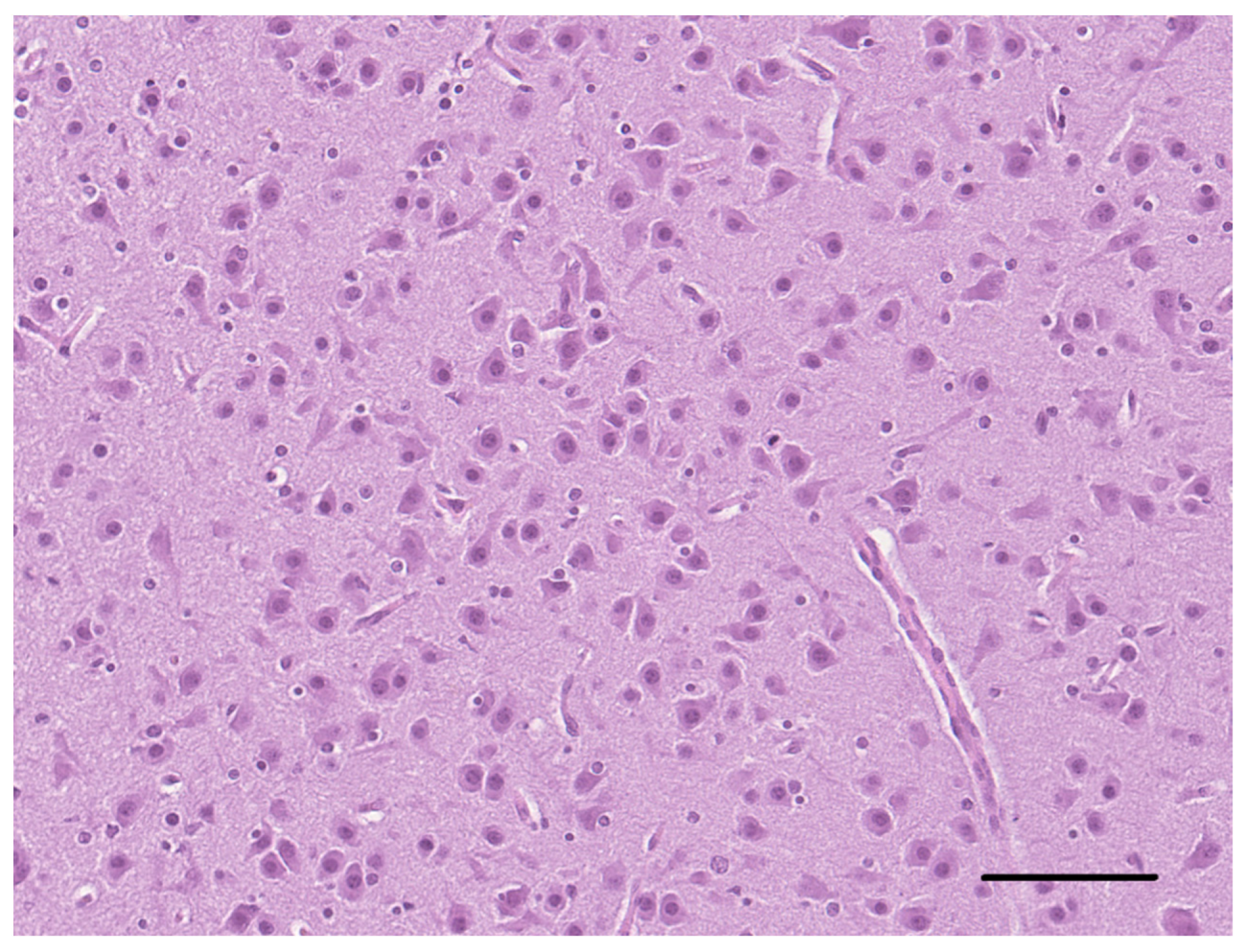
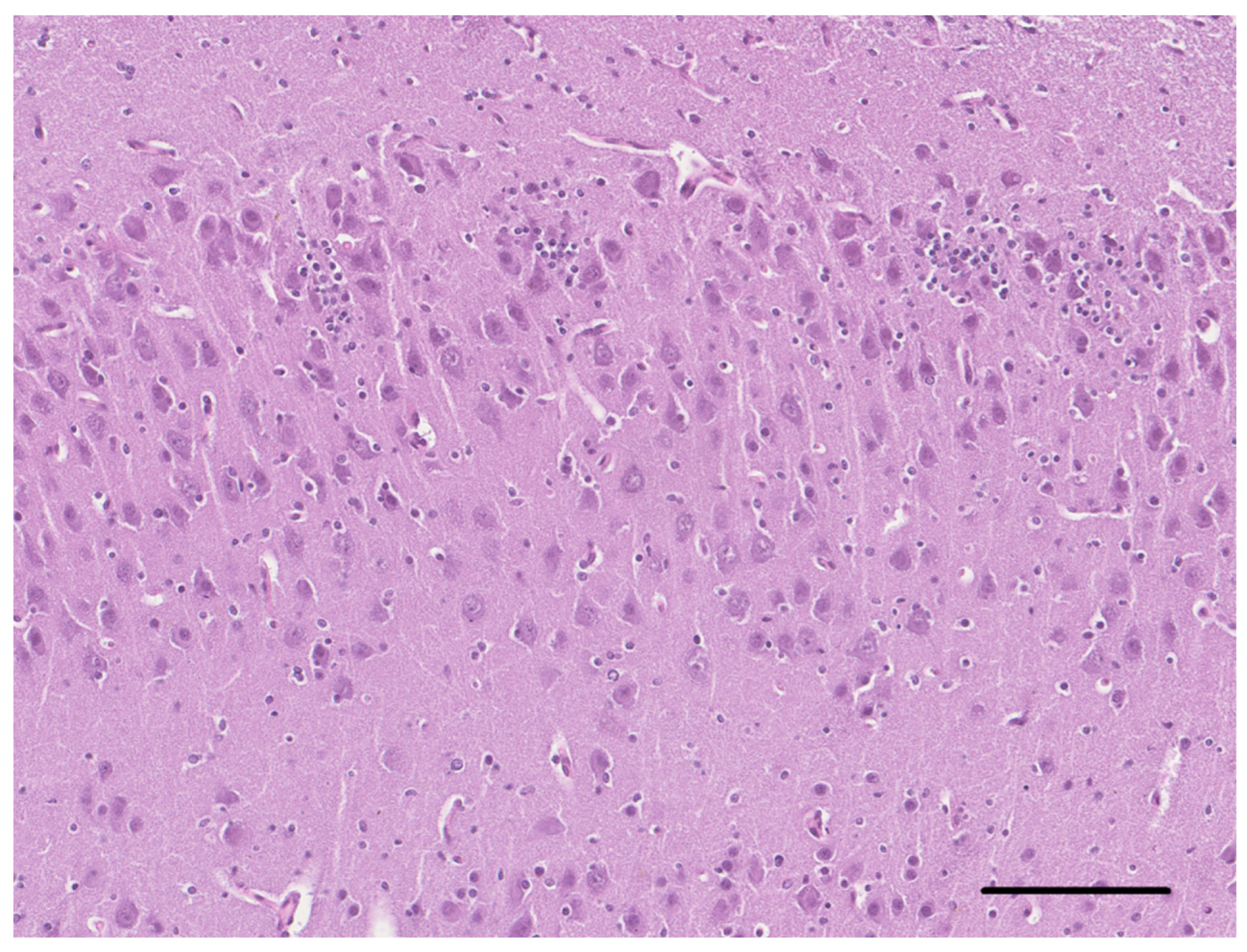
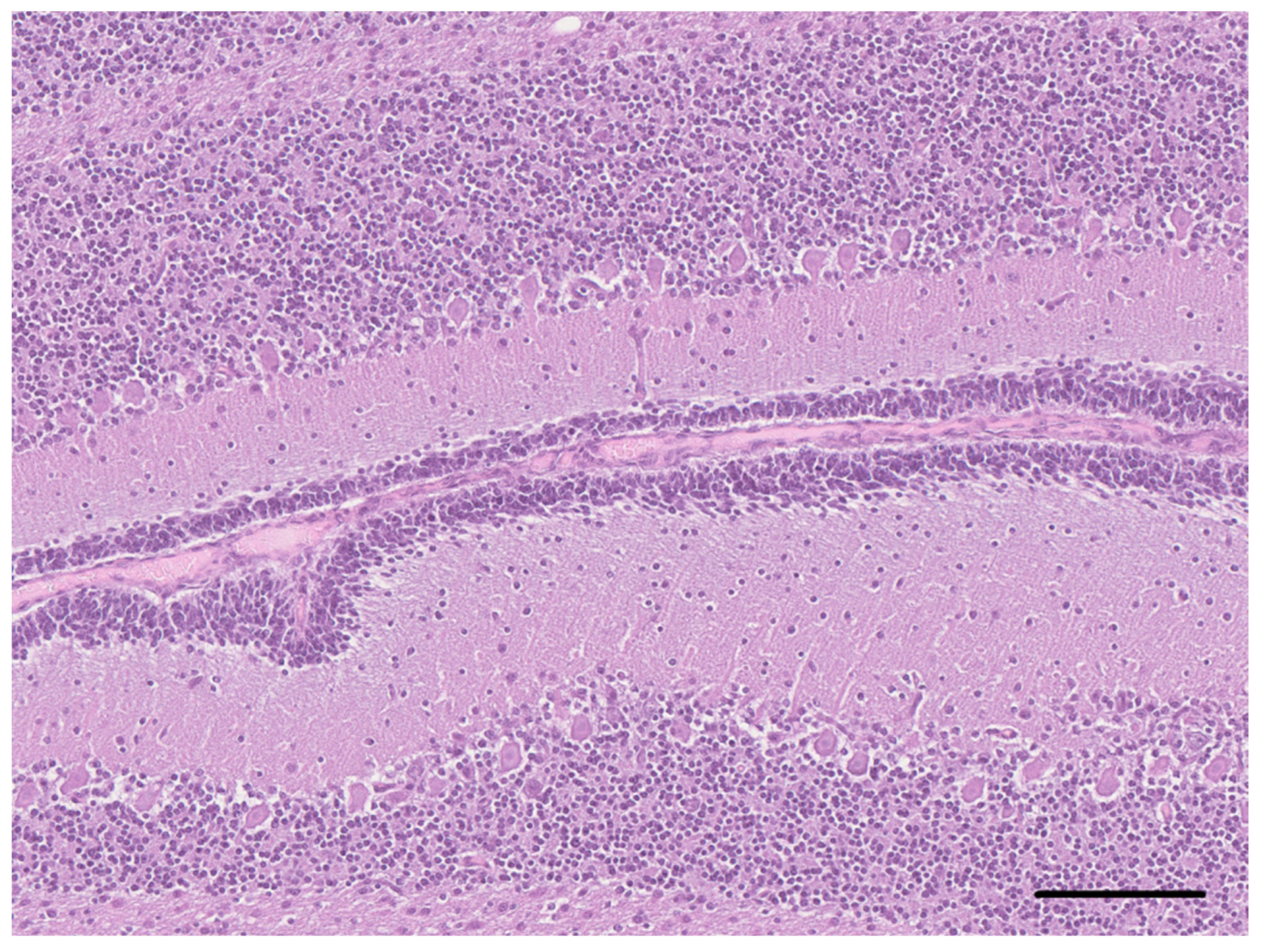
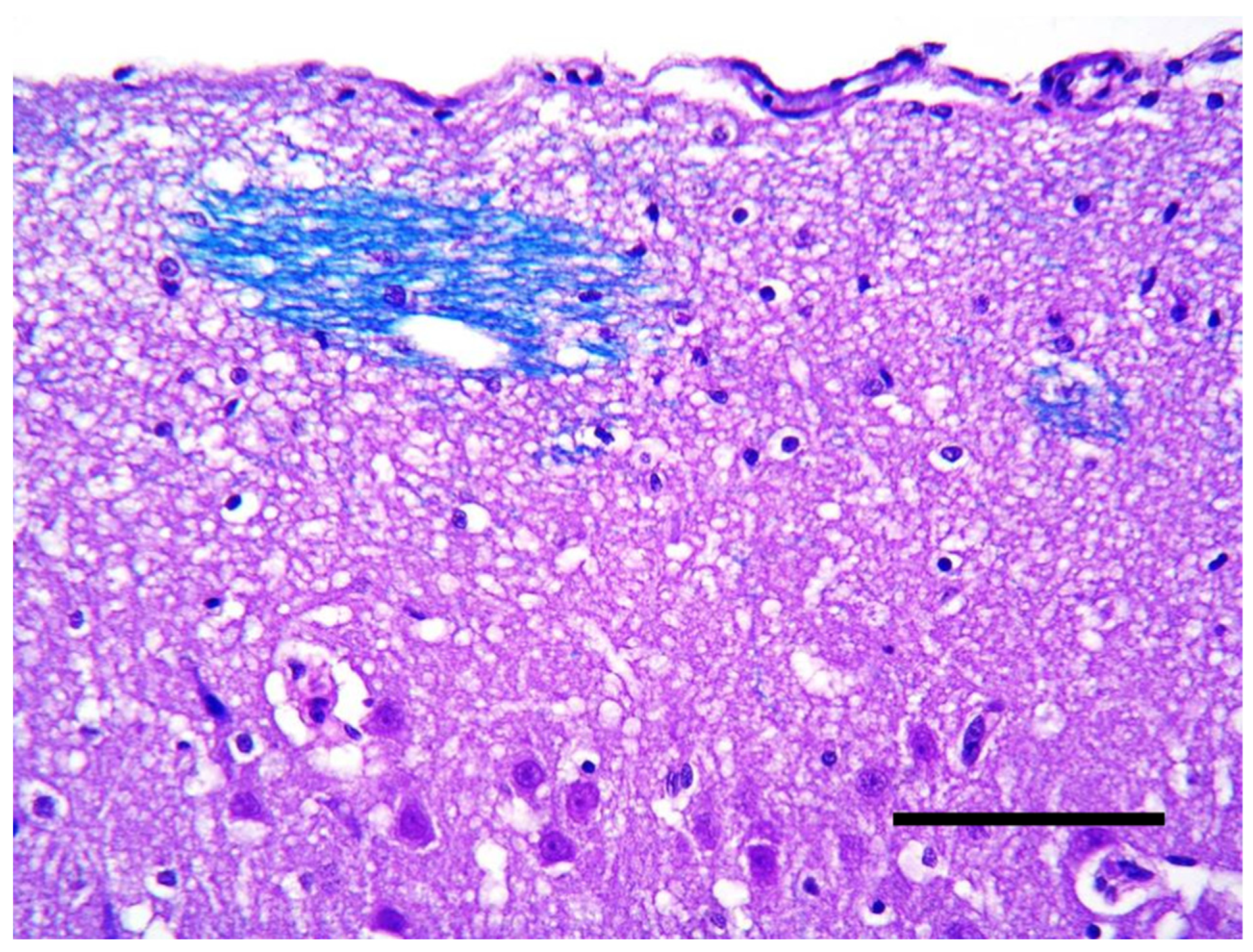
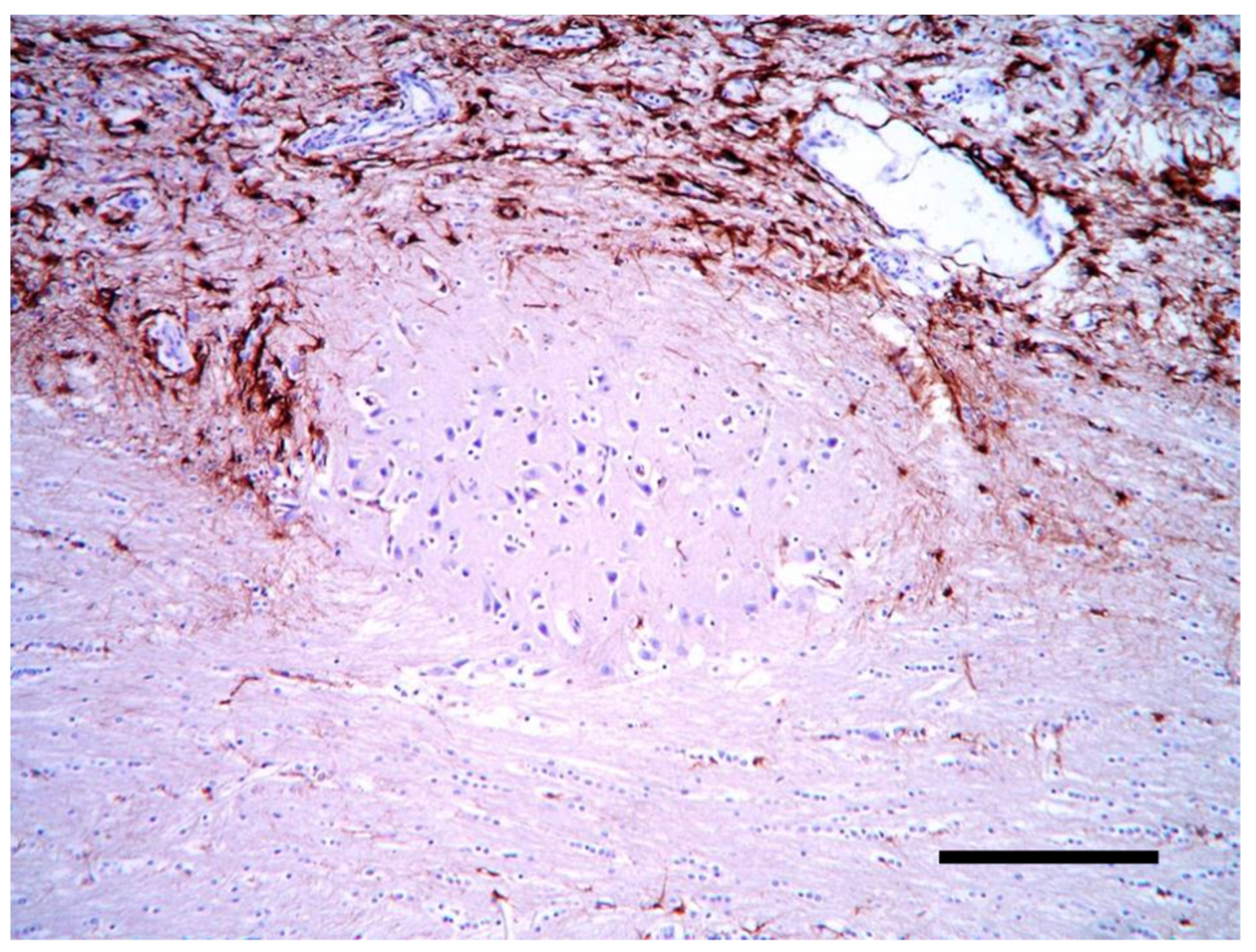
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, D.C.; Falconer, M.A.; Bruton, C.J.; Corsellis, J.A. Focal dysplasia of the cerebral cortex in epilepsy. J. Neurol. Neurosurg. Psychiatry 1971, 34, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.R.; Bergman, R.L. Seizures in young dogs and cats: Pathophysiology and diagnosis. Compendium Vet. 2005, 27, 447–460. [Google Scholar]
- Cantile, C.; Chianini, F.; Arispici, M.; Fatzer, R. Necrotizing meningoencephalitis associated with cortical hippocampal hamartia in a Pekingese dog. Vet. Pathol. 2001, 38, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.F.; Laranjeira, M.G.; Schweigert, A.; de Melo, G.D. Porencephaly and cortical dysplasia as cause of seizures in a dog. BMC Vet. Res. 2012, 8, 1–6. [Google Scholar] [CrossRef]
