Bioinformatics Analysis Identifying Key Biomarkers in Bladder Cancer
Abstract
1. Summary
2. Data Description
2.1. Database Analyses
2.2. Literature Research
3. Methods
3.1. Data Source Identification and Data Mining
3.2. Acquisition of the Hub Genes
3.3. Functional and Clinical Analysis of the Data
3.4. Literature Retrieval and Oncomine Meta-Analysis
4. User Notes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulkarni, G.S.; Black, P.C.; Sridhar, S.S.; Kapoor, A.; Zlotta, A.R.; Shayegan, B.; Rendon, R.A.; Chung, P.; van der Kwast, T.; Alimohamed, N.; et al. Canadian Urological Association guideline: Muscle-invasive bladder cancer. Can. Urol. Assoc. J. 2019, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; Greenberg, R.E.; et al. NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J. Natl. Compr. Cancer Netw. JNCCN 2018, 16, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Comperat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) 2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M.S.; Christodouleas, J.P.; Smith, A.; Abdallah, A.; William, H.; Khaled, H.M.; Hwang, W.T.; Baumann, B.C. Adjuvant Sandwich Chemotherapy Plus Radiotherapy vs. Adjuvant Chemotherapy Alone for Locally Advanced Bladder Cancer After Radical Cystectomy: A Randomized Phase 2 Trial. JAMA Surg. 2018, 153, e174591. [Google Scholar] [CrossRef]
- Berndt-Paetz, M.; Weimann, A.; Sieger, N.; Schastak, S.; Riyad, Y.M.; Griebel, J.; Arthanareeswaran, V.K.A.; Stolzenburg, J.U.; Neuhaus, J. Tetrahydroporphyrin-tetratosylat (THPTS): A near-infrared photosensitizer for targeted and efficient photodynamic therapy (PDT) of human bladder carcinoma. An in vitro study. Photodiagnosis Photodyn. Ther. 2017, 18, 244–251. [Google Scholar] [CrossRef]
- Kutwin, P.; Konecki, T.; Cichocki, M.; Falkowski, P.; Jablonowski, Z. Photodynamic Diagnosis and Narrow-Band Imaging in the Management of Bladder Cancer: A Review. Photomed. Laser Surg. 2017, 35, 459–464. [Google Scholar] [CrossRef]
