Results of a Clinical Trial Showing Changes to the Faecal Microbiome in Racing Thoroughbreds after Feeding a Nutritional Supplement
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Study
2.2. Sample Size for Clinical Trial
2.3. Subject Recruitment and Study Protocol
2.4. Nutritional Supplement
2.5. Blinding
2.6. Faecal Sampling
2.7. Laboratory Analysis
2.8. Statistical Analysis
- (1)
- Increase in the phylum Firmicutes (F) of ≥9%;
- (2)
- Increase in the class Clostridia of ≥24%;
- (3)
- Decrease in the phylum Bacteroidetes (B) of ≥5%;
- (4)
- Increase in the F:B of ≥16%;
- (5)
- Increase in the phylum Actinobacteria of ≥2%.
3. Results
3.1. Pilot Study
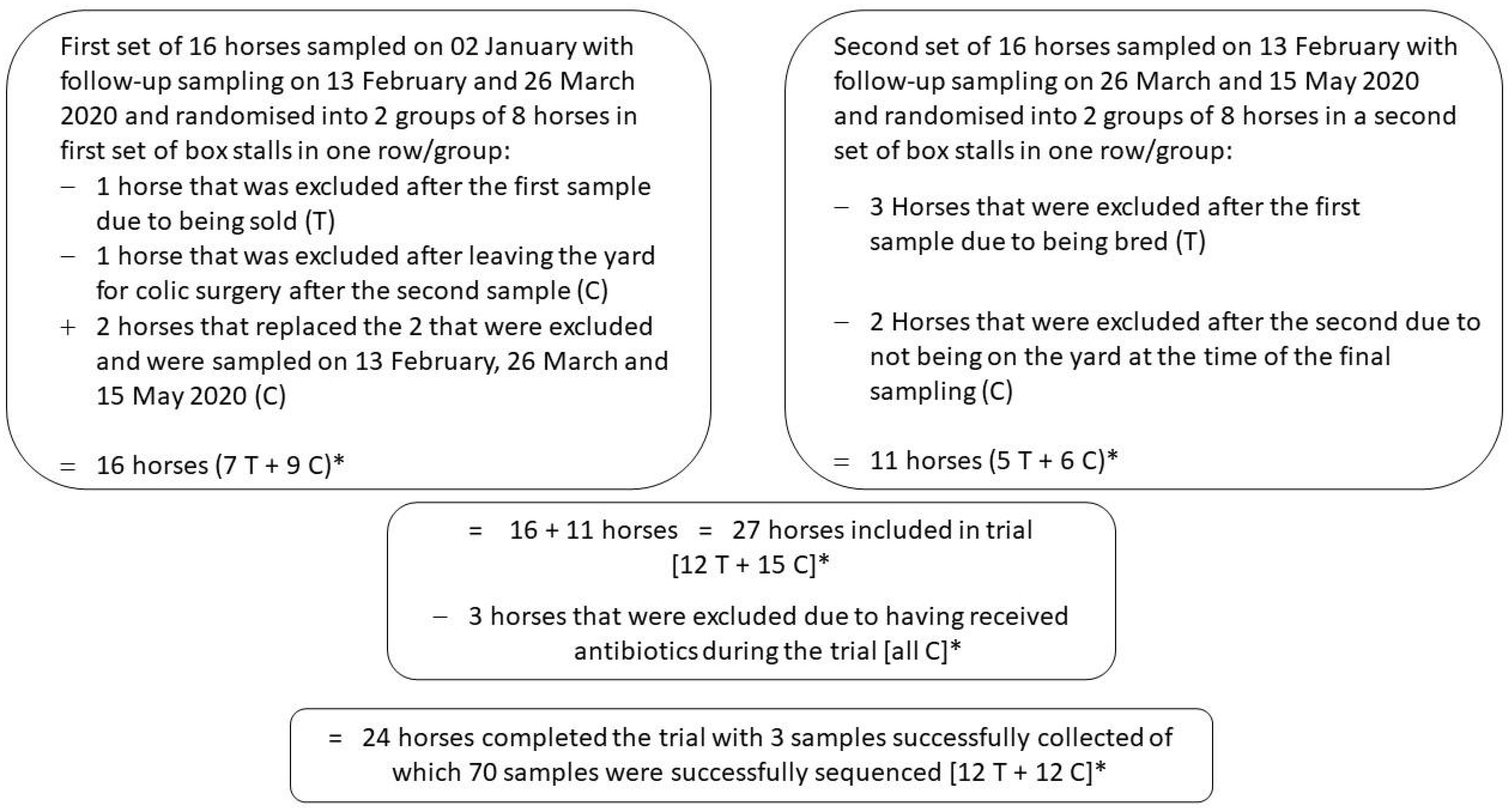
3.2. Clinical Trial Subjects
3.3. Baseline Microbiome for Treatment and Control Groups
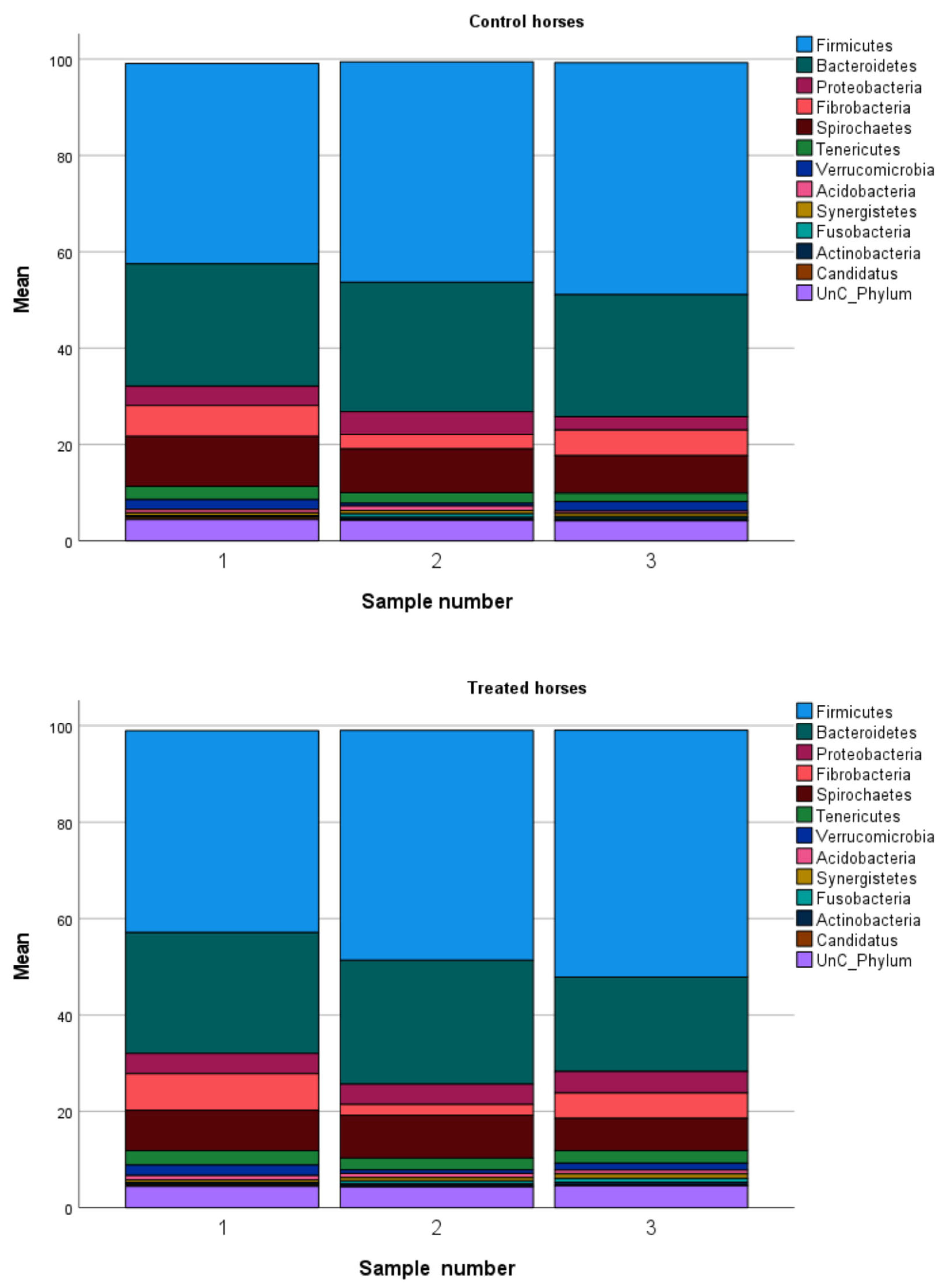
3.4. Microbiome Composition at 12 Weeks and Percent Change in Composition from Baseline for Treatment and Control Groups
3.5. Outcome for 20 Horses on a Per Protocol Analysis
3.6. Intention to Treat Analysis for 22 Horses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Proudman, C.J.; Duncan, S.H.; Flint, H.J.; Dyer, J.; Shirazi-Beechey, S.P. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. Apr. 2012, 107, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.L.; Swecker, W.S., Jr.; Jensen, R.V.; Ponder, M.A. Characterization of the fecal bacteria communities of forage-fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS Microbiol. Lett. 2012, 326, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dougal, K.; Harris, P.A.; Edwards, A.; Pachebat, J.A.; Blackmore, T.M.; Worgan, H.J.; Newbold, C.J. A comparison of the microbiome and the metabolome of different regions of the equine hindgut. FEMS Microbiol. Ecol. 2012, 82, 642–652. [Google Scholar] [CrossRef]
- Sorensen, R.J.; Drouillard, J.S.; Douthit, T.L.; Ran, Q.; Marthaler, D.G.; Kang, Q.; Vahl, C.I.; Lattimer, J.M. Effect of hay type on cecal and fecal microbiome and fermentation parameters in horses. J. Anim. Sci. 2021, 99, skaa407. [Google Scholar] [CrossRef]