- Casey, K.M.; Bollen, A.W.; Winger, K.M.; Vernau, K.M.; Dickinson, P.J.; Higgins, R.J.; Sisó, S. Bilaterally symmetric focal cortical dysplasia in a golden retriever dog. J. Comp. Pathol. 2014, 151, 375–379. [Google Scholar] [CrossRef]
- Klang, A.; Leschnik, M.; Schmidt, P.; Pakozdy, A. Bilateral hippocampal malformation and concurrent granulomatous meningoencephalitis in a dog with refractory epilepsy. J. Comp. Pathol. 2014, 150, 424–428. [Google Scholar] [CrossRef]
- Saito, M.; Sharp, N.J.; Kortz, G.D.; de Lahunta, A.; Leventer, R.J.; Tokuriki, M.; Thrall, D.E. Magnetic resonance imaging features of lissencephaly in 2 Lhasa Apsos. Vet. Radiol. Ultrasound. 2002, 43, 331–337. [Google Scholar] [CrossRef]
- Greene, C.E.; Vandevelde, M.; Braund, K. Lissencephaly in two Lhasa Apso dogs. J. Am. Vet. Med. Assoc. 1976, 169, 405–410. [Google Scholar]
- Shimbo, G.; Tagawa, M.; Oohashi, E.; Yanagawa, M.; Miyahara, K. Lissencephaly in a Pekingese. J. Vet. Med. Sci. 2017, 79, 1694–1697. [Google Scholar] [CrossRef]
- Lee, K.I.; Lim, C.Y.; Kang, B.T.; Park, H.M. Clinical and MRI findings of lissencephaly in a mixed breed dog. J. Vet. Med. Sci. 2011, 73, 1385–1388. [Google Scholar] [CrossRef]
