Isolation, Characterization, and Agent-Based Modeling of Mesenchymal Stem Cells in a Bio-construct for Myocardial Regeneration Scaffold Design †
Abstract
1. Introduction
2. Methods
2.1. In Vitro Experiments
2.1.1. MSCs Isolation
2.1.2. Cell Culture and Differentiation
2.1.3. Cell Seeding
2.1.4. Cell Viability Scaffolds
2.1.5. Fluorescent Labeling
2.1.6. Cell Migration Scaffolds
2.1.7. Cell Viability Assay
2.1.8. Cell Migration Assay
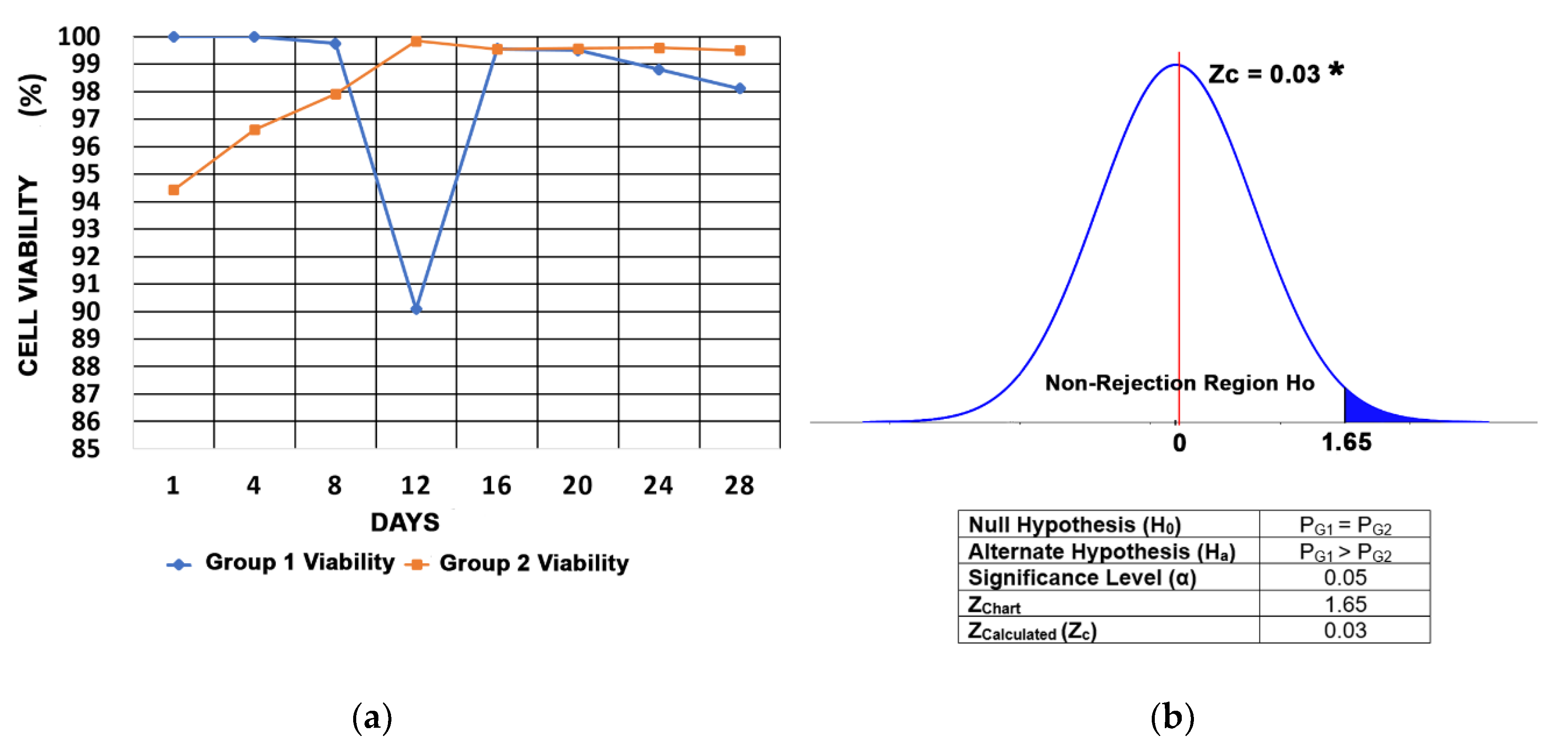
2.1.9. Statistical Analysis
2.2. In Silico Approach: Agent-Based Modeling
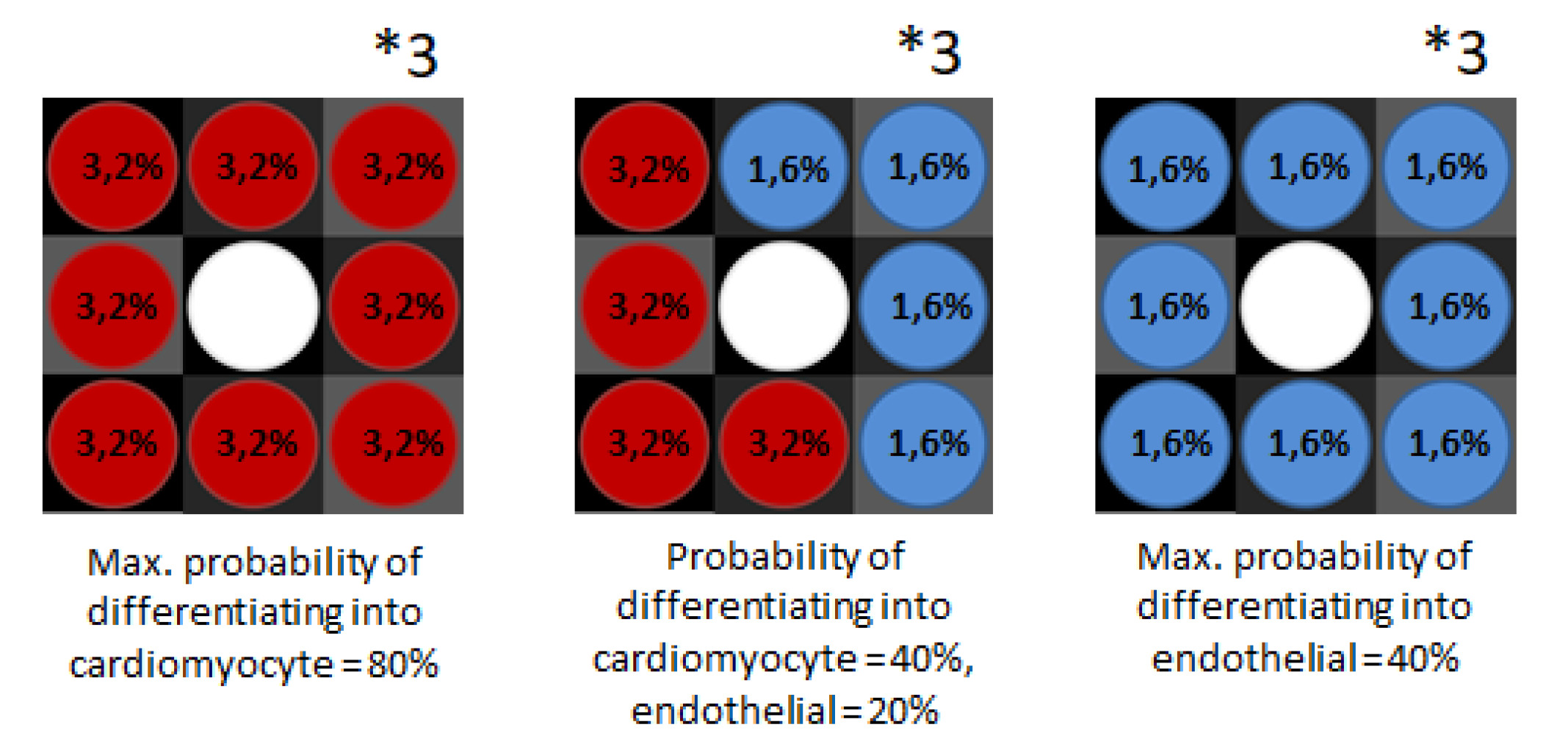
2.2.1. Agents’ Attributes
2.2.2. ‘Setup’ Method
2.2.3. ‘Go’ Method
2.2.4. Cellular Interactions Put in Methods
3. Results
3.1. MSCs Isolation From Rat Bone Marrow
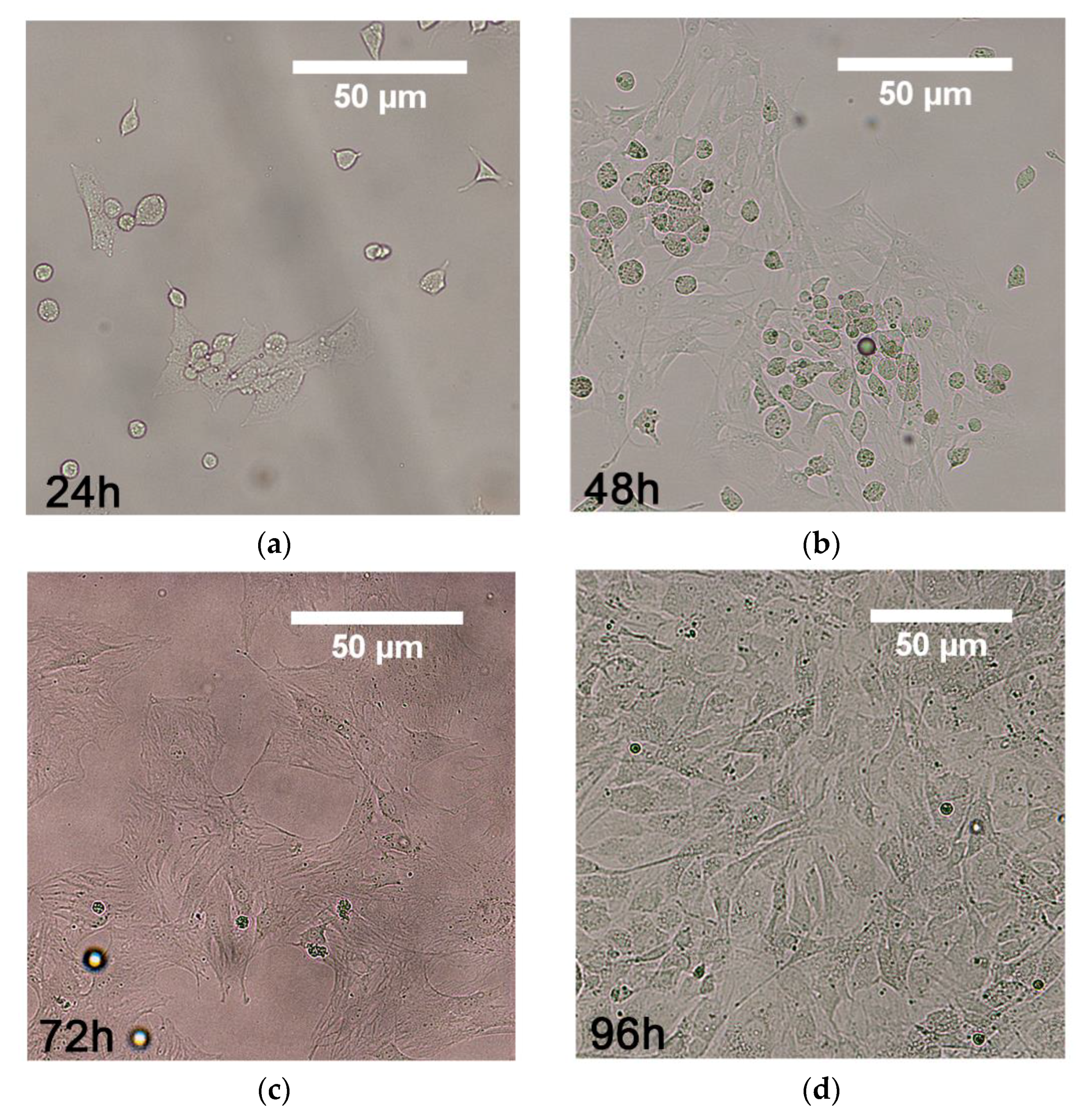
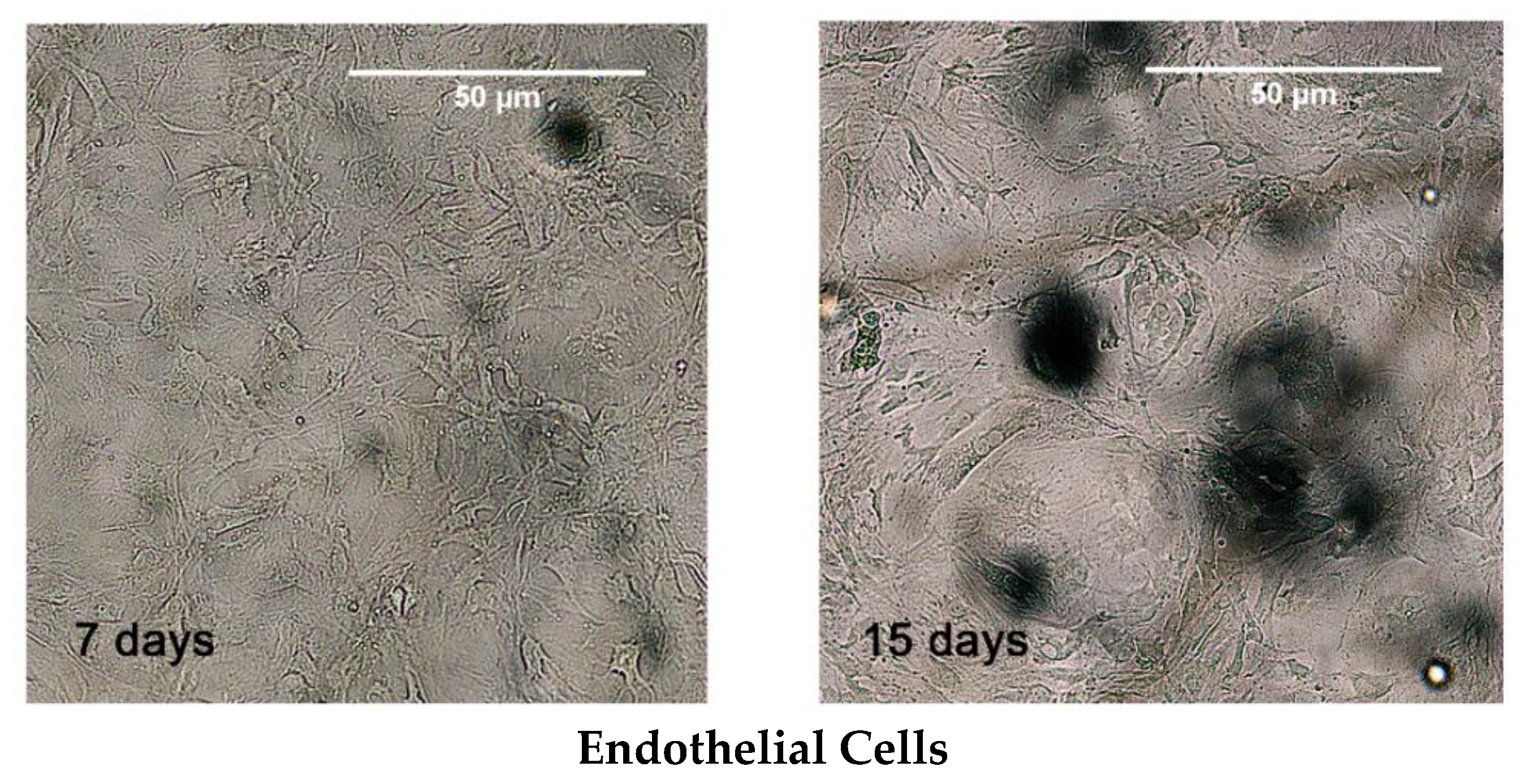
3.2. MSCs Culture and Differentiation
3.3. Cell Seeding
3.4. Cell Viability
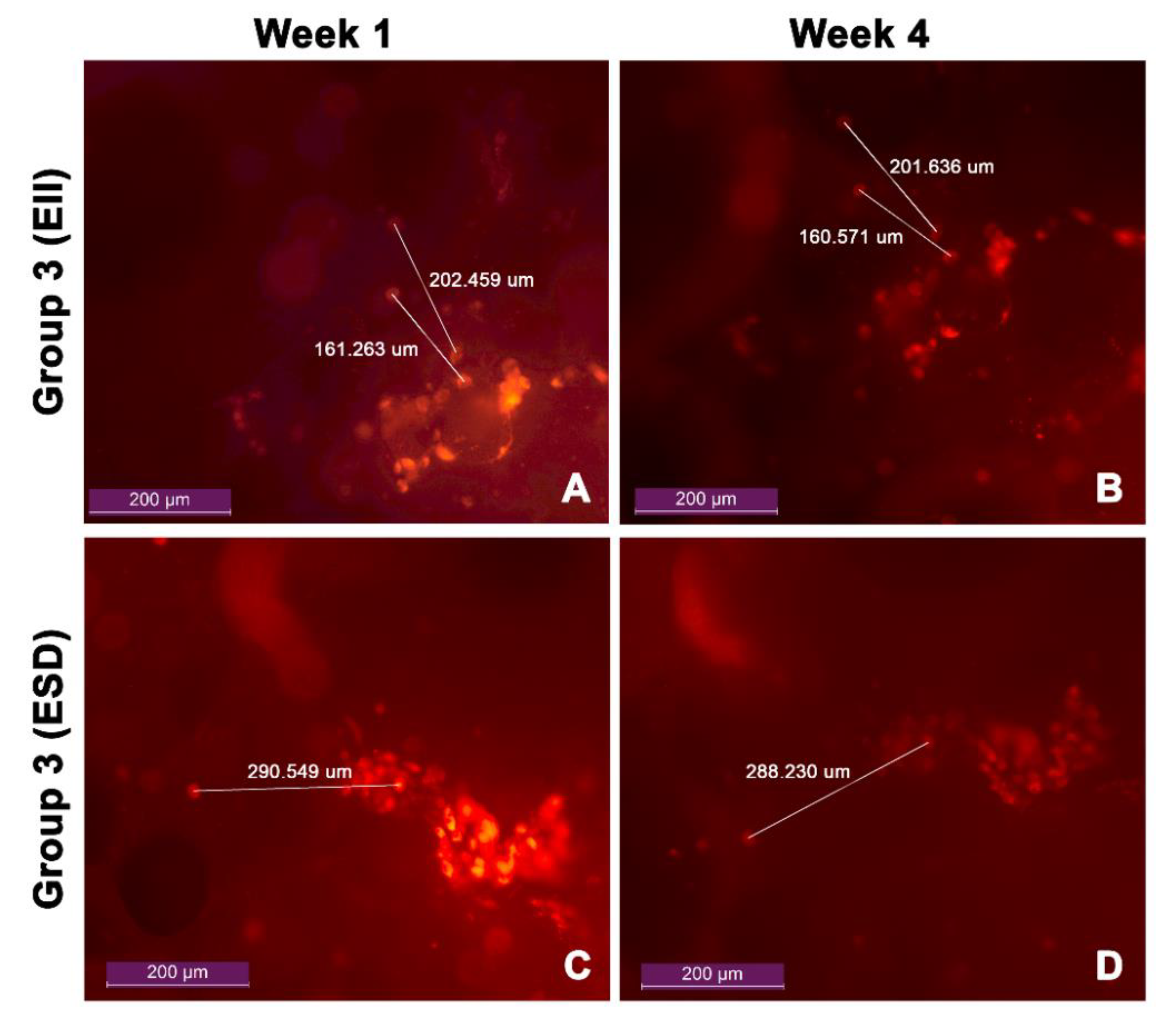
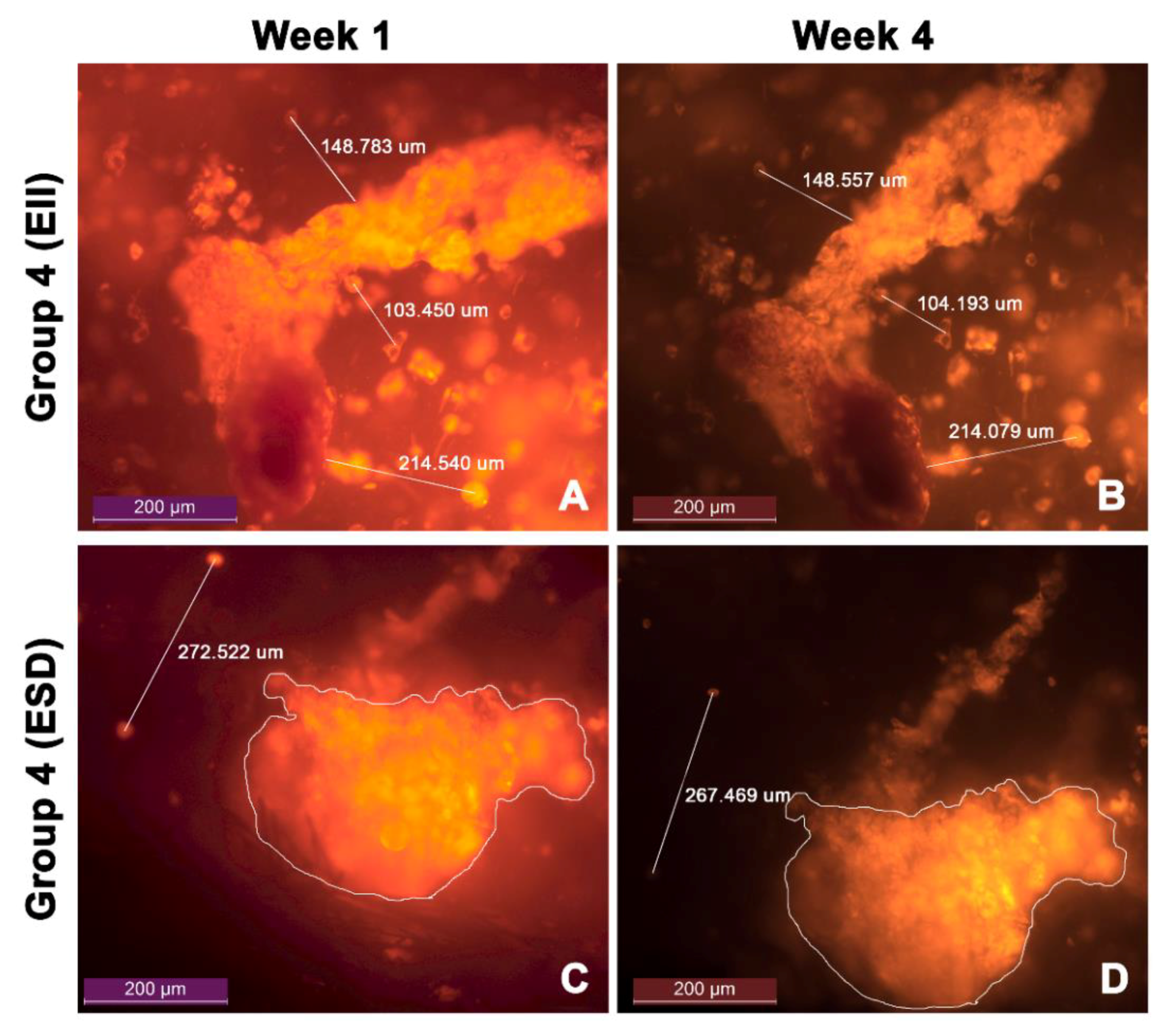
