Molecular Investigation of Theileria and Babesia Species in Domestic Mammals from Sardinia, Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and Screening of Piroplasmida by PCR Amplification
2.3. Purification and Sequencing
3. Results
Prevalence of Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilgic, H.B.; Bakırcı, S.; Kose, O.; Unlu, A.H.; Hacılarlıoglu, S.; Eren, H.; Weir, W.; Karagenc, T. Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasites Vectors 2017, 27, 211. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Scimeca, R.C.; Reichard, M.V.; Mosqueda, J. Babesiosis and Theileriosis in North America. Pathogens 2022, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- Uilenberg, G. Babesia-A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Moreau, E.; Bonnet, S.; Plantard, O.; Malandrin, L. Babesia and its hosts: Adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet. Res. 2009, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Mealey, R.H.; Kappmeyer, L.S.; White, S.N.; Kumpula-McWhirter, N.; Pelzel, A.M.; Grause, J.F.; Bunn, T.O.; Schwartz, A.; Traub-Dargatz, J.L.; et al. Re-emergence of the apicomplexan Theileria equi in the United States: Elimination of persistent infection and transmission risk. PLoS ONE 2012, 7, e44713. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, S.; Calderini, P.; Cassini, R.; Galuppi, R.; Tampieri, M.P.; Pietrobelli, M.; Cancrini, G. Human exposure to piroplasms in Central and Northern Italy. Vet. Ital. 2014, 50, 41–47. [Google Scholar] [PubMed]
- Lempereur, L.; Beck, R.; Fonseca, I.; Marques, C.; Duarte, A.; Santos, M.; Zúquete, S.; Gomes, J.; Walder, G.; Domingos, A.; et al. Guidelines for the Detection of Babesia and Theileria Parasites. Vector Borne Zoonotic Dis. 2017, 17, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Alberti, A.; Zobba, R.; Foxi, C.; Masala, G. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in ticks from domestic and wild hosts in Sardinia. Acta Trop. 2019, 196, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Zobba, R.; Chisu, V.; Pinna Parpaglia, M.L.; Spezzigu, A.; Masala, G.; Schianchi, E.; Alberti, A. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in Sardinian ruminants. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100453. [Google Scholar] [CrossRef]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.C. Presence of potentially pathogenic Babesia spp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006, 13, 65–70. [Google Scholar] [PubMed]
- Antunes, S.; Couto, J.; Ferrolho, J.; Sanches, G.S.; Merino Charrez, J.O.; De la Cruz Hernández, N.; Mazuz, M.; Villar, M.; Shkap, V.; de la Fuente, J.; et al. Transcriptome and Proteome Response of Rhipicephalus annulatus Tick Vector to Babesia bigemina Infection. Front. Physiol. 2019, 2, 318. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, H.; Perera, P.K.; Ghafar, A.; Abbas, T.; Gasser, R.B.; Jabbar, A. An appraisal of oriental theileriosis and the Theileria orientalis complex, with an emphasis on diagnosis and genetic characterisation. Parasitol. Res. 2020, 119, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control. Int. J. Environ. Res. Public Health 2019, 16, 1736. [Google Scholar] [CrossRef] [PubMed]