- Fraser, A.R.; le Chevoir, M.A.; Long, S.N. Lissencephaly in an adult Australian Kelpie. Aust. Vet. J. 2016, 94, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, D.N.; Pinto, G.B.A.; Thomé, E.F.; de Vasconcelos Machado, V.M.; Amorim, R.M. Lissencephaly in Shih Tzu dogs. Acta Vet. Scand. 2020, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Lahunta, A.; Glass, E.N.; Kent, M. Veterinary Neuroanatomy and Clinical Neurology, 5th ed.; Elsevier Health Sciences: St. Louis, MS, USA, 2021; ISBN 9780323696111. [Google Scholar]
- Herrmann, A.; Hecht, W.; Herden, C. Lissencephaly and microencephaly combined with hypoplasia of corpus callosum and cerebellum in a domestic cat. Tierarztliche Prax. Ausg. K Kleintiere Heimtiere 2011, 39, 116–120. [Google Scholar] [PubMed]
- Bhatti, S.F.; De Risio, L.; Muñana, K.; Penderis, J.; Stein, V.M.; Tipold, A.; Berendt, M.; Farquhar, R.G.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force consensus proposal: Medical treatment of canine epilepsy in Europe. BMC Vet. Res. 2015, 11, 176. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Kuzniecky, R.I.; Jackson, G.D.; Guerrini, R.; Dobyns, W.B. A developmental and genetic classification for malformations of cortical development. Neurology 2005, 65, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.A.; Birchansky, S.; Pacheco, E.; Jayakar, P.; Resnick, T.J.; Dean, P.; Duchowny, M.S. Distinct clinicopathologic subtypes of cortical dysplasia of Taylor. Neurology 2005, 11, 55–61. [Google Scholar] [CrossRef]
- Imad, N.; Harvey, B.S.; Ingmar, B. The international consensus classification of Focal Cortical Dysplasia—A critical update 2018. Neuropathol. Appl. Neurobiol. 2018, 44, 18–31. [Google Scholar] [CrossRef]
- Philip, H.; Iffland, I.I.; Peter, B.C. Focal Cortical Dysplasia: Gene Mutations, Cell Signaling, and Therapeutic Implications. Annu. Rev. Pathol. 2017, 12, 547–571. [Google Scholar] [CrossRef]
- Feng, C.; Zhao, H.; Tian, M.; Lu, M.; Wen, J. Detecting focal cortical dysplasia lesions from FLAIR-negative images based on cortical thickness. Biomed. Eng. Online 2020, 19, 13. [Google Scholar] [CrossRef]
