UAV-Based 3D Point Clouds of Freshwater Fish Habitats, Xingu River Basin, Brazil
Abstract
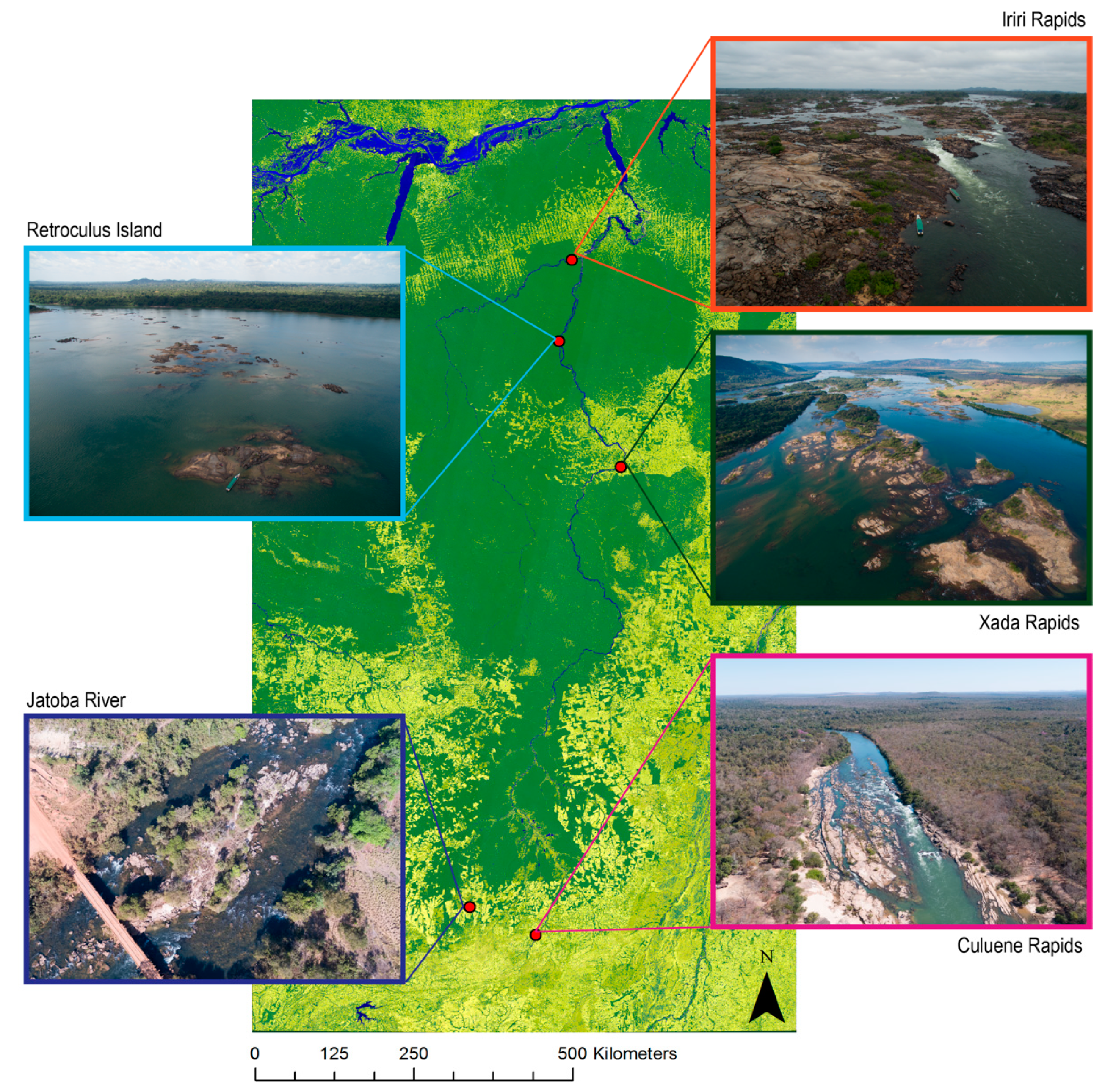
1. Summary
2. Data Description
3. Methods
4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalacska, M.; Lucanus, O.; Sousa, L.M.; Vieira, T.; Arroyo-Mora, J.P. Freshwater fish habitat complexity maping using above and underwater structure-from-motion photogrammetry. Remote Sens. 2018, 10, 1912. [Google Scholar] [CrossRef]
- Du Preez, C. A new arc-chord ratio (ACR) rugosity index for quantifying three-dimensional landscape structural complexity. Landsc. Ecol. 2015, 30, 181–192. [Google Scholar] [CrossRef]
- Walbridge, S.; Slocum, N.; Pobuda, M.; Wright, D.J. Unified Geomorphological Analysis Workflows with Benthic Terrain Modeler. Geosciences 2018, 8, 94. [Google Scholar] [CrossRef]
- Reichert, J.; Backes, A.R.; Schubert, P.; Wilke, T. The power of 3D fractal dimensions for comparative shape and structural complexity analyses of irregularly shaped organisms. Methods Ecol. Evol. 2017, 8, 1650–1658. [Google Scholar] [CrossRef]
- Leon, J.X.; Roelfsema, C.M.; Saunders, M.I.; Phinn, S.R. Measuring coral reef terrain roughness using ‘Structure-from-Motion’ close-range photogrammetry. Geomorphology 2015, 242, 21–28. [Google Scholar] [CrossRef]
- McCormick, M.I. Comparison of field methods for measuring surface-topography and their associations with a tropical reef fish assemblage. Mar. Ecol. Prog. Ser. 1994, 112, 87–96. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Brazier, R.E.; Anderson, K. Ultra-fine grain landscape-scale quantification of dryland vegetation structure with drone-acquired structure-from-motion photogrammetry. Remote Sens. Environ. 2016, 183, 129–143. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Micheletti, N.; Chandler, J.H.; Lane, S.N. Structure from Motion (SfM) Photogrammetry. In Geomorphological Techniques; British Society for Geomorphology: London, UK, 2015; pp. 2–12. [Google Scholar]