- Cerri, S.; Taminiau, B.; de Lusancay, A.H.C.; Lecoq, L.; Amory, H.; Daube, G.; Cesarini, C. Effect of oral administration of omeprazole on the microbiota of the gastric glandular mucosa and feces of healthy horses. J. Vet. Intern. Med. 2020, 34, 2727–2737. [Google Scholar] [CrossRef]
- Garber, A.; Hastie, P.; Murray, J.-A. Factors influencing equine gut microbiota: Current knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef]
- O’Donnell, M.M.; Harris, H.M.B.; Jeffery, I.B.; Claesson, M.J.; Younge, B.; O’Toole, P.W.; Ross, R.P. The core faecal bacterial microbiome of I rish T horoughbred racehorses. Lett. Appl. Microbiol. 2013, 57, 492–501. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Organization FaAOotUNaWH. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; World Health Organization: Córdoba, Argentina, 2001. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Heaton, C.P.; Cavinder, C.A.; Paz, H.A.; Rude, B.J.; Smith, T.; Memili, E.; Harris, P.; Liburt, N.; Krotky, A. Are prebiotics beneficial for digestion in mature and senior horses? J. Equine Vet. Sci. 2019, 76, 87–88. [Google Scholar] [CrossRef]
- Respondek, F.; Goachet, A.; Julliand, V. Effects of dietary short-chain fructooligosaccharides on the intestinal microflora of horses subjected to a sudden change in diet. J. Anim. Sci. 2008, 86, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.E.; Maddox, T.W.; Berg, A.; Antczak, P.; Ketley, J.M.; Williams, N.J.; Archer, D.C. Variation in faecal microbiota in a group of horses managed at pasture over a 12-month period. Sci. Rep. 2018, 8, 8510. [Google Scholar] [CrossRef] [PubMed]
- Theelen, M.J.P.; Luiken, R.E.C.; Wagenaar, J.A.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Rossen, J.W.A.; Zomer, A.L. The Equine Faecal Microbiota of Healthy Horses and Ponies in The Netherlands: Impact of Host and Environmental Factors. Animals 2021, 11, 1762. [Google Scholar] [CrossRef]
- Fouhy, F.; Deane, J.; Rea, M.C.; O’Sullivan, Ó.; Ross, R.P.; O’Callaghan, G.; Plant, B.J.; Stanton, C. The Effects of Freezing on Faecal Microbiota as Determined Using MiSeq Sequencing and Culture-Based Investigations. PLoS ONE 2015, 10, e0119355. [Google Scholar] [CrossRef]