- Jayakar, P. Invasive EEG monitoring in children: When, where, and what? J. Clin. Neurophysiol. 1999, 16, 408–418. [Google Scholar] [CrossRef]
- Phemister, R.D.; Young, S. The postnatal development of the canine cerebellar cortex. J. Comp. Neurol. 1968, 134, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Garcia-Tapia, D.; Riedesel, E.; Ellinwood, N.M.; Jens, J.K. Normal canine brain maturation at magnetic resonance imaging. Vet. Radiol. Ultrasound 2010, 51, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Nardou, R.; Ferrari, D.C.; Ben-Ari, Y. Mechanisms and effects of seizures in the immature brain. Semin. Fetal Neonatal Med. 2013, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Devisme, L.; Bouchet, C.; Gonzalès, M.; Alanio, E.; Bazin, A.; Bessières, B.; Bigi, N.; Blanchet, P.; Bonneau, D.; Bonnières, M.; et al. Cobblestone lissencephaly: Neuropathological subtypes and correlations with genes of dystroglycanopathies. Brain 2012, 135, 469–482. [Google Scholar] [CrossRef]
- Fry, A.E.; Cushion, T.D.; Pilz, D.T. The genetics of lissencephaly. Am. J. Med. Genet. C Semin. Med. Genet. 2014, 166, 198–210. [Google Scholar] [CrossRef]
- Kato, M. Genotype-phenotype correlation in neuronal migration disorders and cortical dysplasias. Front. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Schiller, S.; Rosewich, H.; Grünewald, S.; Gärtner, J. Inborn errors of metabolism leading to neuronal migration defects. J. Inherit. Metab. Dis. 2020, 43, 145–155. [Google Scholar] [CrossRef]
- Guerrini, R.; Carrozzo, R. Epileptogenic brain malformations: Clinical presentation, malformative patterns and indications for genetic testing. Seizure 2001, 10, 532–547. [Google Scholar] [CrossRef]
- Razek, A.A.; Kandell, A.Y.; Elsorogy, L.G.; Elmongy, A.; Basett, A.A. Disorders of cortical formation: MR imaging features. Am. J. Neuroradiol. 2009, 30, 4–11. [Google Scholar] [CrossRef]
- Chen, W.; Jin, B.; Aung, T.; He, C.; Chen, C.; Wang, S.; Ding, Y.; Ding, F.; Wang, C.; Li, H.; et al. Response to antiseizure medications in epileptic patients with malformation of cortical development. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211050027. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchetto, A.; Gallucci, A.; Biggio, F.; Cantile, C. Malformation of the Cortical Development Associated with Severe Clusters of Epileptic Seizures. Vet. Sci. 2023, 10, 7. https://doi.org/10.3390/vetsci10010007
Cocchetto A, Gallucci A, Biggio F, Cantile C. Malformation of the Cortical Development Associated with Severe Clusters of Epileptic Seizures. Veterinary Sciences. 2023; 10(1):7. https://doi.org/10.3390/vetsci10010007
Chicago/Turabian StyleCocchetto, Aurora, Antonella Gallucci, Federica Biggio, and Carlo Cantile. 2023. "Malformation of the Cortical Development Associated with Severe Clusters of Epileptic Seizures" Veterinary Sciences 10, no. 1: 7. https://doi.org/10.3390/vetsci10010007
APA StyleCocchetto, A., Gallucci, A., Biggio, F., & Cantile, C. (2023). Malformation of the Cortical Development Associated with Severe Clusters of Epileptic Seizures. Veterinary Sciences, 10(1), 7. https://doi.org/10.3390/vetsci10010007