- Power, N.E.; Izawa, J. Comparison of Guidelines on Non-Muscle Invasive Bladder Cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer (Amst. Neth.) 2016, 2, 27–36. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Guo, Q.; Zhang, S.; Zhao, Y.; Wang, H.; Li, T.; Gong, Y.; Wang, Y.; Zhang, T.; et al. Increased expression of TRIP13 drives the tumorigenesis of bladder cancer in association with the EGFR signaling pathway. Int. J. Biol. Sci. 2019, 15, 1488–1499. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Chen, M.; Wang, S.; Wen, X.; Zhang, S. Identification of key candidate genes and biological pathways in bladder cancer. PeerJ 2018, 6, e6036. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Zhang, C.; Berndt-Paetz, M.; Neuhaus, J. Identification of Key Biomarkers in Bladder Cancer: Evidence from a Bioinformatics Analysis. Diagnostics 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, K.; Czerwinska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. (Pozn. Pol.) 2015, 19, A68–A77. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, L.; Chen, J.; Zhong, Y.; Dai, Z. Identification of potential crucial genes and construction of microRNA-mRNA negative regulatory networks in osteosarcoma. Hereditas 2018, 155, 21. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics (Oxf. Engl.) 2008, 24, 282–284. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, J.; Chen, G.; Li, M.; Wu, F.X.; Pan, Y. ClusterViz: A Cytoscape APP for Cluster Analysis of Biological Network. IEEE/ACM Trans. Comput. Biol. Bioinform. 2015, 12, 815–822. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics (Oxf. Engl.) 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Zaravinos, A.; Lambrou, G.I.; Boulalas, I.; Delakas, D.; Spandidos, D.A. Identification of common differentially expressed genes in urinary bladder cancer. PLoS ONE 2011, 6, e18135. [Google Scholar] [CrossRef]
- Zaravinos, A.; Lambrou, G.I.; Volanis, D.; Delakas, D.; Spandidos, D.A. Spotlight on differentially expressed genes in urinary bladder cancer. PLoS ONE 2011, 6, e18255. [Google Scholar] [CrossRef]