- Tedjo, D.I.; Jonkers, D.M.; Savelkoul, P.H.; Masclee, A.A.; van Best, N.; Pierik, M.J.; Penders, J. The Effect of Sampling and Storage on the Fecal Microbiota Composition in Healthy and Diseased Subjects. PLoS ONE 2015, 10, e0126685. [Google Scholar] [CrossRef]
- Song, S.J.; Amir, A.; Metcalf, J.L.; Amato, K.R.; Xu, Z.Z.; Humphrey, G.; Knight, R. Preservation Methods Differ in Fecal Microbiome Stability, Affecting Suitability for Field Studies. MSystems 2016, 1, e00021-16. [Google Scholar] [CrossRef]
- Cardona, S.; Eck, A.; Cassellas, M.; Gallart, M.; Alastrue, C.; Dore, J.; Azpiroz, F.; Roca, J.; Guarner, F.; Manichanh, C. Storage conditions of intestinal microbiota matter in metagenomic analysis. BMC Microbiol. 2012, 12, 158. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Klaenhammer, T.R.; Ringel, Y. Characterization of the Fecal Microbiota Using High-Throughput Sequencing Reveals a Stable Microbial Community during Storage. PLoS ONE 2012, 7, e46953. [Google Scholar] [CrossRef]
- Choo, J.M.; Leong, L.E.; Rogers, G.B. Sample storage conditions significantly influence faecal microbiome profiles. Sci. Rep. 2015, 5, 16350. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.; Casella, G.; Simell, O.; Krischer, J.; Wasserfall, C.H.; Schatz, D.; Atkinson, M.A.; Neu, J.; Triplett, E.W. Influence of Fecal Sample Storage on Bacterial Community Diversity. Open Microbiol. J. 2009, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Zhou, N.; Gordon, J.I.; Knight, R.; Fierer, N. Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol. Lett. 2010, 307, 80–86. [Google Scholar] [CrossRef]
- Dominianni, C.; Wu, J.; Hayes, R.B.; Ahn, J. Comparison of methods for fecal microbiome biospecimen collection. BMC Microbiol. 2014, 14, 103. [Google Scholar] [CrossRef]
- Nel Van Zyl, K.; Whitelaw, A.C.; Newton-Foot, M. The effect of storage conditions on microbial communities in stool. PLoS ONE 2020, 15, e0227486. [Google Scholar] [CrossRef] [PubMed]
| Bacteria | Baseline Samples | After 2 Months of Supplementation | % Change in Relative Abundance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | |
| Firmicutes:Bactoidetes Ratio | 1.16 | 1.16 | 0.49 | 0.67 | 1.63 | 3.14 | 2.00 | 2.80 | 1.26 | 7.29 | 1.98 | 0.84 | 2.46 | 0.58 | 5.66 |
| Phylum: | |||||||||||||||
| Firmicutes | 32.94 | 31.95 | 4.59 | 28.73 | 39.12 | 46.84 | 47.58 | 10.38 | 33.45 | 58.75 | 13.90 | 12.87 | 8.95 | 4.72 | 25.16 |
| Bacteroidetes | 31.69 | 31.73 | 10.22 | 20.59 | 42.69 | 20.66 | 23.97 | 8.65 | 8.06 | 26.65 | −11.03 | −12.08 | 5.11 | −16.04 | −3.91 |
| Spirochaetes | 7.19 | 7.39 | 4.75 | 1.63 | 12.35 | 7.77 | 8.17 | 2.27 | 4.78 | 9.97 | −0.01 | −2.30 | 5.74 | −3.78 | 8.35 |
| Fibrobacteria | 2.4 | 1.52 | 2.53 | 0.44 | 6.11 | 0.10 | 0.10 | 0.04 | 0.04 | 0.14 | −2.30 | −1.40 | 2.56 | −6.07 | −0.33 |
| Proteobacteria | 9.18 | 10.19 | 3.17 | 4.64 | 11.69 | 9.17 | 9.04 | 3.36 | 5.62 | 12.99 | 0.58 | 1.75 | 4.02 | −5.01 | 3.84 |
| Tenericutes | 1.34 | 1.40 | 0.5 | 0.76 | 1.81 | 0.68 | 0.71 | 0.09 | 0.54 | 0.74 | −0.67 | −0.77 | 0.49 | −1.12 | −0.02 |
| Verrucomicrobia | 3.77 | 2.37 | 3.47 | 1.42 | 8.93 | 6.76 | 7.04 | 2.28 | 4.06 | 8.88 | 2.99 | 3.86 | 4.83 | −3.24 | 7.46 |
| Acidobacteria | 0.03 | 0.03 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | −0.02 | −0.02 | 0.02 | −0.04 | 0.00 |
| Synergistetes | 0.31 | 0.32 | 0.03 | 0.27 | 0.35 | 0.14 | 0.13 | 0.07 | 0.07 | 0.22 | −0.18 | −0.18 | 0.06 | −0.24 | −0.11 |
| Actinobacteria | 0.86 | 0.91 | 0.34 | 0.41 | 1.22 | 0.69 | 0.68 | 0.28 | 0.39 | 1.03 | −0.17 | −0.11 | 0.56 | −0.83 | 0.38 |
| Fusobacteria | 0.004 | 0.002 | 1.07 | 5.64 | 8.24 | 0.01 | 0.01 | 0.01 | 0.004 | 0.02 | 0.01 | 0.01 | 0.01 | −0.01 | 0.02 |
| Candidatus | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Unclassified phylum | 7.06 | 7.19 | 0.005 | 0.000 | 0.01 | 4.93 | 4.88 | 0.38 | 4.52 | 5.43 | |||||
| Class: | |||||||||||||||
| Clostridia | 28.37 | 27.51 | 4.07 | 24.4 | 34.06 | 42.35 | 42.33 | 9.09 | 31.28 | 53.46 | 13.98 | 11.40 | 8.62 | 6.88 | 26.24 |
| Erysipelotrichia | 1.02 | 0.75 | 0.83 | 0.36 | 2.23 | 1.71 | 1.72 | 0.80 | 0.73 | 2.68 | 0.69 | 0.63 | 1.16 | −0.48 | 1.99 |
| 10 Control Horses | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Baseline Samples | after 12 Weeks of Supplementation | % Change in Relative Abundance | ||||||||||||
| Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | |
| SSD | 2.82 | 2.82 | 0.12 | 2.67 | 3.04 | 2.90 | 2.97 | 0.23 | 2.41 | 3.14 | 3.24 | 4.14 | 11.19 | −16.87 | 17.67 |
| Number of Species | 697.20 * | 696.00 | 68.06 | 617.00 | 805.00 | 865.40 | 898.50 | 87.02 | 747.00 | 961.00 | 24.53 ##° | 23.34 ##° | 11.14 | 4.85 | 42.46 |
| Firmicutes:Bactoidetes Ratio | 1.80 | 1.73 | 0.80 | 0.79 | 3.38 | 2.20 | 1.79 | 1.40 | 0.81 | 5.99 | 33.28 | 14.95 | 63.56 | −63.03 | 140.61 |
| Phylum: | |||||||||||||||
| Firmicutes | 41.61 | 42.33 | 9.95 | 26.20 | 55.12 | 48.16 | 47.22 | 8.53 | 36.47 | 63.29 | 20.79 | 19.31 | 29.50 | −28.76 | 66.89 |
| Bacteroidetes | 25.37 | 25.60 | 6.69 | 16.31 | 36.75 | 25.34 | 24.53 # | 8.29 | 10.57 | 44.92 | 4.30 | −13.11 | 41.08 | −35.19 | 92.71 |
| Spirochaetes | 10.33 | 9.29 | 5.26 | 4.81 | 24.10 | 7.77 | 6.18 | 5.16 | 1.88 | 17.91 | −2.20 | −50.02 | 89.64 | −78.83 | 172.14 |
| Fibrobacteria | 6.45 | 3.74 | 6.79 | 1.42 | 23.90 | 5.29 | 4.09 | 4.47 | 1.25 | 16.33 | 68.21 | −27.37 | 188.67 | −86.57 | 466.89 |
| Proteobacteria | 4.02 | 3.75 | 0.84 | 3.06 | 5.70 | 2.81 | 2.26 | 1.82 | 1.43 | 7.46 | −24.57 | −50.82 | 56.05 | −74.91 | 112.54 |
| Tenericutes | 2.76 | 2.99 | 1.26 | 0.46 | 4.70 | 1.76 | 1.77 | 0.73 | 0.39 | 2.85 | −1.08 | −35.05 | 112.17 | −89.46 | 304.35 |
| Verrucomicrobia | 2.06 | 0.99 | 2.15 | 0.23 | 7.24 | 1.97 | 0.93 | 2.65 | 0.36 | 9.12 | 17.67 | −5.72 | 84.79 | −72.65 | 164.35 |
| Acidobacteria | 0.66 | 0.68 | 0.13 | 0.49 | 0.89 | 0.48 | 0.33 | 0.37 | 0.18 | 1.31 | −25.05 | −48.89 | 56.28 | −74.33 | 74.67 |
| Synergistetes | 0.61 | 0.45 | 0.45 | 0.00 | 1.41 | 0.67 | 0.54 | 0.36 | 0.25 | 1.37 | 20.75 | 10.47 | 68.04 | −72.48 | 147.31 |
| Fusobacteria | 0.07 | 0.04 | 0.05 | 0.03 | 0.16 | 0.41 | 0.13 | 0.85 | 0.01 | 2.80 | 844.31 | 103.13 | 2095.05 | −95.97 | 6729.27 |
| Actinobacteria | 0.28 ** | 0.25 | 0.08 | 0.21 | 0.49 | 0.35 | 0.33 | 0.12 | 0.22 | 0.60 | 36.93 | 27.46 | 63.90 | −53.47 | 147.92 |
| Candidatus | 0.45 | 0.38 | 0.31 | 0.09 | 1.23 | 0.13 | 0.10 | 0.08 | 0.04 | 0.31 | −57.18 ° | −77.51 ° | 39.39 | −93.06 | 35.11 |