- House, J.E.; Brambilla, V.; Bidaut, L.M.; Christie, A.P.; Pizarro, O.; Madin, J.S.; Dornelas, M. Moving to 3D: Relationships between coral planar area, surface area and volume. PeerJ 2018, 6, 19. [Google Scholar] [CrossRef]
- Palma, M.; Casado, M.R.; Pantaleo, U.; Cerrano, C. High Resolution Orthomosaics of African Coral Reefs: A Tool for Wide-Scale Benthic Monitoring. Remote Sens. 2017, 9, 705. [Google Scholar] [CrossRef]
- Young, G.C.; Dey, S.; Rogers, A.D.; Exton, D. Cost and time-effective method for multiscale measures of rugosity, fractal dimension, and vector dispersion from coral reef 3D models. PLoS ONE 2017, 12, e0175341. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Roman, C.; Pizarro, O.; Eustice, R.; Can, A. Towards high-resolution imaging from underwater vehicles. Int. J. Robot. Res. 2007, 26, 55–74. [Google Scholar] [CrossRef]
- Ferrari, R.; Bryson, M.; Bridge, T.; Hustache, J.; Williams, S.B.; Byrne, M.; Figueira, W. Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob. Chang. Boil. 2016, 22, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.P.; Pennino, M.G.; de Nobrega, M.F.; Oliveira, J.E.L.; Mendes, L.D. Identifying fish diversity hot-spots in data-poor situations. Mar. Environ. Res. 2017, 129, 365–373. [Google Scholar] [CrossRef]
- Gonzalez-Rivero, M.; Harborne, A.R.; Herrera-Reveles, A.; Bozec, Y.M.; Rogers, A.; Friedman, A.; Ganase, A.; Hoegh-Guldberg, O. Linking fishes to multiple metrics of coral reef structural complexity using three-dimensional technology. Sci. Rep. 2017, 7, 13965. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tokeshi, M. Effects of habitat complexity on benthic assemblages in a variable environment. Freshw. Boil. 2004, 49, 1164–1178. [Google Scholar] [CrossRef]
- ASPRS. LAS Specification v 1.2; ASPRS: Bethesda, MD, USA, 2008. [Google Scholar]