- Borisov, N.; Tkachev, V.; Suntsova, M.; Kovalchuk, O.; Zhavoronkov, A.; Muchnik, I.; Buzdin, A. A method of gene expression data transfer from cell lines to cancer patients for machine-learning prediction of drug efficiency. Cell Cycle (Georget. Tex.) 2018, 17, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lin, T.; He, W.; Han, J.; Zhu, D.; Hu, K.; Li, W.; Zheng, Z.; Huang, J.; Xie, W. Knockdown of a novel lincRNA AATBC suppresses proliferation and induces apoptosis in bladder cancer. Oncotarget 2015, 6, 1064–1078. [Google Scholar] [CrossRef]
- Lu, M.; Ge, Q.; Wang, G.; Luo, Y.; Wang, X.; Jiang, W.; Liu, X.; Wu, C.L.; Xiao, Y.; Wang, X. CIRBP is a novel oncogene in human bladder cancer inducing expression of HIF-1alpha. Cell Death Dis. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, G.; Peng, J.; Qian, G.; Jiang, W.; Xie, C.; Xiao, Y.; Wang, X. Knockdown of SIRT1 Suppresses Bladder Cancer Cell Proliferation and Migration and Induces Cell Cycle Arrest and Antioxidant Response through FOXO3a-Mediated Pathways. Biomed. Res. Int. 2017, 2017, 3781904. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhong, G.; Jiang, N.; Wang, B.; Fan, X.; Chen, C.; Chen, X.; Huang, J.; Lin, T. Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. J. Clin. Investig. 2018, 128, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG database. Novartis Found. Symp. 2002, 247, 91–101. [Google Scholar]
- Amin, M.B. Histological variants of urothelial carcinoma: Diagnostic, therapeutic and prognostic implications. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2009, 22 (Suppl. 2), S96–S118. [Google Scholar] [CrossRef]
- Han, Y.; Jin, X.; Zhou, H.; Liu, B. Identification of key genes associated with bladder cancer using gene expression profiles. Oncol. Lett. 2018, 15, 297–303. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, L.; Song, Z.; Xiong, M.; Zhang, Y.; Yang, Y.; Chen, K.; Chen, Z. The identification of new biomarkers for bladder cancer: A study based on TCGA and GEO datasets. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Jia, Z.; Ai, X.; Sun, F.; Zang, T.; Guan, Y.; Gao, F. Identification of new hub genes associated with bladder carcinoma via bioinformatics analysis. Tumori 2015, 101, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zheng, Q.; Tian, Y.; Ji, Z.; Ye, H. Identification of a nine-gene panel as a prognostic indicator for recurrence with muscle-invasive bladder cancer. J. Surg. Oncol. 2019, 119, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Yuan, P.C. Molecular network-based identification of competing endogenous RNAs in bladder cancer. PLoS ONE 2019, 14, e0220118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, L.; Zang, Y.; Xu, Z. Identification of Core Genes and Key Pathways via Integrated Analysis of Gene Expression and DNA Methylation Profiles in Bladder Cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 3024–3033. [Google Scholar] [CrossRef]
- Blaveri, E.; Simko, J.P.; Korkola, J.E.; Brewer, J.L.; Baehner, F.; Mehta, K.; Devries, S.; Koppie, T.; Pejavar, S.; Carroll, P.; et al. Bladder cancer outcome and subtype classification by gene expression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 4044–4055. [Google Scholar] [CrossRef]
- Dyrskjot, L.; Kruhoffer, M.; Thykjaer, T.; Marcussen, N.; Jensen, J.L.; Moller, K.; Orntoft, T.F. Gene expression in the urinary bladder: A common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004, 64, 4040–4048. [Google Scholar] [CrossRef]
- Lee, J.S.; Leem, S.H.; Lee, S.Y.; Kim, S.C.; Park, E.S.; Kim, S.B.; Kim, S.K.; Kim, Y.J.; Kim, W.J.; Chu, I.S. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2660–2667. [Google Scholar] [CrossRef]
- Modlich, O.; Prisack, H.B.; Pitschke, G.; Ramp, U.; Ackermann, R.; Bojar, H.; Vogeli, T.A.; Grimm, M.O. Identifying superficial, muscle-invasive, and metastasizing transitional cell carcinoma of the bladder: Use of cDNA array analysis of gene expression profiles. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 3410–3421. [Google Scholar] [CrossRef]