| Unclassified_phylum | 4.43 | 4.42 | 0.95 | 3.12 | 6.46 | 4.14 | 3.59 | 1.55 | 2.65 | 7.44 | −0.78 | −20.08 | 54.00 | −44.91 | 138.54 |
| Class: | |||||||||||||||
| Clostridia | 29.50 | 31.70 | 6.29 | 20.54 | 37.72 | 37.42 | 36.19 | 10.76 | 22.96 | 59.19 | 31.98 ° | 39.52 ° | 40.98 | −36.39 | 82.06 |
| Erysipelotrichia | 6.73 | 6.70 | 3.79 | 1.59 | 14.34 | 4.80 | 4.46 | 2.92 | 1.50 | 11.34 | 13.81 | −20.65 | 105.33 | −87.71 | 196.91 |
| 10 Treated Horses | |||||||||||||||
| SSD | 2.87 | 2.91 | 0.16 | 2.60 | 3.10 | 2.97 | 2.97 | 0.09 | 2.83 | 3.15 | 3.57 | 3.83 | 5.07 | −4.17 | 9.61 |
| Number of Species | 801.40 * | 791.50 | 89.48 | 687 | 934 | 843.10 | 817.50 | 168.99 | 590 | 1098 | 5.88 ## | −0.52 ## | 22.36 | −22.47 | 44.10 |
| Firmicutes:Bactoidetes Ratio | 1.77 | 1.85 | 0.63 | 0.77 | 3.11 | 3.14 | 2.68 | 1.54 | 1.37 | 6.02 | 84.74 °° | 70.79 °° | 87.19 | −4.23 | 295.36 |
| Phylum: | |||||||||||||||
| Firmicutes | 42.36 | 44.66 | 6.83 | 29.49 | 50.70 | 51.16 | 50.39 | 6.86 | 41.37 | 64.94 | 22.85 °° | 20.19 °° | 20.19 | −2.06 | 63.58 |
| Bacteroidetes | 25.75 | 23.89 | 6.47 | 16.29 | 40.41 | 19.05 | 19.31 # | 6.53 | 8.88 | 30.20 | −24.57 °° | −24.93 °° | 22.43 | −70.31 | 3.21 |
| Spirochaetes | 8.54 | 8.37 | 2.43 | 5.45 | 13.42 | 6.98 | 7.47 | 4.00 | 1.48 | 13.64 | −17.44 | −31.14 | 49.81 | −76.91 | 58.24 |
| Fibrobacteria | 6.63 | 5.62 | 4.80 | 1.32 | 14.11 | 5.82 | 2.56 | 7.44 | 0.98 | 23.72 | 54.00 | −52.46 | 209.45 | −85.33 | 531.36 |
| Proteobacteria | 3.87 | 3.12 | 1.92 | 2.01 | 7.51 | 4.42 | 3.32 | 2.78 | 1.62 | 9.75 | 25.05 | 3.55 | 72.36 | −54.14 | 152.59 |
| Tenericutes | 3.02 | 3.21 | 1.16 | 1.60 | 5.30 | 2.47 | 1.67 | 2.75 | 0.12 | 9.48 | −23.68 | −39.86 | 75.14 | −94.28 | 171.63 |
| Verrucomicrobia | 2.19 | 1.92 | 1.46 | 0.64 | 4.76 | 1.65 | 1.33 | 1.30 | 0.26 | 3.77 | 20.25 | −21.23 | 110.29 | −94.45 | 180.62 |
| Acidobacteria | 0.74 | 0.57 | 0.53 | 0.36 | 2.17 | 0.75 | 0.57 | 0.48 | 0.24 | 1.63 | 31.38 | −10.42 | 102.95 | −72.26 | 251.29 |
| Synergistetes | 0.61 | 0.63 | 0.18 | 0.29 | 0.93 | 0.96 | 0.87 | 0.37 | 0.58 | 1.68 | 72.03 | 12.57 * | 113.41 | −18.05 | 323.79 |