3.5. Cell Migration
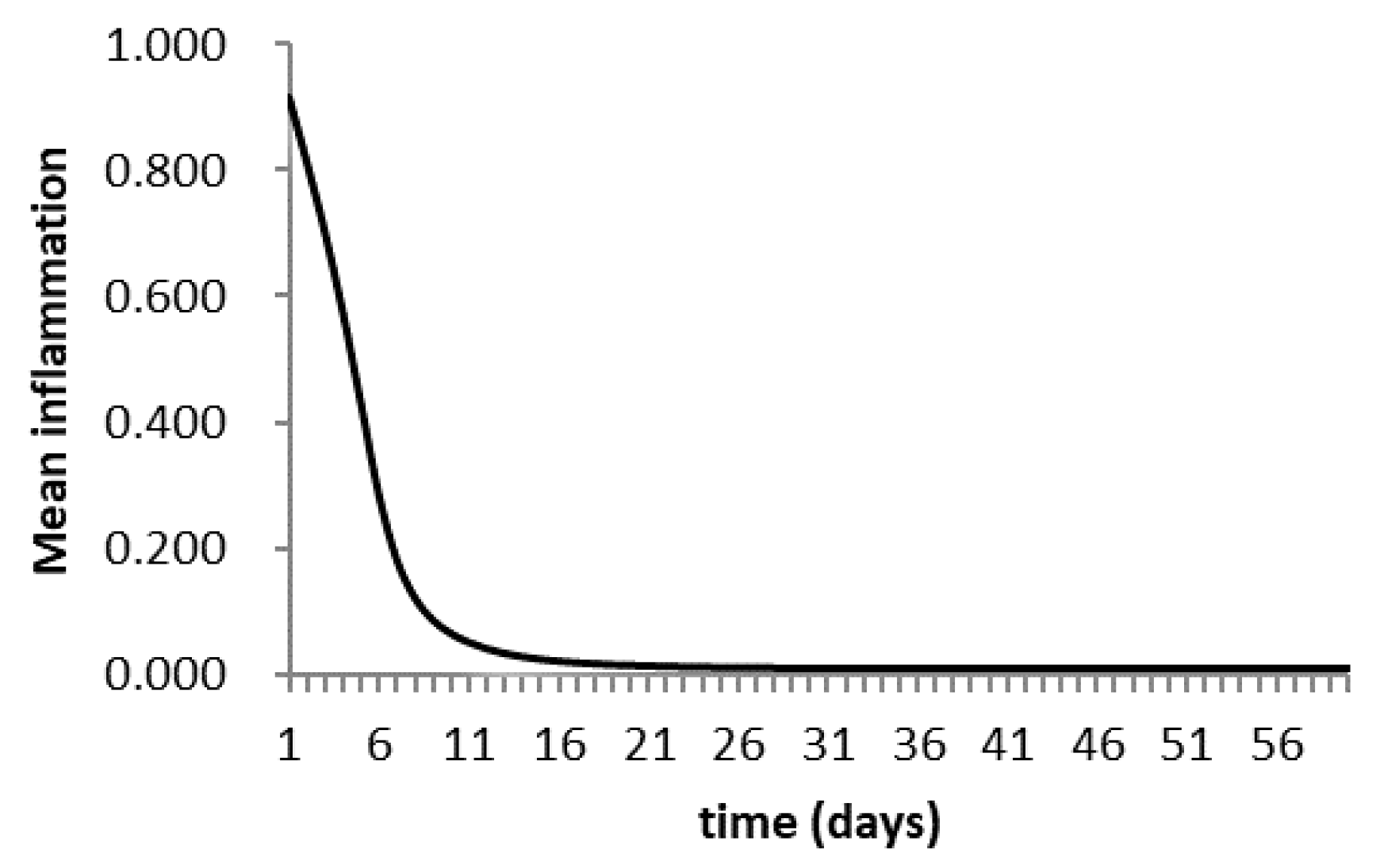
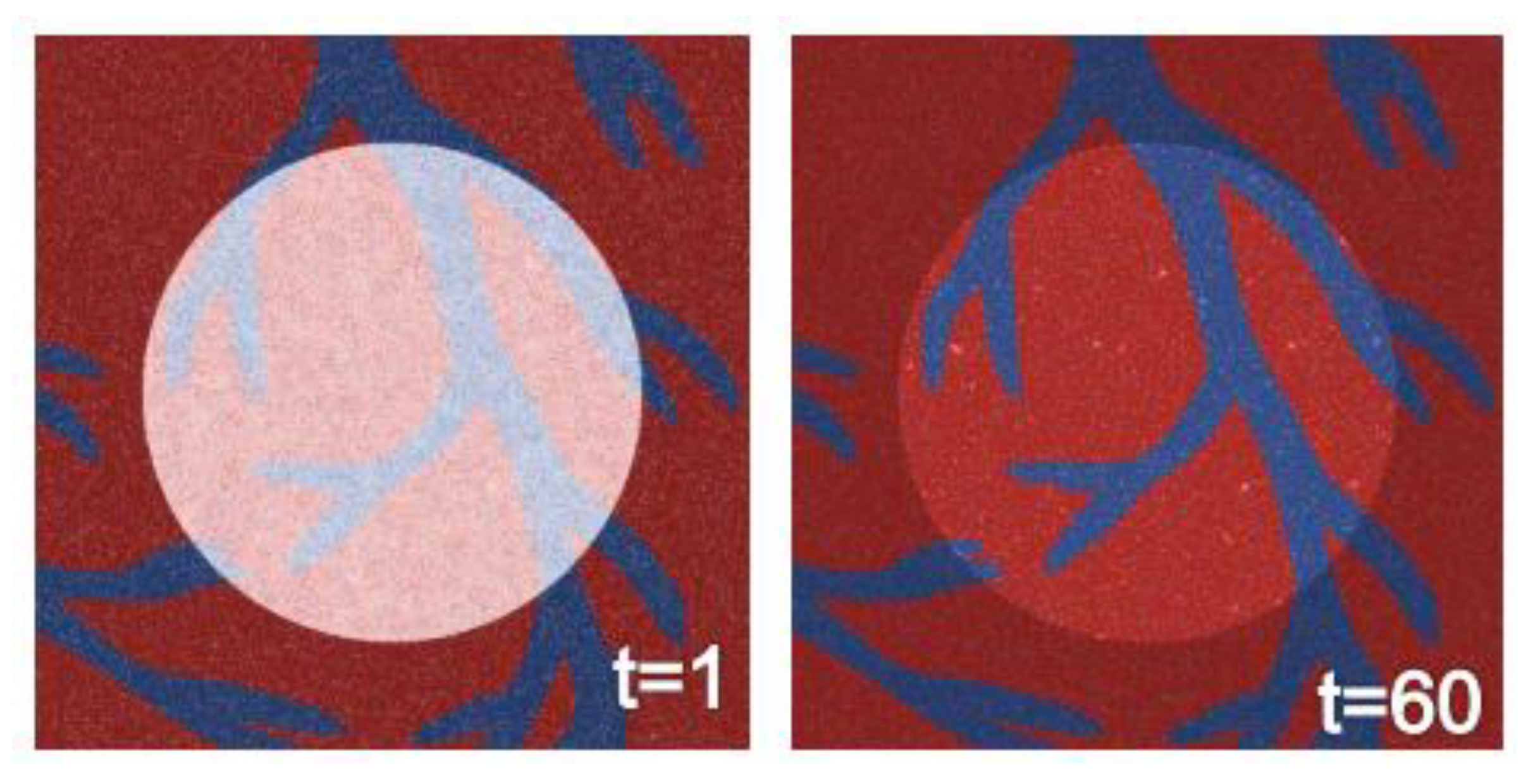
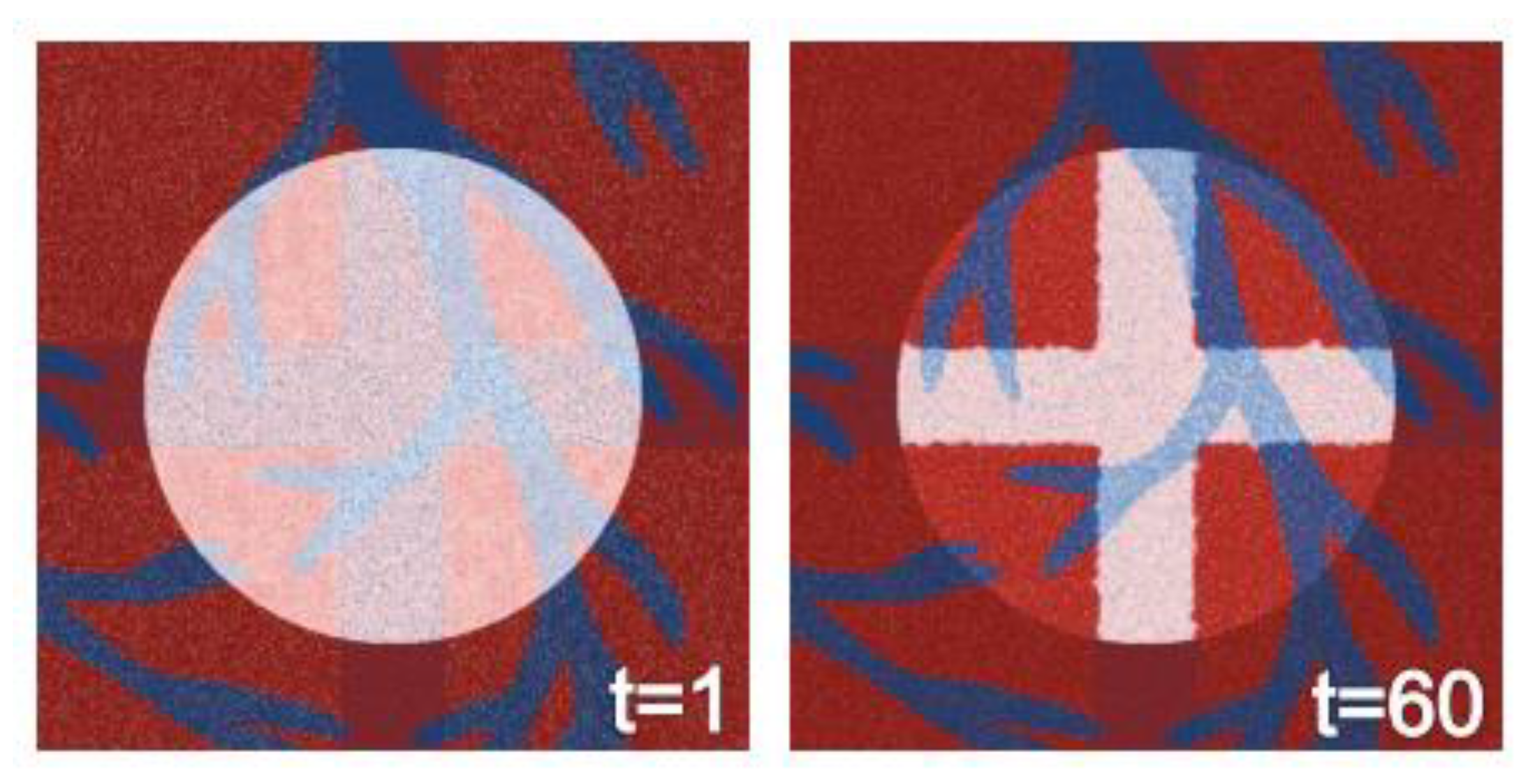
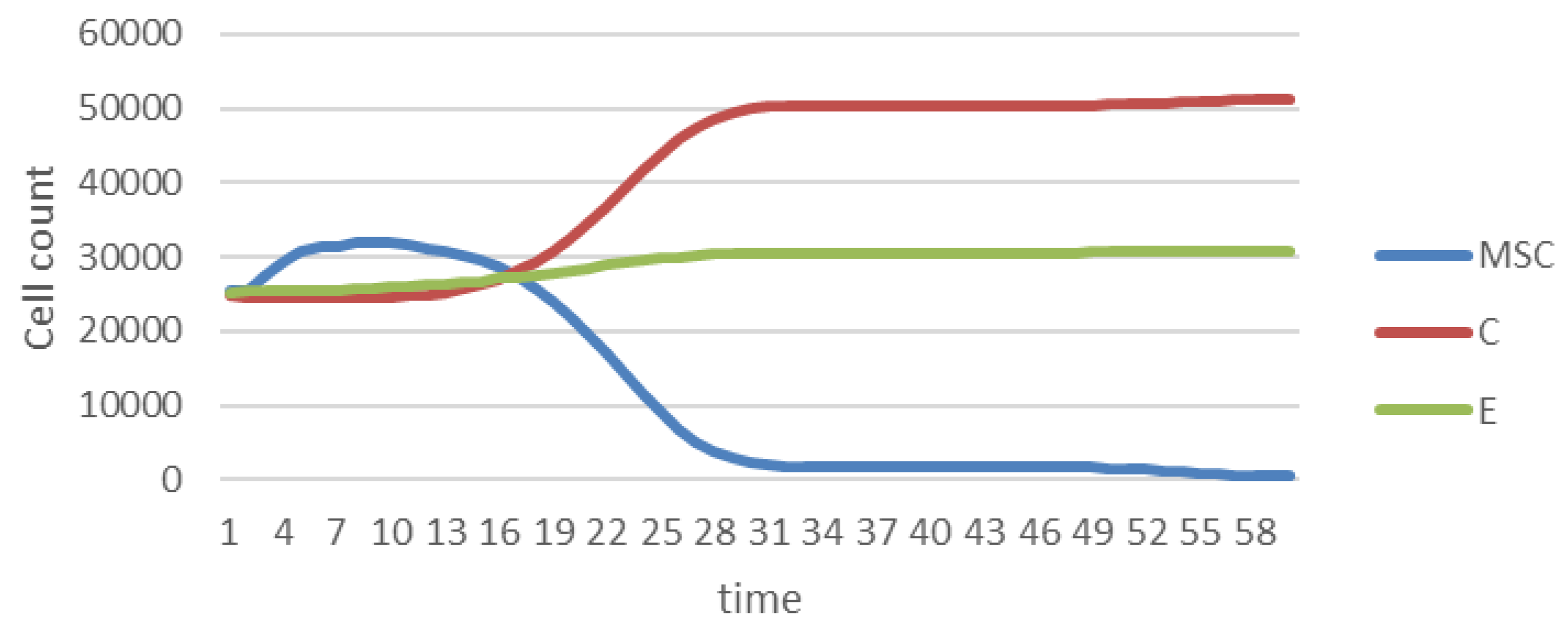
3.6. Agent-Based Modeling of Cells in A Scaffold Implanted on the Infarcted Myocardium
4. Discussion
4.1. In Vitro Experimentation
4.2. Agent-Based Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malliaras, K.; Marbán, E. Cardiac cell therapy: Where we’ve been, where we are, and where we should be headed. Br. Med Bull. 2011, 98, 161–185. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. spine J. 2018, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef]
- Domenech, M.; Polo-Corrales, L.; Ramirez-Vick, J.E.; Freytes, D.O. Tissue Engineering Strategies for Myocardial Regeneration: Acellular Versus Cellular Scaffolds? Tissue Eng. Part B Rev. 2016, 22, 438–458. [Google Scholar] [CrossRef] [PubMed]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comput. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Brodland, G.W. How computational models can help unlock biological systems. Semin. Cell Dev. Biol. 2015, 47–48, 62–73. [Google Scholar] [CrossRef]
- Briers, D.; Haghighi, I.; White, D.; Kemp, M.L.; Belta, C. Pattern synthesis in a 3D agent-based model of stem cell differentiation. In Proceedings of the 2016 IEEE 55th Conference on Decision and Control (CDC), Las Vegas, NV, USA, 12–14 December 2016. [Google Scholar]
- Tanaka, N.; Yamashita, T.; Sato, A.; Vogel, V.; Tanaka, Y. Simple agarose micro-confinement array and machine-learning-based classification for analyzing the patterned differentiation of mesenchymal stem cells. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Inverno, M.; Saunders, R. Agent-Based Modelling of Stem Cell Self- organisation in a Niche. Engineering Self-Organising Systems: Methodologies and Applications; Springer: Berlin, Germany, 2005; pp. 52–68. [Google Scholar]
- Garzoni, L.R.; Rossi, M.I.D.; de Barros, A.P.D.N. Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp. Cell Res. 2009, 315, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Lopez, D.V.; Pena-Reyes, C.; Rojas, A.J. Agent-based modeling of mesenchymal stem cells on a 3D-printed bio-device for the regenerative treatment of the infarcted myocardium. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; pp. 2033–2040. [Google Scholar]
- Wilensky, U. What is NetLogo? The NetLogo 6.0.2 User Manual; Northwestern University: Evanston, IL, USA, 1999. [Google Scholar]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation. Circulation Res. 2010, 107, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Koziński, M.; Magielski, P. Course of inflammatory activation during acute myocardial infarction in patients with preserved left ventricular systolic function. Folia Med. Copernic. 2014, 2, 6–18. [Google Scholar]
- Martire, A.; Bedada, F.B.; Uchida, S. Mesenchymal stem cells attenuate inflammatory processes in the heart and lung via inhibition of TNF signaling. Basic Res. Cardiol. 2016, 111, 54. [Google Scholar] [CrossRef] [PubMed]