- Sanchez-Carbayo, M.; Socci, N.D.; Lozano, J.; Saint, F.; Cordon-Cardo, C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 778–789. [Google Scholar] [CrossRef]
- Li, L.; Lei, Q.; Zhang, S.; Kong, L.; Qin, B. Screening and identification of key biomarkers in hepatocellular carcinoma: Evidence from bioinformatic analysis. Oncol. Rep. 2017, 38, 2607–2618. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef] [PubMed]

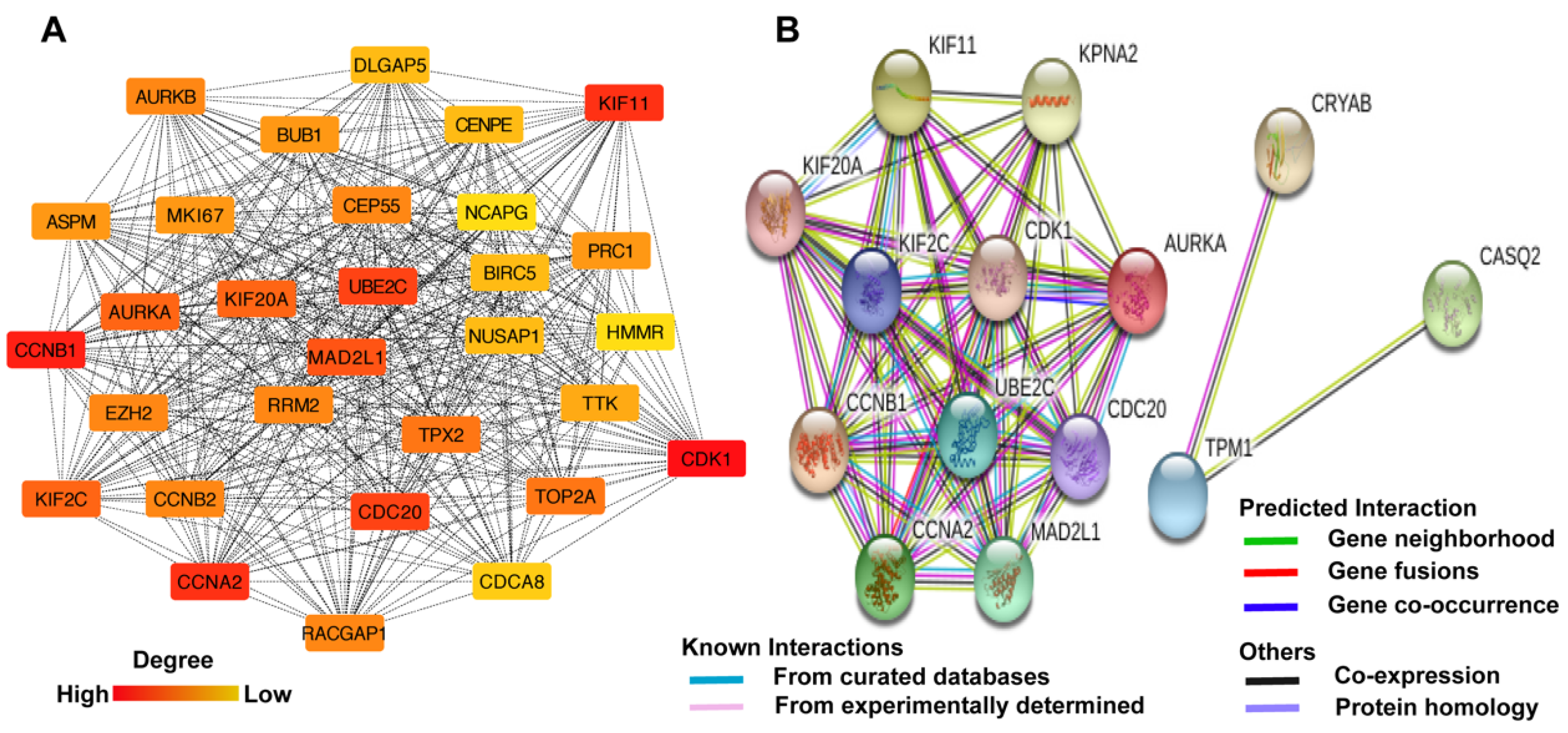
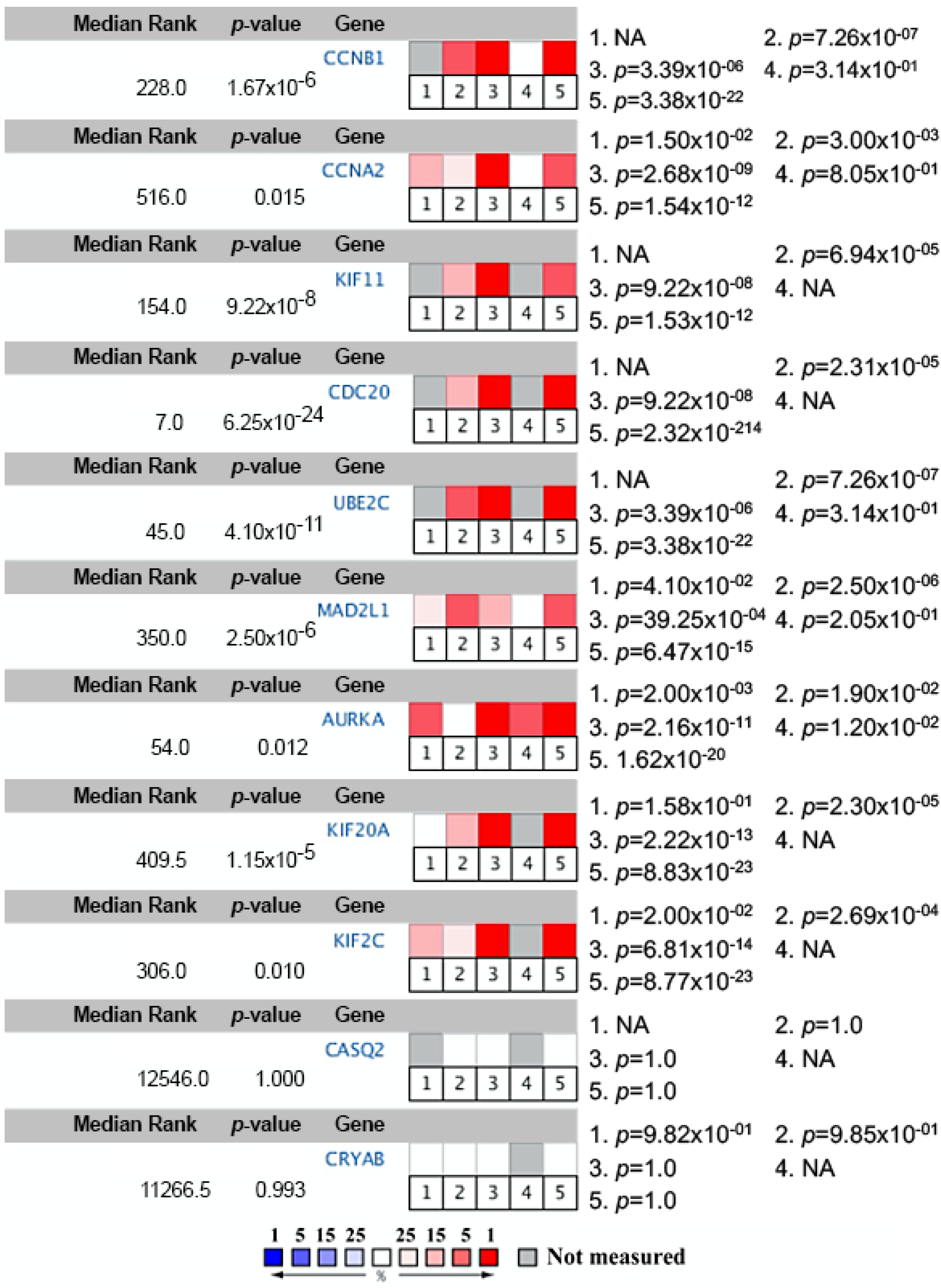
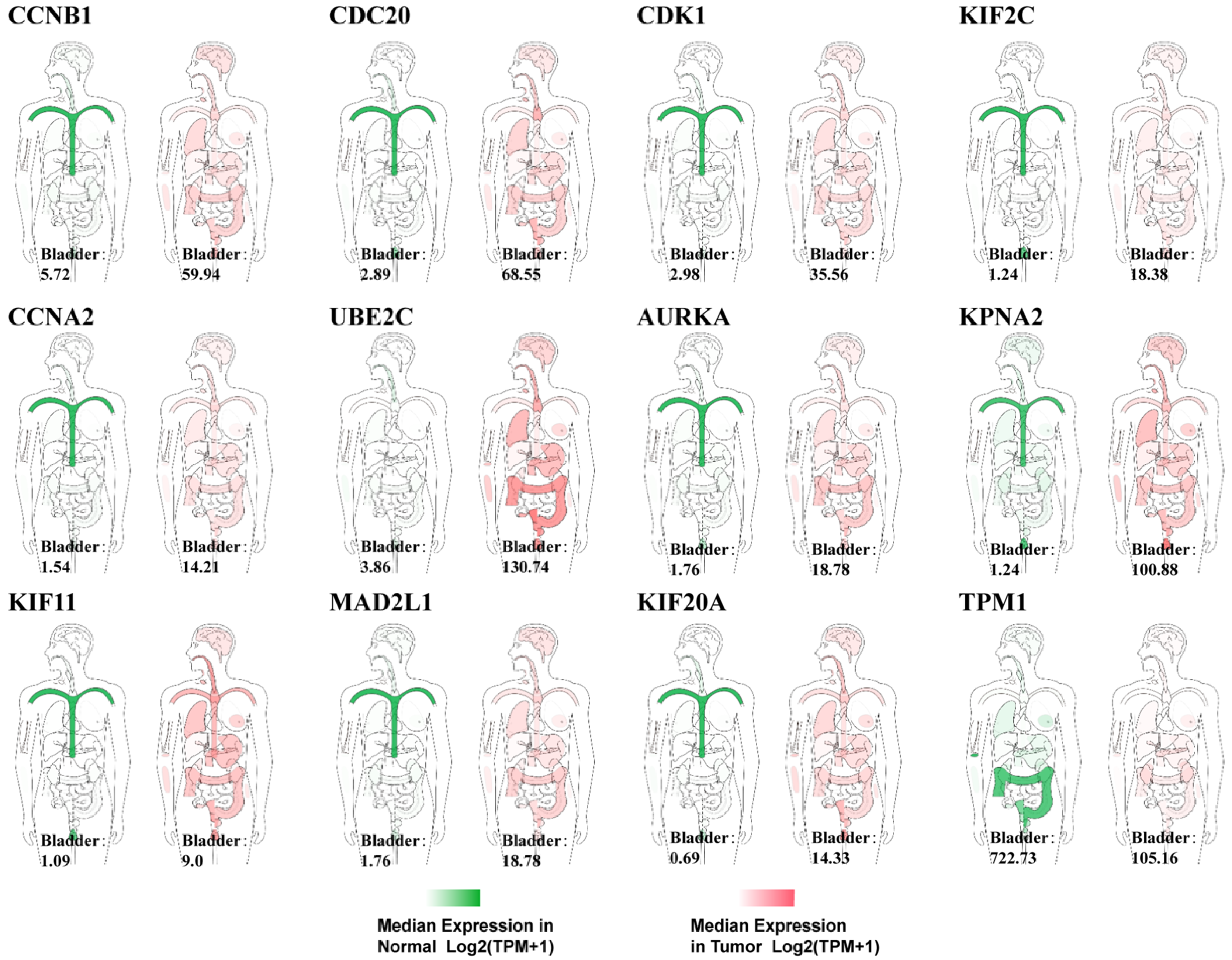
| Dataset | Number of Noncancerous Bladder Tissue Samples | Number of Cancer Tissue Samples | Number of DEGs Extracted from Dataset | Number of DEGs after FUNRICH Mapping |
|---|---|---|---|---|
| GSE27448 [19,20] | 5 | 10 | 5251 | 4701 |
| GSE52519 [21] | 3 | 9 | 751 | 742 |
| GSE61615 [22] | 2 | 2 | 842 | 736 |
| GSE76211 [23,24] | 3 | 3 | 770 | 658 |
| GSE100926 [25] | 3 | 3 | 223 | 194 |
| TCGA-BLCA [13] | 19 | 406 | 2873 | 2537 |
| Target Genes | Race of Patients | Gender of Patients | Histological Subtypes | Molebular Subtypes | ||||
|---|---|---|---|---|---|---|---|---|
| CDK1 | CAU (↑) vs. ASI | p = 8.829 × 10−4 | N vs. M (↑) | p < 1.000 × 10−12 | N vs. PT (↑) | p = 2.109 × 10−15 | N vs. NET (↑) | p = 5.922 × 10−8 |
| AFA (↑) vs. ASI | p = 3.379 × 10−3 | N vs. F (↑) | p = 1.554 × 10−15 | N vs. NPT (↑) | p = 1.624 × 10−12 | N vs. BST (↑) | p = 1.624 × 10−12 | |
| PT vs. NPT (↑) | p = 3.872 × 10−2 | N vs. LT (↑) | p = 5.809 × 10−7 | |||||
| N vs. LIT (↑) | p = 2.824 × 10−12 | |||||||
| N vs. LPT (↑) | p = 1.625 × 10−12 | |||||||
| CCNB1 | CAU (↑) vs. ASI | p = 7.479 × 10−7 | N vs. M (↑) | p = 5.311 × 10−13 | N vs. PT (↑) | p = 2.949 × 10−10 | N vs. NET (↑) | p = 1.620 × 10−5 |
| AFA (↑) vs. ASI | p = 5.221 × 10−4 | N vs. F (↑) | p = 2.907 × 10−12 | N vs. NPT (↑) | p = 2.631 × 10−14 | N vs. BST (↑) | p = 1.624 × 10−12 | |
| PT vs. NPT (↑) | p = 2.944 × 10−3 | N vs. LT (↑) | p = 2.202 × 10−4 | |||||