| Fusobacteria | 0.07 | 0.07 | 0.04 | 0.02 | 0.17 | 0.53 | 0.14 | 0.71 | 0.01 | 1.82 | 1252.22 | 130.01 | 2124.69 | −92.35 | 5725.00 |
| Actinobacteria | 0.38 ** | 0.37 | 0.11 | 0.21 | 0.56 | 0.51 | 0.42 | 0.29 | 0.30 | 1.25 | 36.53 | 8.29 °° | 57.12 | −24.69 | 150.00 |
| Candidatus | 0.38 | 0.33 | 0.16 | 0.19 | 0.66 | 0.17 | 0.13 | 0.21 | 0.00 | 0.73 | −29.11 | −55.17 | 113.81 | −99.26 | 284.21 |
| UnC_Phylum | 4.47 | 4.62 | 0.81 | 3.18 | 5.58 | 4.61 | 4.48 | 1.13 | 3.17 | 6.94 | 6.77 | 4.36 | 32.51 | −40.50 | 54.65 |
| Class: | |||||||||||||||
| Clostridia | 30.89 | 32.95 | 8.08 | 20.26 | 45.60 | 42.02 | 41.69 | 8.63 | 29.88 | 59.98 | 40.24 °° | 35.61 °° | 25.86 | −8.18 | 78.54 |
| Erysipelotrichia | 5.96 | 4.93 | 2.95 | 2.29 | 13.03 | 3.40 | 3.80 | 1.69 | 0.37 | 6.01 | −37.28 °° | −37.99 °° | 33.61 | −94.12 | 28.78 |
| Group | 1st Definition of Change * | Total | |
| Yes | No | ||
| W = Treatment | 8 | 2 | 10 |
| X = Control | 2 | 8 | 10 |
| Total | 10 | 10 | 20 |
| Group | 2nd Definition of Change ** | Total | |
| Yes | No | ||
| W = Treatment | 8 | 2 | 10 |
| X = Control | 0 | 10 | 10 |
| Total | 10 | 10 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, V.J.; LeBlanc, N.; Penell, J. Results of a Clinical Trial Showing Changes to the Faecal Microbiome in Racing Thoroughbreds after Feeding a Nutritional Supplement. Vet. Sci. 2023, 10, 27. https://doi.org/10.3390/vetsci10010027
Adams VJ, LeBlanc N, Penell J. Results of a Clinical Trial Showing Changes to the Faecal Microbiome in Racing Thoroughbreds after Feeding a Nutritional Supplement. Veterinary Sciences. 2023; 10(1):27. https://doi.org/10.3390/vetsci10010027
Chicago/Turabian StyleAdams, Vicki J., Neil LeBlanc, and Johanna Penell. 2023. "Results of a Clinical Trial Showing Changes to the Faecal Microbiome in Racing Thoroughbreds after Feeding a Nutritional Supplement" Veterinary Sciences 10, no. 1: 27. https://doi.org/10.3390/vetsci10010027
APA StyleAdams, V. J., LeBlanc, N., & Penell, J. (2023). Results of a Clinical Trial Showing Changes to the Faecal Microbiome in Racing Thoroughbreds after Feeding a Nutritional Supplement. Veterinary Sciences, 10(1), 27. https://doi.org/10.3390/vetsci10010027