- Cano, G.; García-Rodríguez, J.; Orts, S. Predicción de solubilidad de fármacos usando máquinas de soporte vectorial sobre unidades de procesamiento gráfico. Rev. Int. Methodos. Numer. Calc. Diseno. 2007, 33, 97–102. [Google Scholar] [CrossRef]
- Tan, J.; et al. Ablation of TNF-α receptors influences mesenchymal stem cell-mediated cardiac protection against ischemia. Shock 2010, 3, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Høyem, M.R.; Måløy, F.; Jakobsen, P.; Brandsdal, B.O. Stem cell regulation: Implications when differentiated cells regulate symmetric stem cell division. J. Theor. Biol. 2015, 380, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef]
- Garijo, N.; Manzano, R.; Osta, R.; Perez, M.A. Stochastic cellular automata model of cell migration, proliferation and differentiation: Validation with in vitro cultures of muscle satellite cells. J. Theor. Biol. 2012, 314, 1–9. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Yu, H.-S.; Won, J.-E.; Jin, G.-Z.; Kim, H.-W. Construction of mesenchymal stem cell-containing collagen gel with a macrochanneled polycaprolactone scaffold and the flow perfusion culturing for bone tissue engineering. Biores. Open Access 2012, 1, 124–136. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Krampe, B.; Al-Rubeai, M. Cell death in mammalian cell culture: Molecular mechanisms and cell line engineering strategies. Cytotechnology 2010, 62, 175–188. [Google Scholar] [CrossRef]
- Janeczek Portalska, K.; Leferink, A.; Groen, N. Endothelial Differentiation of Mesenchymal Stromal Cells. PLoS ONE 2012, 7, e46842. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.E.; Haugh, J.M. Directed migration of mesenchymal cells: Where signaling and the cytoskeleton meet. Curr Opin. Cell Biol. 2014, 30, 74–82. [Google Scholar] [CrossRef] [PubMed]





| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Test | Cell Viability | Cell migration | ||