| N vs. LIT (↑) | p = 4.952 × 10−7 | |||||||
| N vs. LPT (↑) | p = 3.502 × 10−9 | |||||||
| CCNA2 | CAU (↑) vs. ASI | p = 5.447 × 10−8 | N vs. M (↑) | p = 5.311 × 10−9 | N vs. PT (↑) | p = 4.309 × 10−7 | N vs. NET (↑) | p = 5.481 × 10−7 |
| AFA (↑) vs. ASI | p = 9.961 × 10−4 | N vs. F (↑) | p = 3.062 × 10−8 | N vs. NPT (↑) | p = 4.395 × 10−10 | N vs. BST (↑) | p = 1.863 × 10−12 | |
| PT vs. NPT (↑) | p = 6.169 × 10−4 | N vs. LT (↑) | p = 2.176 × 10−4 | |||||
| N vs. LIT (↑) | p = 6.473 × 10−5 | |||||||
| N vs. LPT (↑) | p = 4.175 × 10−6 | |||||||
| KIF11 | CAU (↑) vs. ASI | p = 5.620 × 10−7 | N vs. M (↑) | p = 5.836 × 10−9 | N vs. PT (↑) | p = 8.989 × 10−8 | N vs. NET (↑) | p = 4.693 × 10−7 |
| AFA (↑) vs. ASI | p = 1.065 × 10−6 | N vs. F (↑) | p = 1.036 × 10−7 | N vs. NPT (↑) | p = 9.950 × 10−10 | N vs. BST (↑) | p = 2.290 × 10−13 | |
| PT vs. NPT (↑) | p = 6.997 × 10−3 | N vs. LT (↑) | p = 1.922 × 10−5 | |||||
| N vs. LIT (↑) | p = 4.399 × 10−5 | |||||||
| N vs. LPT (↑) | p = 5.848 × 10−7 | |||||||
| CDC20 | CAU (↑) vs. ASI | p = 5.038 × 10−3 | N vs. M (↑) | p < 1.000 × 10−12 | N vs. PT (↑) | p = 1.691 × 10−12 | N vs. NET | p = 2.644 × 10−8 |
| AFA vs. ASI | p = 3.077 × 10−3 | N vs. F (↑) | p < 1.000 × 10−12 | N vs. NPT (↑) | p < 1.000 × 10−12 | N vs. BST | p < 1.000 × 10−12 | |
| PT vs. NPT (↑) | p = 9.644 × 10−5 | N vs. LT | p = 8.558 × 10−8 | |||||
| N vs. LIT | p = 2.198 × 10−11 | |||||||
| N vs. LPT | p = 3.194 × 10−14 | |||||||
| UBE2C | CAU (↑) vs. ASI | p = 6.099 × 10−3 | N vs. M (↑) | p < 1.000 × 10−12 | N vs. PT (↑) | p = 1.624 × 10−12 | N vs. NET (↑) | p = 1.045 × 10−8 |
| N vs. F (↑) | p < 1.000 × 10−12 | N vs. NPT (↑) | p = 1.624 × 10−12 | N vs. BST (↑) | p = 1.624 × 10−12 | |||
| PT vs. NPT (↑) | p = 1.429 × 10−2 | N vs. LT (↑) | p = 1.664 × 10−10 | |||||
| N vs. LIT (↑) | p = 1.497 × 10−9 | |||||||
| N vs. LPT (↑) | p = 1.624 × 10−12 | |||||||
| MAD2L1 | CAU (↑) vs. ASI | p = 1.627 × 10−7 | N vs. M (↑) | p = 4.241 × 10−14 | N vs. PT (↑) | p = 1.634 × 10−10 | N vs. NET (↑) | p = 4.815 × 10−7 |
