Rabies in Bats (Chiroptera, Mammalia) in Brazil: Prevalence and Potential Risk Factors Based on Twenty Years of Research in the Northwestern Region of São Paulo, Brazil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
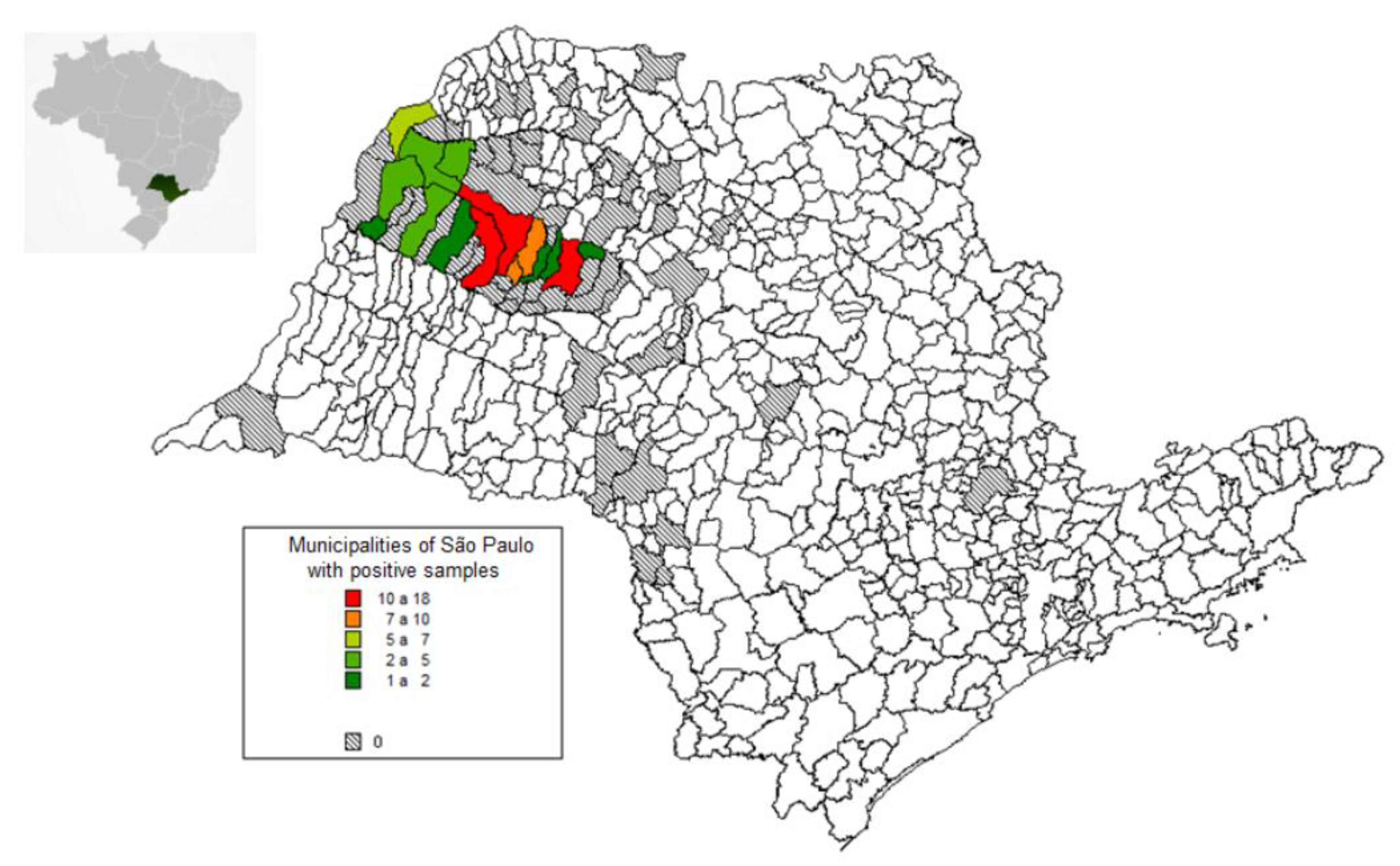
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Expert Consultation on Rabies; Third Report. WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2018; No. 1012; Available online: https://apps.who.int/iris/handle/10665/272364 (accessed on 6 September 2022).