- Dandois, J.P.; Olano, M.; Ellis, E.C. Optimal Altitude, Overlap, and Weather Conditions for Computer Vision UAV Estimates of Forest Structure. Remote Sens. 2015, 7, 13895–13920. [Google Scholar] [CrossRef]
- Kalacska, M.; Chmura, G.L.; Lucanus, O.; Berube, D.; Arroyo-Mora, J.P. Structure from motion will revolutionize analyses of tidal wetland landscapes. Remote Sens. Environ. 2017, 199, 14–24. [Google Scholar] [CrossRef]
- Lowe, D.G. Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 2004, 60, 91–110. [Google Scholar] [CrossRef]
- Strecha, C.; von Hansen, W.; Van Gool, L.; Fua, P.; Thoennessen, U. On Benchmarking camera calibration and multi-view stereo for high resolution imagery. In Proceedings of the 2008 IEEE Conference on Computer Vision and Pattern Recognition, Anchorage, AK, USA, 23–28 June 2008. [Google Scholar]
- Strecha, C.; Bronstein, A.M.; Bronstein, M.M.; Fua, P. LDAHash: Improved Matching with Smaller Descriptors. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 34, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Vautherin, J.; Rutishauser, S.; Schneider-Zapp, K.; Choi, H.F.; Chovancova, V.; Glass, A.; Strecha, C. Photogrammetric accuracy and modeling of rolling shutter cameras. ISPRS J. Photogramm. Remote Sens. 2016, 3, 139–146. [Google Scholar] [CrossRef]
- Tamminga, A.; Hugenholtz, C.; Eaton, B.; Lapointe, M. Hyperspatial remote sensing of channel reach morphology and hydraulic fish habitat using an unmanned aerial vehicle (UAV): A first assessment in the context of river research and management. River Res. Appl. 2015, 31, 379–391. [Google Scholar] [CrossRef]
- Westaway, R.M.; Lane, S.N.; Hicks, D.M. The development of an automated correction procedure for digital photogrammetry for the study of wide, shallow, gravel-bed rivers. Earth Surf. Process. Landf. 2000, 25, 209–226. [Google Scholar] [CrossRef]
- Woodget, A.S.; Carbonneau, P.E.; Visser, F.; Maddock, I.P. Quantifying submerged fluvial topography using hyperspatial resolution UAS imagery and structure from motion photogrammetry. Earth Surf. Process. Landf. 2015, 40, 47–64. [Google Scholar] [CrossRef]