| AFA (↑) vs. ASI | p = 4.381 × 10−4 | N vs. F (↑) | p = 1.488 × 10−12 | N vs. NPT (↑) | p = 1.625 × 10−12 | N vs. BST (↑) | p < 1.000 × 10−12 | |
| PT vs. NPT (↑) | p = 3.153 × 10−4 | N vs. LT (↑) | p = 1.755 × 10−6 | |||||
| N vs. LIT (↑) | p = 1.364 × 10−7 | |||||||
| N vs. LPT (↑) | p = 1.625 × 10−10 | |||||||
| AURKA | CAU (↑) vs. ASI | p = 1.565 × 10−7 | N vs. M (↑) | p < 1.000 × 10−12 | N vs. PT (↑) | p = 6.550 × 10−15 | N vs. NET (↑) | p = 1.539 × 10−7 |
| AFA (↑) vs. ASI | p = 1.629 × 10−4 | N vs. F (↑) | p < 1.000 × 10−12 | N vs. NPT (↑) | p < 1.000 × 10−12 | N vs. BST (↑) | p < 1.000 × 10−12 | |
| PT vs. NPT (↑) | p = 2.996 × 10−3 | N vs. LT (↑) | p = 2.259 × 10−10 | |||||
| N vs. LIT (↑) | p = 1.487 × 10−11 | |||||||
| N vs. LPT (↑) | p = 1.674 × 10−12 | |||||||
| KIF20A | CAU (↑) vs. ASI | p = 6.312 × 10−7 | N vs. M (↑) | p = 4.398 × 10−8 | N vs. PT (↑) | p = 2.500 × 10−7 | N vs. NET (↑) | p = 4.343 × 10−7 |
| AFA (↑) vs. ASI | p = 1.179 × 10−4 | N vs. F (↑) | p = 1.725 × 10−6 | N vs. NPT (↑) | p = 7.345 × 10−9 | N vs. BST (↑) | p = 1.373 × 10−9 | |
| N vs. LT (↑) | p = 7.949 × 10−5 | |||||||
| N vs. LIT (↑) | p = 8.399 × 10−4 | |||||||
| N vs. LPT (↑) | p = 7.846 × 10−7 | |||||||
| KIF2C | CAU (↑) vs. ASI | p = 1.844 × 10−5 | N vs. M (↑) | p = 1.624 × 10−12 | N vs. PT (↑) | p = 7.327 × 10−15 | N vs. NET (↑) | p = 1.387 × 10−7 |
| AFA (↑) vs. ASI | p = 1.828 × 10−5 | N vs. F (↑) | p < 1.000 × 10−12 | N vs. NPT (↑) | p = 1.624 × 10−12 | N vs. BST (↑) | p = 1.624 × 10−12 | |
| PT vs. NPT (↑) | p = 2.143 × 10−3 | N vs. LT (↑) | p = 1.563 × 10−10 | |||||
| N vs. LIT (↑) | p = 4.494 × 10−13 | |||||||
| N vs. LPT (↑) | p = 1.625 × 10−12 | |||||||
| KPNA2 | CAU (↑) vs. ASI | p = 2.028 × 10−8 | N vs. M (↑) | p = 1.364 × 10−11 | N vs. PT (↑) | p = 2.543 × 10−9 | N vs. NET (↑) | p = 1.269 × 10−8 |
| AFA (↑) vs. ASI | p = 1.019 × 10−4 | N vs. F (↑) | p = 2.920 × 10−11 | N vs. NPT (↑) | p = 2.059 × 10−12 | N vs. BST (↑) | p = 1.626 × 10−12 | |