- Carvalho, M.F.; Vigilato, M.A.N.; Pompei, J.C.; Rocha, F.; Vokaty, A.; Molina-Flores, B.; Cosivi, O.; Vilas, V.J.D.R. Rabies in the Americas: 1998–2014. PLoS Negl. Trop. Dis. 2017, 12, e0006271. [Google Scholar] [CrossRef]
- Vargas, A.; Romano, A.P.M.; Mérchan-Hamann, E. Raiva humana no Brasil: Estudo descritivo, 2000–2017. Epidemiol. Serv. Saúde 2019, 28, e2018275. [Google Scholar] [CrossRef] [PubMed]
- BRASIL; Ministério da Saúde; Secretaria de Vigilância em Saúde; Raiva. Tabela 4-Casos de Raiva Humana por Município de Ocorrência, Espécie Animal Agressora e Variante Genética. Brasil. 2010 a 2022 *. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/r/raiva/imagens/arquivos-2022/tabela-4_2022.pdf (accessed on 21 June 2022).
- Escobar, L.E.; Peterson, T.A.; Favi, M.; Yung, V.; Medina-Vogel, G. Bat-borne rabies in Latin America. Rev. Inst. Med. Trop. São Paulo 2015, 57, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, D.K.A.; Favaro, A.B.B.C.; Carvalho, C.; Picolo, M.R.; Hernandez, J.C.B.; Lot, M.S.; Albas, A.; Araújo, D.B.; Pedro, W.A.; Queiroz, L.H. Rabies surveillance in bats in Northwestern State of São Paulo. Rev. Soc. Bras. Med. Trop. 2014, 47, 709–715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Correa, M.M.O.; Lazar, A.; Dias, D.; Bonvicino, C.R. Quirópteros hospedeiros de zoonoses no Brasil. Bol. Soc. Bras. Mastozool. 2013, 67, 23–28. Available online: http://www.arca.fiocruz.br/handle/icict/11604 (accessed on 18 July 2018).
- Sodré, M.M.; Gama, A.R.; Almeida, M.F. Update list of bat species positive for rabies in Brazil. Rev. Inst. Med. Trop São Paulo. 2010, 52, 75–81. [Google Scholar] [CrossRef]
- Pacheco, S.M.; Sodré, M.; Gama, A.R.; Bredt, A.; Cavallini, E.D.; Marques, S.R.V.; Guimarães, M.M.; Bianconi, G. Morcegos urbanos: Status do conhecimento e plano de ação para a conservação no Brasil. Chiropt. Neotropical. 2010, 16, 629–647. [Google Scholar]
- Almeida, M.F.; Rosa, A.D.; Sodré, M.M.; Martorelli, L.F.A.; Netto, J.T. Fauna de Morcegos (Mammalia, Chiroptera) e a ocorrência de vírus da raiva na cidade de São Paulo, Brasil. Rev. Vet. Zootec. 2015, 22, 89–100. [Google Scholar]
- Albas, A.; Souza, E.A.N.; Lourenço, R.A.; Favoretto, S.R.; Sodré, M.M. Perfil antigênico do vírus da raiva isolado de diferentes espécies de morcegos não hematófagos da Região de Presidente Prudente, Estado de São Paulo. Rev. Soc. Bras. Med. Trop. 2009, 42, 15–17. [Google Scholar] [CrossRef]
- Almeida, M.F.; Martorelli, L.F.A.; Sodré, M.M.; Kataoka, A.P.A.G.; Rosa, A.R.; Oliveira, M.L.; Amatuzzi, E. Diagnóstico e sorologia de raiva em morcegos do Estado de São Paulo, Brasil. Rev. Soc. Bras. Med. Trop. 2011, 44, 140–145. [Google Scholar] [CrossRef][Green Version]
- Cabral, C.C.; Morais, A.C.N.; Dias, A.V.A.B.; Araújo, M.G.; Moreira, W.C.; Mattos, G.L.M. Circulation of rabies virus in non-hematophagous bats in city of Rio de Janeiro, Brazil, during 2001–2010. Rev. Soc. Bras. Med. Trop. 2012, 45, 180–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha, E.M.S.; Queiroz, L.H.; Lara, M.C.C.S.H.; Nassar, A.F.C.; Albas, A.; Sodré, M.M.; Pedro, W.A. Bat rabies in the north-northwestern of the state of São Paulo, Brazil: 1997–2002. Rev. Saúde Pública 2006, 40, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.H.; Carvalho, C.C.; Buso, D.S.; Ferrari, C.I.L.; Pedro, W.A. Perfil epidemiológico da raiva na região Noroeste do Estado de São Paulo no período de 1993 a 2007. Rev. Soc. Bras. Med. Trop. 2009, 42, 9–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheffer, K.C.; Carrieri, M.L.; Albas, A.; Santos, H.C.P.; Korait, I.; Ito, F.H. Vírus da raiva em quirópteros naturalmente infectados no Estado de São Paulo, Brasil. Rev. Saúde Pública 2007, 41, 389–395. [Google Scholar] [CrossRef][Green Version]
- Secretaria Estadual de Saúde; Instituto Pasteur. Raiva Aspectos Gerais e Clínica. Manual 8; Instituto Pasteur: São Paulo, Brazil, 2009; 49p. [Google Scholar]
- Dean, D.J.; Abelseth, M.K.; Atanasiu, P. The fluorescent antibody test. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.X., Kaplan, M.M., Koprowisk, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; p. 47. [Google Scholar]
- Koprowisk, H. The mouse inoculation test. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.X., Kaplan, M.M., Koprowisk, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; p. 476. [Google Scholar]
- World Health Organization (WHO). Laboratory Techiques in Rabies, 4th ed.; WHO: Geneva, Switzerland, 1996; 476p. [Google Scholar]
- Vizotto, L.D.; Taddei, V.A. Chave para determinação de quirópteros brasileiros. Rev. Fac. Filos. Cien. Letr. S. José R. Preto Bolm. Cienc. 1973, 1, 1–72. [Google Scholar]
- Gregorin, R.; Taddei, V.A. Chave Artificial para a Identificação de Molossídeos Brasileiros (Mammalia, Chiroptera). Mastozool. Neotrop. 2002, 9, 13–32. [Google Scholar]
- Reis, N.R.; Peracchi, A.L.; Batista, C.B.; Lima, I.P.; Pereira, A.D. História Natural dos Morcegos Brasileiros/Chave de Identificação de Espécies, 1st ed.; Technical Books Editora Ltda.: Rio de Janeiro, Brazil, 2017; 416p. [Google Scholar]
- Pedro, W.A.; Taddei, V.A. Taxonomic assemblage of bats from Panga Reserve, southeastern Brazil: Abundance patterns and trophic relations in the Phyllostomidae (Chiroptera). Boletim do Museu de Biologia Mello Leitão 1997, 6, 3–21. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 24 July 2018).
- MAPINFO Corporation. MapInfo Professional; Versão 7.5; MAPINFO Corporation: New York, NY, USA, 2003. [Google Scholar]
- Organizacion Panamericana de La Salud. Eliminación de la Rabia Humana Transmitida por Perros en América Latina: Análisis de la Situación, Año 2004; WHO: Washington, DC, USA, 2005; Available online: https://www3.paho.org/spanish/ad/dpc/vp/rabia-sit.pdf (accessed on 14 August 2018).
- Vigilato, M.A.N.; Clavijo, A.; Knobl, T.; Tamayo Silva, H.M.; Cosivi, O.; Schneider, M.C.; Leanes, L.F.; Belotto, A.J.; Espinal, M.A. Progress towards eliminating canine rabies: Policies and perspectives from Latin America and the Caribbean. Philos. Trans. R. Soc. B 2013, 368, 20120143. [Google Scholar] [CrossRef]
- Ribeiro, J.; Staudacher, C.; Martins, C.M.; Ullman, L.S.; Ferreira, F.; Araujo Neto, J.P.; Biondo, A.W. Bat rabies surveillance and risk factors for rabies spillover in an urban area of Southern Brazil. BMC Vet. Res. 2018, 14, 173. [Google Scholar] [CrossRef]