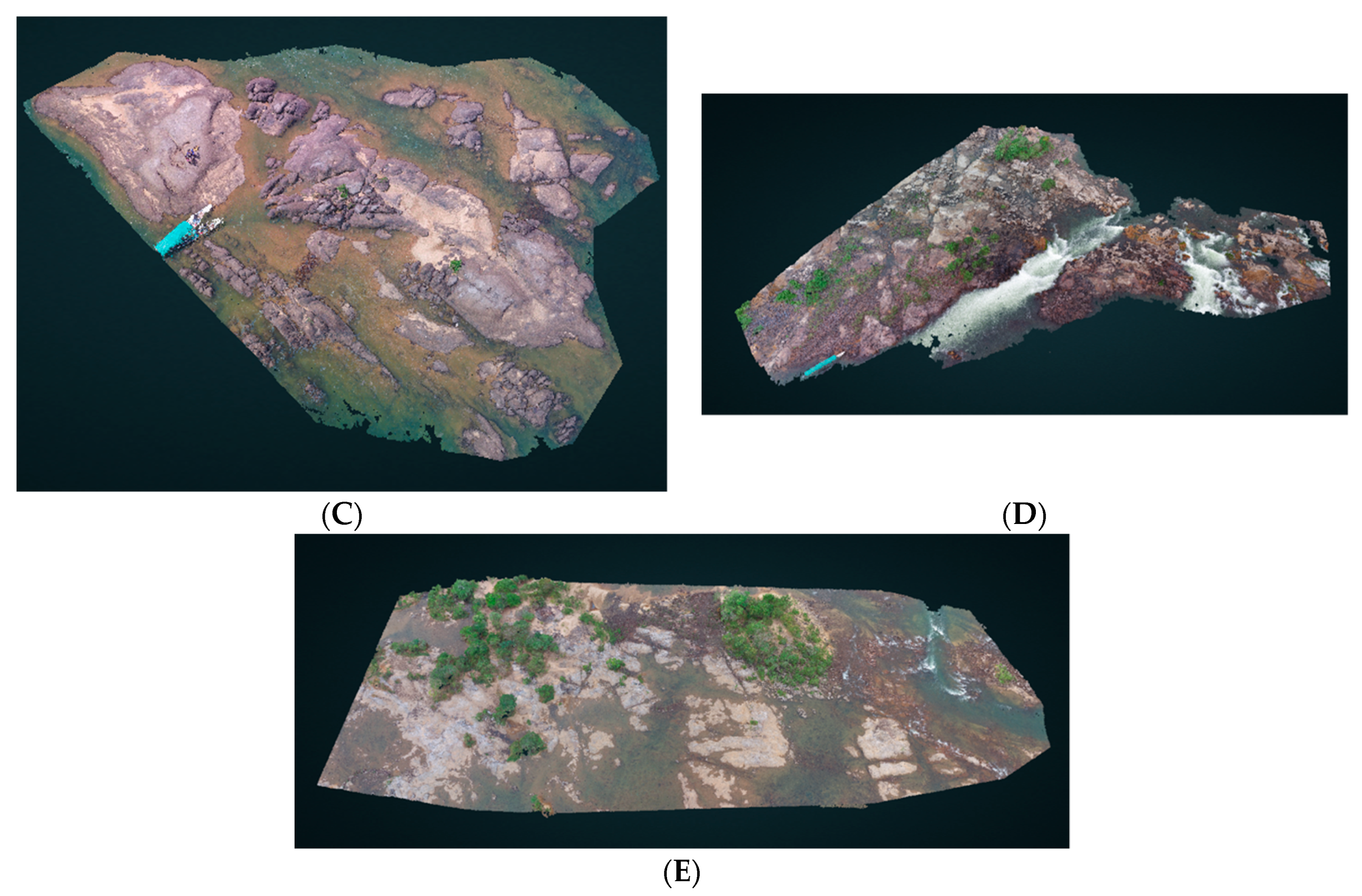
| File | Location | Size | Interactive version |
|---|---|---|---|
| Jatoba.las | Jatoba river | 1.12 GB | http://bit.ly/riojatoba |
| Culuene_HD.las | Culuene rapids | 801.59 MB | http://bit.ly/culuene |
| Retroculus_island.las | Retroculus island | 714.93 MB | http://bit.ly/retroculus |
| Xada_HD.las | Xada rapids | 459.31 MB | http://bit.ly/xadarapids |
| Iriri_HD.las | Iriri rapids | 2.48 GB | http://bit.ly/iriri3D |
| Location | UAV | Camera | Date | GSD (cm) | No. Photographs | Area (ha) |
|---|---|---|---|---|---|---|
| Jatoba river | Inspire 2 | X5S | 2 August 2017 | 1.20 | 375 | 2.80 |
| Culuene rapids | Inspire 2 | X5S | 1 August 2017 | 1.75 | 283 | 4.54 |
| Retroculus island | Inspire 1 | X3 | 8 August 2016 | 1.43 | 208 | 0.52 |
| Xada rapids | Inspire 1 | X3 | 11 August 2016 | 2.38 | 420 | 4.62 |
| Iriri rapids | Inspire 1 | X3 | 6 August 2016 | 1.46 | 425 | 2.77 |
| Location | Camera Speed (m/s) | Displacement During Readout (m) | Rolling Shutter Readout Time (ms) |
|---|---|---|---|
| Jatoba river | 2.1 | 0.13 | 60.63 |
| Culuene rapids | 3.4 | 0.27 | 80.06 |
| Retroculus island | 2.0 | 0.16 | 80.90 |
| Xada rapids | 2.4 | 0.15 | 63.58 |
| Iriri rapids | 2.0 | 0.14 | 72.29 |
| Location | Median Matches per Image | Avg Point Cloud Density (/m3) | Median Keypoints per Image | Camera Optimization (%) | Total Processing Time | Total Number of Points (Dense Point Cloud) |
|---|---|---|---|---|---|---|
| Jatoba river | 17,958.3 | 1044.0 | 72,446 | 0.33 | 8 h:49 min:58 s | 35,432,692 |
| Culuene rapids | 23,869.7 | 620.6 | 70,893 | 1.96 | 6 h:16 min:04s | 24,695,393 |
| Retroculus Isl. | 23,059.0 | 1782.1 | 5342 | 1.36 | 21 min:44 s | 22,033,200 |
| Xada rapids | 18,118.6 | 469.3 | 41,835 | 3.42 | 1 h:57 min:08 s | 14,129,408 |
| Iriri rapids * | 15,684.2 | 5863.4 | 42,048 | 0.12 | 25 h:58 min:06 s | 78,332,198 |