| PT vs. NPT (↑) | p = 3.995 × 10−4 | N vs. LT (↑) | p = 6.761 × 10−7 | |||||
| N vs. LIT (↑) | p = 9.536 × 10−8 | |||||||
| N vs. LPT (↑) | p = 5.829 × 10−8 | |||||||
| TPM1 | CAU (↑) vs. ASI | p = 2.199 × 10−11 | N vs. M (↓) | p = 3.825 × 10−3 | N vs. PT (↓) | p = 3.180 × 10−3 | N vs. NET (↓) | p = 3.638 × 10−3 |
| AFA (↑) vs. ASI | p = 7.136 × 10−3 | N vs. F (↓) | p = 4.022 × 10−3 | N vs. NPT (↓) | p = 4.757 × 10−3 | N vs. BST (↓) | p = 4.404 × 10−3 | |
| PT vs. NPT (↑) | p = 1.321 × 10−6 | N vs. LT (↓) | p = 3.798 × 10−3 | |||||
| N vs. LIT (↓) | p = 7.343 × 10−3 | |||||||
| N vs. LPT (↓) | p = 2.404 × 10−3 | |||||||
| CASQ2 | CAU (↑) vs. ASI | p = 2.406 × 10−7 | N vs. M (↓) | p = 4.142 × 10−3 | N vs. PT (↓) | p = 3.699 × 10−3 | N vs. NET (↓) | p = 3.638 × 10−3 |
| AFA (↑) vs. ASI | p = 4.344 × 10−2 | N vs. F (↓) | p = 3.979 × 10−3 | N vs. NPT (↓) | p = 4.741 × 10−3 | N vs. BST (↓) | p = 4.404 × 10−3 | |
| CAU vs. AFA (↑) | p = 2.058 × 10−2 | PT vs. NPT (↑) | p = 1.379 × 10−3 | N vs. LT (↓) | p = 3.798 × 10−3 | |||
| N vs. LIT (↓) | p = 7.343 × 10−3 | |||||||
| N vs. LPT (↓) | p = 2.404 × 10−3 | |||||||
| CRYAB | CAU (↑) vs. ASI | p = 4.492 × 10−8 | PT vs. NPT (↑) | p = 4.366 × 10−4 | ||||
| AFA (↑) vs. ASI | p = 1.972 × 10−2 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Berndt-Paetz, M.; Neuhaus, J. Bioinformatics Analysis Identifying Key Biomarkers in Bladder Cancer. Data 2020, 5, 38. https://doi.org/10.3390/data5020038
Zhang C, Berndt-Paetz M, Neuhaus J. Bioinformatics Analysis Identifying Key Biomarkers in Bladder Cancer. Data. 2020; 5(2):38. https://doi.org/10.3390/data5020038
Chicago/Turabian StyleZhang, Chuan, Mandy Berndt-Paetz, and Jochen Neuhaus. 2020. "Bioinformatics Analysis Identifying Key Biomarkers in Bladder Cancer" Data 5, no. 2: 38. https://doi.org/10.3390/data5020038
APA StyleZhang, C., Berndt-Paetz, M., & Neuhaus, J. (2020). Bioinformatics Analysis Identifying Key Biomarkers in Bladder Cancer. Data, 5(2), 38. https://doi.org/10.3390/data5020038