| Cell distribution | Homogeneous | Homogeneous | Crosswise | |
| Scaffolds number | 24 | 12 | ||
| Cell concentration | 750 cells/μL | 5000 cells/μL | 2250 cells/μL | |
| MSCs | Cardiomyocytes | Endothelial Cells | |
|---|---|---|---|
| Fluorophore | Dil | DAPI | DiO |
| Cell distribution | 5 μL/10 mL | 2 μL/10 mL | 9 μL/10 mL |
| Average Distance (μm) | Coefficient of Variation | |
|---|---|---|
| Group 3 (EII) | 160.92 ± 0.48 | 0.30% |
| 202.05 ± 0.58 | 0.29% | |
| Group 3 (ESD) | 289.39 ± 0.58 | 0.57% |
| Group 4 (EII) | 103.48 ± 0.54 | 0.52% |
| 149.01 ± 1.38 | 0.92% | |
| 214.56 ± 1.30 | 0.61% | |
| Group 4 (ESD) | 269.13 ± 2.35 | 0.87% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez López, D.V.; Melo Escobar, M.I.; Peña-Reyes, C.A.; Rojas Arciniegas, Á.J.; Neuta Arciniegas, P.A. Isolation, Characterization, and Agent-Based Modeling of Mesenchymal Stem Cells in a Bio-construct for Myocardial Regeneration Scaffold Design. Data 2019, 4, 71. https://doi.org/10.3390/data4020071
Ramírez López DV, Melo Escobar MI, Peña-Reyes CA, Rojas Arciniegas ÁJ, Neuta Arciniegas PA. Isolation, Characterization, and Agent-Based Modeling of Mesenchymal Stem Cells in a Bio-construct for Myocardial Regeneration Scaffold Design. Data. 2019; 4(2):71. https://doi.org/10.3390/data4020071
Chicago/Turabian StyleRamírez López, Diana Victoria, María Isabel Melo Escobar, Carlos A. Peña-Reyes, Álvaro J. Rojas Arciniegas, and Paola Andrea Neuta Arciniegas. 2019. "Isolation, Characterization, and Agent-Based Modeling of Mesenchymal Stem Cells in a Bio-construct for Myocardial Regeneration Scaffold Design" Data 4, no. 2: 71. https://doi.org/10.3390/data4020071
APA StyleRamírez López, D. V., Melo Escobar, M. I., Peña-Reyes, C. A., Rojas Arciniegas, Á. J., & Neuta Arciniegas, P. A. (2019). Isolation, Characterization, and Agent-Based Modeling of Mesenchymal Stem Cells in a Bio-construct for Myocardial Regeneration Scaffold Design. Data, 4(2), 71. https://doi.org/10.3390/data4020071