| Location | X: μ ± σ | Y: μ ± σ | Z: μ ± σ |
|---|---|---|---|
| Jatoba river | 0.00 ± 0.99 | 0.00 ± 0.89 | 0.01 ± 0.32 |
| Culuene rapids | 0.00 ± 1.34 | 0.00 ± 1.86 | 0.00 ± 2.37 |
| Retroculus Island | 0.01 ± 0.52 | 0.00 ± 0.50 | 0.00 ± 1.01 |
| Xada rapids | 0.00 ± 0.89 | 0.00 ± 0.87 | 0.00 ± 0.69 |
| Iriri rapids | 0.00 ± 0.60 | 0.00 ± 0.60 | 0.00 ± 1.00 |
| Location | X (m) | Y (m) | Z (m) | Omega (°) | Phi (°) | Kappa (°) |
|---|---|---|---|---|---|---|
| Jatoba river | 0.008 ± 0.003 | 0.007 ± 0.003 | 0.004 ± 0.001 | 0.012 ± 0.004 | 0.010 ± 0.004 | 0.003 ± 0.001 |
| Culuene rapids | 0.012 ± 0.009 | 0.012 ± 0.008 | 0.005 ± 0.003 | 0.012 ± 0.008 | 0.010 ± 0.007 | 0.004 ± 0.002 |
| Retroculus Isl. | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.006 ± 0.002 | 0.006 ± 0.002 | 0.003 ± 0.001 |
| Xada rapids | 0.004 ± 0.002 | 0.004 ± 0.002 | 0.003 ± 0.001 | 0.005 ± 0.002 | 0.005 ± 0.002 | 0.002 ± 0.001 |
| Iriri rapids | 0.054 ± 0.032 | 0.053 ± 0.026 | 0.022 ± 0.010 | 0.102 ± 0.048 | 0.097 ± 0.059 | 0.025 ± 0.008 |
| Location | No. 2D Keypoint Observations for Bundle Block Adjustment | No. 3D pts for Bundle Block Adjustment | Mean Reprojection Error (pixels) |
|---|---|---|---|
| Jatoba river | 7,021,320 | 2,165,893 | 0.205 |
| Culuene rapids | 6,436,758 | 1,293,024 | 0.198 |
| Retroculus Island | 1,353,973 | 270,069 | 0.212 |
| Xada rapids | 7,668,646 | 1,442,876 | 0.259 |
| Iriri rapids | 7,720,544 | 1,848,706 | 0.199 |
| Location | Focal Length | Principal Point x | Principal Point y | R1 | R2 | R3 | T1 | T2 |
|---|---|---|---|---|---|---|---|---|
| Jatoba river | I = 15.000 O = 15.065 σ = 0.006 | I = 8.75 O = 8.824 σ = 0.000 | I = 6.556 O = 6.635 σ = 0.002 | I = 0.000 O = −0.005 σ = 0.000 | I = 0.000 O = −0.004 σ = 0.000 | I = −0.000 O = 0.010 σ = 0.001 | I = 0.000 O = 0.001 σ = 0.000 | I = 0.000 O = 0.002 σ = 0.000 |