- Carvalho, C.C.; Gonçalves, J.F.; Franco, R.; Casagrande, D.K.A.; Pedro, W.A.; Queiroz, L.H. Caracterização da fauna de morcegos (Mammalia, Chiroptera) e ocorrência de vírus rábico na região noroeste do estado de São Paulo, Brasil. Rev. Vet. Zootec. 2011, 8, 490–503. [Google Scholar]
- Albas, A.; Souza, E.A.N.; Picolo, M.R.; Favoretto, S.R.; Gama, A.R.; Sodré, M.M. Os morcegos e a raiva na região oeste do Estado de São Paulo. Rev. Soc. Bras. Med. Trop. 2011, 44, 201–205. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, T.; Rodrigues, R.C.A.; Castagna, C.; Presotto, D.; De Nadai, D.V.; Frage, A.; Braga, G.B.; Guilloux, A.G.A.; Alves, A.J.S.; Martins, C.M.; et al. Assessing the rabies control and surveillance systems in Brazil: An experience of measures toward bats after the halt of massive vaccination of dogs and cats in Campinas, São Paulo. Prev. Vet. Med. 2013, 111, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.A.; Rocha, F.; Ulloa-Stanojlovic, F.M.; Nitsche, A.; Castagna, C.; de Lucca, T.; Rodrigues, R.C.A. Spatiotemporal distribution of a non-hematophagous bat community and rabies virus circulation: A proposal for urban rabies surveillance in Brazil. Epidemiol. Infect. 2019, 147, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.B.; Acharya, L.; Audet, D.; Hickey, M.B.C.; Merriman, C.; Obrist, M.K. Phyllostomid bats (Chiroptera: Phyllostomidae) as indicators of habitat disruption in the neotropics. Biotropica 1992, 24, 440–446. [Google Scholar] [CrossRef]
- Pereira, A.S.; Casseb, L.M.N.; Barbosa, T.F.S.; Begot, A.L.; Brito, R.M.O.; Vasconcelos, P.F.C.; da Rosa, E.S.T. Rabies Virus in Bats, State of Pará, Brazil, 2005–2011. Vector Borne Zoonotic. Dis. 2017, 6, 576–581. [Google Scholar] [CrossRef]
- Munoz-Romo, M.; Herrera, E.A.; Knut, T.H. Roosting behaviour and group stability of the big fruit-eating bat Artibeus lituratus (Chiroptera: Phyllostomidae). Mamm. Biol. 2008, 73, 214–221. [Google Scholar] [CrossRef]
- Souza, L.C.; Langoni, H.; Silva, R.C.; Lucheis, S.B. Vigilância epidemiológica da raiva na região de Botucatu-SP: Importância dos quirópteros na manutenção do vírus na natureza. Ars Vet. 2005, 21, 62–68. [Google Scholar]
| Variable | OR | Default Error | p Value | |
|---|---|---|---|---|
| Food habit | Insectivorous Frugivorous | 1 4.20 | 1.341 | <0.0001 |
| Family | Molossidae Phyllostomidae | 1 5.87 | 1.442 | 0.001 |
| Molossidae Vespertilionidae | 1 20.9 | 1.389 | 0.001 | |
| Phyllostomidae Vespertilionidae | 1 3.56 | 1.374 | 0.001 | |
| Season | Rainy Dry | 1 2.33 | 1.312 | 0.009 |
| Sex | Female Male | 1 1.17 | 1.296 | 0.960 |
| Family, Gender and Species (Food Habit **) | Sample n (%) | Positive n (%) | * FmP, GnP and SpP |
|---|---|---|---|
| Molossidae | 4149 (74.4) | 21 (27.6) | 0.5 |
| Cynomops | 29 (0.5) | 1 (1.3) | 3.4 |
| Cynomops planirostris (I) | 29 (0.5) | 1 (1.3) | 3.4 |
| Eumops | 524 (9.4) | 1 (1.3) | 0.2 |
| Eumops auripendulus (I) | 11 (0.2) | 0 | 0 |
| Eumops glaucinus (I) | 474 (8.5) | 1 (1.3) | 0 |
| Eumops perotis (I) | 39 (0.7) | 0 | 0.2 |
| Molossops | 8 (0.1) | 0 | 0 |
| Molossops temminckii (I) | 8 (0.1) | 0 | 0 |
| Molossus | 3565 (64,0) | 19 (25) | 0.5 |
| Molossus molossus (I) | 1620 (29.1) | 5 (6.6) | 0.3 |
| Molossus rufus (I) | 1945 (34.9) | 14 (18.4) | 0.7 |
| Nyctinomops | 20 (0.4) | 0 | 0 |
| Nyctinomops laticaudatus (I) | 18 (0.3) | 0 | 0 |