- Zobba, R.; Ardu, M.; Niccolini, S.; Chessa, B.; Manna, L.; Cocco, R.; Pinna Parpaglia, M.L. Clinical and Laboratory Findings in Equine Piroplasmosis. JEVS 2008, 28, 301–308. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Bovine babesiosis. In Terrestrial Animal Health Code, 27th ed.; World Organisation for Animal Health: Paris, France, 2018; Volume II, Available online: https://aanzfta.asean.org/AECSP/ASEAN-SPS-Guide/files/media/2020/09/The-OIE-Terrestrial-Animal-Health-Code-Volume-2.pdf (accessed on 19 October 2022).
- Brown, C.G.; Ilhan, T.; Kirvar, E.; Thomas, M.; Wilkie, G.; Leemans, I.; Hooshmand-Rad, P. Theileria lestoquardi and T. annulata in cattle, sheep, and goats. In vitro and in vivo studies. Ann. N. Y. Acad. Sci. 1998, 849, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zeb, J.; Shams, S.; Din, I.U.; Ayaz, S.; Khan, A.; Nasreen, N.; Khan, H.; Khan, M.A.; Senbill, H. Molecular epidemiology and associated risk factors of Anaplasma marginale and Theileria annulata in cattle from North-western Pakistan. Vet. Parasitol. 2020, 279, 109044. [Google Scholar] [CrossRef] [PubMed]
- Preston, P.M.; Jackson, L.A.; Sutherland, I.A.; Brown, D.J.; Schofield, J.; Bird, T.; Sanderson, A.; Brown, C.G. Theileria annulata: Attenuation of a schizont-infected cell line by prolonged in vitro culture is not caused by the preferential growth of particular host cell types. Exp. Parasitol. 2001, 98, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Gargano, V.; Blanda, V.; Gambino, D.; La Russa, F.; Di Cataldo, S.; Gentile, A.; Schirò, G.; Torina, A.; Millán, J.; Vicari, D. Serological Survey and Molecular Characterization of Theileria annulata in Sicilian Cattle. Pathogens 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
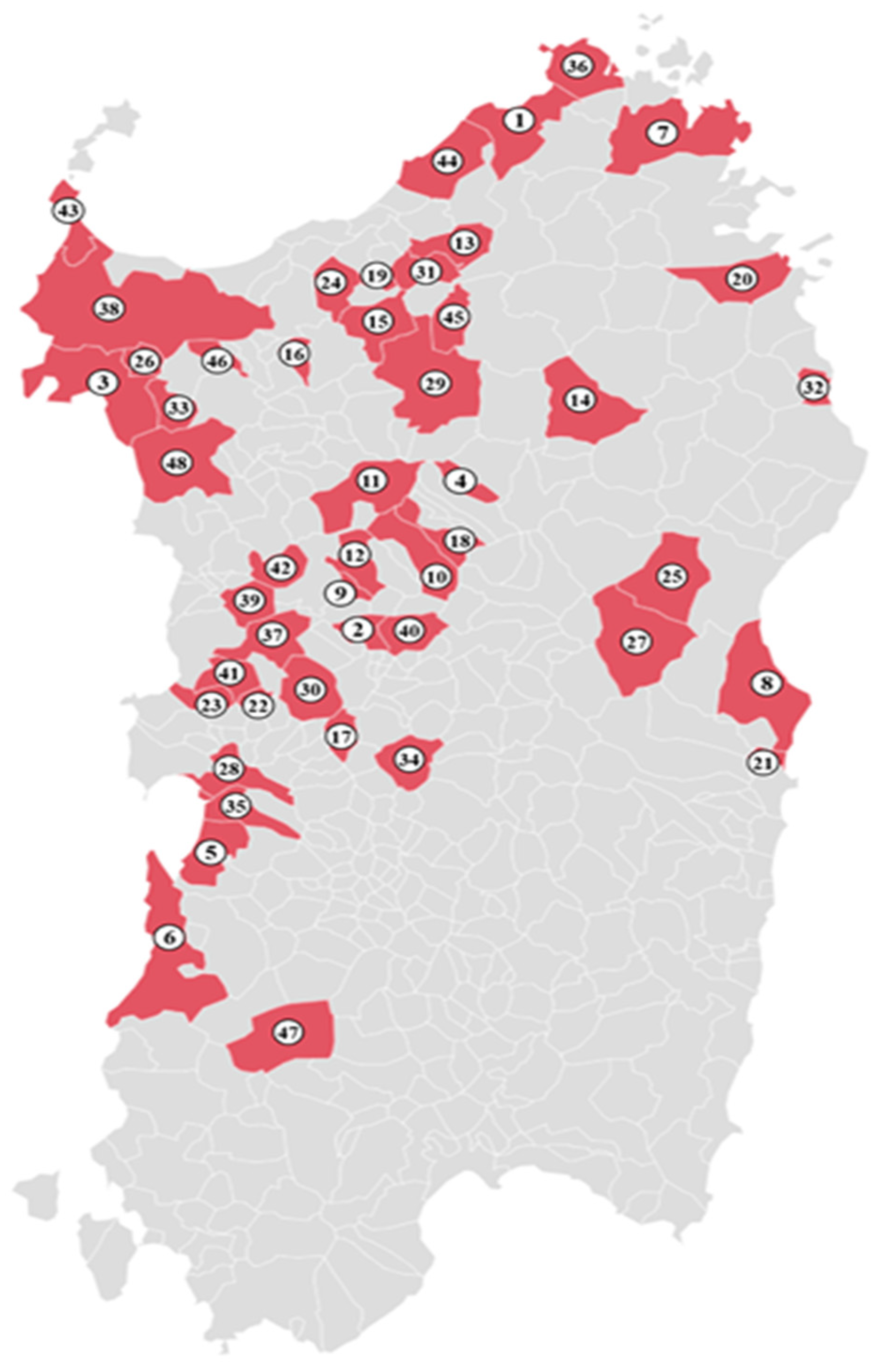
| Animal Hosts | No. of Examined Animals | No. of Infected Animals (%) | No. of Good Quality Sequences | Pathogens Sequenced (Identificative Number of Collection Site) | 1st Hit GenBank Accession Number (ID%) |
|---|---|---|---|---|---|
| Cattle | 72 | 49 (68%) | 24/49 | T. orientalis/sergenti/buffeli (13, 27, 31, 36, 44, 45) | MN611176 |
| 7/49 | T. annulata (17, 22, 37) | MT341858 | |||
| 3/49 | B. bigemina (7) | MH050356 | |||
| Horse | 80 | 60 (75%) | 36/60 | T. equi (2, 3, 5, 10, 16, 19, 23, 25, 28, 33, 34, 35, 36, 39, 43, 47) | MT463613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisu, V.; Serra, E.; Foxi, C.; Chessa, G.; Masala, G. Molecular Investigation of Theileria and Babesia Species in Domestic Mammals from Sardinia, Italy. Vet. Sci. 2023, 10, 59. https://doi.org/10.3390/vetsci10010059
Chisu V, Serra E, Foxi C, Chessa G, Masala G. Molecular Investigation of Theileria and Babesia Species in Domestic Mammals from Sardinia, Italy. Veterinary Sciences. 2023; 10(1):59. https://doi.org/10.3390/vetsci10010059
Chicago/Turabian StyleChisu, Valentina, Elisa Serra, Cipriano Foxi, Giovanna Chessa, and Giovanna Masala. 2023. "Molecular Investigation of Theileria and Babesia Species in Domestic Mammals from Sardinia, Italy" Veterinary Sciences 10, no. 1: 59. https://doi.org/10.3390/vetsci10010059
APA StyleChisu, V., Serra, E., Foxi, C., Chessa, G., & Masala, G. (2023). Molecular Investigation of Theileria and Babesia Species in Domestic Mammals from Sardinia, Italy. Veterinary Sciences, 10(1), 59. https://doi.org/10.3390/vetsci10010059