| Culuene rapids | I = 15.000 O = 14.751 σ = 0.004 | I = 8.75 O = 8.854 σ = 0.000 | I = 6.556 O = 6.764 σ = 0.002 | I = 0.000 O = −0.006 σ = 0.000 | I = 0.000 O = −0.004 σ = 0.000 | I = −0.000 O = 0.010 σ = 0.000 | I = 0.000 O = 0.001 σ = 0.000 | I = 0.000 O = 0.002 σ = 0.000 |
| Retroculus Island | I = 3.61 O = 3.659 σ = 0.002 | I = 3.159 O = 3.157 σ = 0.000 | I = 2.369 O = 2.356 σ = 0.000 | I = −0.13 O = −0.131 σ = 0.000 | I = 0.106 O = 0.108 σ = 0.000 | I = −0.016 O = −0.014 σ = 0.000 | I = 0.000 O = −0.001 σ = 0.000 | I = 0.000 O = 0.000 σ = 0.000 |
| Xada rapids | I = 3.61 O = 3.486 σ = 0.001 | I = 3.159 O = 3.156 σ = 0.000 | I = 2.369 O = 2.352 σ = 0.000 | I = −0.13 O = −0.119 σ = 0.000 | I = 0.106 O = 0.087 σ = 0.000 | I = −0.016 O = −0.009 σ = 0.000 | I = 0.000 O = −0.001 σ = 0.000 | I = 0.000 O = 0.000 σ = 0.000 |
| Iriri rapids | I = 3.551 O = 3.547 σ = 0.000 | I = 3.085 O = 3.084 σ = 0.000 | I = 2.314 O = 2.300 σ = 0.000 | I = −0.13 O = −0.119 σ = 0.000 | I = 0.106 O = 0.104 σ = 0.001 | I = −0.016 O = −0.013 σ = 0.000 | I = 0.000 O = −0.001 σ = 0.000 | I = 0.000 O = 0.000 σ = 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalacska, M.; Lucanus, O.; Sousa, L.; Vieira, T.; Arroyo-Mora, J.P. UAV-Based 3D Point Clouds of Freshwater Fish Habitats, Xingu River Basin, Brazil. Data 2019, 4, 9. https://doi.org/10.3390/data4010009
Kalacska M, Lucanus O, Sousa L, Vieira T, Arroyo-Mora JP. UAV-Based 3D Point Clouds of Freshwater Fish Habitats, Xingu River Basin, Brazil. Data. 2019; 4(1):9. https://doi.org/10.3390/data4010009
Chicago/Turabian StyleKalacska, Margaret, Oliver Lucanus, Leandro Sousa, Thiago Vieira, and Juan Pablo Arroyo-Mora. 2019. "UAV-Based 3D Point Clouds of Freshwater Fish Habitats, Xingu River Basin, Brazil" Data 4, no. 1: 9. https://doi.org/10.3390/data4010009
APA StyleKalacska, M., Lucanus, O., Sousa, L., Vieira, T., & Arroyo-Mora, J. P. (2019). UAV-Based 3D Point Clouds of Freshwater Fish Habitats, Xingu River Basin, Brazil. Data, 4(1), 9. https://doi.org/10.3390/data4010009