| Nyctinomops macrotis (I) | 2 (0.04) | 0 | 0 |
| Tadarida | 3 (0.05) | 0 | 0 |
| Tadarida brasiliensis (I) | 3 (0.05) | 0 | 0 |
| Noctilionidae | 43 (0.8) | 0 | 0 |
| Noctilio | 43 (0.8) | 0 | 0 |
| Noctilio albiventris (I) | 43 (0.8) | 0 | 0 |
| Phyllostomidae | 856 (15.4) | 18 (23.7) | 2.1 |
| Artibeus | 308 (5.5) | 18 (23.7) | 5.8 |
| Artibeus jamaicensis (F) | 23 (0.4) | 0 | 0 |
| Artibeus lituratus (F) | 231 (4.1) | 18 (23.7) | 8 |
| Artibeus obscurus (F) | 1 (0.02) | 0 | 0 |
| Artibeus planirostris (F) | 53 (0.9) | 0 | 0 |
| Carollia | 50 (0,9) | 0 | 0 |
| Carollia perspicillata (F) | 50 (0,9) | 0 | 0 |
| Chiroderma | 5 (0.1) | 0 | 0 |
| Chiroderma doriae (F) | 5 (0.1) | 0 | 0 |
| Desmodus | 311 (5.6) | 0 | 0 |
| Desmodus rotundus (S) | 311 (5.6) | 0 | 0 |
| Glossophaga | 107 (1.9) | 0 | 0 |
| Glossophaga soricina (N) | 107 (1.9) | 0 | 0 |
| Phyllostomus | 17 (0.3) | 0 | 0 |
| Phyllostomus hastatus (O) | 17 (0.3) | 0 | 0 |
| Platyrrhinus | 44 (0.8) | 0 | 0 |
| Platyrrhinus lineatus (F) | 44 (0.8) | 0 | 0 |
| Uroderma | 9 (0.2) | 0 | 0 |
| Uroderma bilobatum (F) | 9 (0.2) | 0 | 0 |
| Sturnira | 5 (0.1) | 0 | 0 |
| Sturnira lilium (F) | 5 (0.1) | 0 | 0 |
| Vespertilionidae | 525 (9.4) | 37 (48.7) | 7.4 |
| Eptesicus | 157 (2.8) | 16 (21) | 10.2 |
| Eptesicus brasiliensis (I) | 13 (0.2) | 0 | 0 |
| Eptesicus diminutus (I) | 39 (0.7) | 2 (2.6) | 5.1 |
| Eptesicus furinalis (I) | 105 (1.9) | 14 (18.4) | 13.3 |
| Lasiurus | 148 (2.7) | 2 (2.6) | 1.3 |
| Lasiurus blossevillii (I) | 51 (0.9) | 1 (1.3) | 2 |
| Lasiurus cinereus (I) | 21 (0.4) | 0 | 0 |
| Lasiurus ega (I) | 76 (1.4) | 1 (1.3) | 1.3 |
| Myotis | 220 (3.9) | 19 (25) | 8.6 |
| Myotis nigricans (I) | 220 (3.9) | 19 (25) | 8.6 |
| TOTAL | 5573 | 76 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.B.; de Carvalho, C.; Casagrande, D.; Picinato, M.A.d.C.; Pedro, W.A.; Marinho, M.; Queiroz, L.H. Rabies in Bats (Chiroptera, Mammalia) in Brazil: Prevalence and Potential Risk Factors Based on Twenty Years of Research in the Northwestern Region of São Paulo, Brazil. Vet. Sci. 2023, 10, 34. https://doi.org/10.3390/vetsci10010034
Garcia AB, de Carvalho C, Casagrande D, Picinato MAdC, Pedro WA, Marinho M, Queiroz LH. Rabies in Bats (Chiroptera, Mammalia) in Brazil: Prevalence and Potential Risk Factors Based on Twenty Years of Research in the Northwestern Region of São Paulo, Brazil. Veterinary Sciences. 2023; 10(1):34. https://doi.org/10.3390/vetsci10010034
Chicago/Turabian StyleGarcia, Ana Beatriz, Cristiano de Carvalho, Daiene Casagrande, Mirelle Andrea de Carvalho Picinato, Wagner Andre Pedro, Márcia Marinho, and Luzia Helena Queiroz. 2023. "Rabies in Bats (Chiroptera, Mammalia) in Brazil: Prevalence and Potential Risk Factors Based on Twenty Years of Research in the Northwestern Region of São Paulo, Brazil" Veterinary Sciences 10, no. 1: 34. https://doi.org/10.3390/vetsci10010034
APA StyleGarcia, A. B., de Carvalho, C., Casagrande, D., Picinato, M. A. d. C., Pedro, W. A., Marinho, M., & Queiroz, L. H. (2023). Rabies in Bats (Chiroptera, Mammalia) in Brazil: Prevalence and Potential Risk Factors Based on Twenty Years of Research in the Northwestern Region of São Paulo, Brazil. Veterinary Sciences, 10(1), 34. https://doi.org/10.3390/vetsci10010034





