Abstract
Tall fescue toxicosis negatively impacts blood flow, elevates body temperature, and reduces beef cattle’s average daily gain (ADG). In previous studies, isoflavones have diminished the symptoms of tall fescue toxicosis in ruminants. Therefore, this dataset determined the impact of low concentrations of isoflavone doses on animal vasculature, body temperature, ADG, and rumen microbial communities in beef cattle. A 21-day experiment with Angus cattle consisted of four isoflavone doses: 0 g, 2 g, 4 g, and 6 g, along with a control group. Isoflavones were mixed with 0.5 kg of dried distiller’s grains (DDGs). Daily individual rectal temperatures were recorded. Weekly blood serum was collected via coccygeal venipuncture, blood vasculature data were measured via color Doppler ultrasound, and body weight (BW) was recorded. Approximately 100 mL of rumen content was collected at the end of the trial. The pulsatility index (PI) decreased in the control group compared to the 2 g and 4 g groups (p = 0.01). Animals in the isoflavone treatment groups recorded a higher rectal temperature (p < 0.05). ADG was reduced in animals undergoing isoflavone treatments (p < 0.001). Finally, there was no impact on the rumen microbial communities (p > 0.05). Isoflavone supplementation may mitigate tall fescue toxicosis and improve animal performance at greater doses.
Dataset: DOI: 10.6084/m9.figshare.28278602.
Dataset License: CC BY 4.0
1. Summary
Tall fescue is a cool-season, perennial forage widely grown in the southeastern United States due to its high productivity and resilience against environmental stresses and pests. This resilience is primarily attributed to a fungal endophyte, Epichloë coenophiala, which infects tall fescue plants and produces ergot alkaloids, toxins harmful to grazing livestock. Consumption of these alkaloids leads to tall fescue toxicosis, a condition characterized by persistent vasoconstriction, elevated body temperature, and reduced feed intake and growth in cattle; annual losses from tall fescue toxicosis average USD 2 billion for beef cattle producers in the U.S. [1].
Various strategies have been implemented to mitigate the financial impact of tall fescue toxicosis and relieve physiological symptoms in livestock [2,3,4,5,6,7,8,9]. A common approach is incorporating legumes into pastures containing phytoestrogenic isoflavones and antimicrobial properties, inhibiting hyper-ammonia-producing bacteria [10]. Isoflavones like biochanin A can reduce vasoconstriction and alleviate fescue toxicosis symptoms. Aiken et al. [11] found that 30 mg of biochanin A per liter of rumen fluid promoted vasodilation in goats with vasoconstriction due to endophyte-infected tall fescue. This effect is linked to isoflavones’ activity on β-adrenergic receptors in the vascular endothelium, enhancing nitric oxide production [3,11,12].
Additionally, isoflavone supplementation in cattle diets has been shown to promote the growth of beneficial cellulolytic and amylolytic bacteria [13]. However, Aiken et al. [11] cautioned that ergot alkaloid saturation in the bloodstream may hinder biochanin A’s effectiveness. Thus, further research is warranted to explore the dose-response relationships necessary for optimal isoflavone levels that mitigate tall fescue toxicosis symptoms in beef cattle, including vascular health and body temperature. This study aims to determine the minimum dose required for isoflavones to enhance vascular health, livestock well-being, and rumen microbiome.
2. Data Description
This study aimed to determine the optimal dose and frequency of isoflavones to clarify the adverse effects of tall fescue toxicosis on blood vasculature, average daily gain, and rectal temperature. Additionally, it evaluated the effects of endophyte-infected tall fescue and isoflavone supplementation on rumen microbial populations. Enhancing production parameters such as average daily gain in beef cattle grazing endophyte-infected tall fescue could lessen producers’ concerns regarding tall fescue as their primary forage species in their operation. The initial goal was to improve the animal’s blood vasculature, which may further enhance growth and weight gain.
The data in Table 1 describe the vasoconstriction response observed in cattle grazing endophyte-infected tall fescue, a common symptom of tall fescue toxicosis. Vasoconstriction is one of the symptoms cattle experience when grazing endophyte-infected tall fescue. Aiken et al. [14] reported a vasoconstriction response in bovine caudal arteries after a 48 h exposure to endophyte-naïve conditions, with ergovaline concentrations of 0.8 μg/g DM. Similar responses would likely be expected in cattle on a grazing system during summer, as symptoms of tall fescue toxicosis emerge after approximately 21 days of grazing endophyte-infected tall fescue [15].

Table 1.
Effect of isoflavone dose-response on vasculature measurements in Angus cattle.
The responses observed during this experiment differed from those reported by Aiken and Flythe [16], who noted vasodilation of the carotid artery in goats after 3 to 4 days of isoflavone treatment. However, Aiken et al. [11] suggested that a greater density of β-adrenergic receptors in the interosseus region than in the carotid artery might result in more pronounced vasoconstriction in certain areas of the animal’s body. The median coccygeal artery examined may have experienced more significant vasoconstriction than the carotid artery. The differences in results may have also been affected by the low dose of isoflavones administered to the animals. Various studies indicate that infusions of red clover isoflavone products significantly impact the vasculature of animals suffering from tall fescue toxicosis. However, effective doses may vary by species and administration methods. The isoflavone concentration used by Aiken et al. [11] to achieve vasodilation was 30 ppm of biochanin A, based on findings by Flythe et al. [17] concerning the reduction of hyperammonemia-producing bacteria in vitro.
The data in Table 2 describe the impact of isoflavones on thermoregulation, animal performance, and fescue toxicosis indicators. Persistent vasoconstriction prevents the animal from thermoregulating its body temperature, leading to severe heat stress. Animals under any stress exhibit reduced feed intake and growth performance, resulting in economic losses for producers. Despite the aim to enhance animal performance through isoflavone administration, the low doses administered were inadequate for inducing vasodilation, hindering the animals’ ability to dissipate heat throughout the study period. Notably, the heat stress and poor growth performance recorded are not solely attributable to tall fescue toxicosis; other factors, including high environmental temperatures during summer, shade availability in the pasture, and nutrient composition of the forage, also contributed to these outcomes. Many factors are beyond human control, potentially affecting the experiment’s results.

Table 2.
Effect of isoflavone dose-response on rectal temperature, average daily gain (ADG), and prolactin concentration in Angus cattle.
Consumption of endophyte-infected tall fescue significantly reduces beef cattle’s ADG and feed intake. However, this issue can be improved by incorporating legumes into tall fescue pastures. Adding legumes has been suggested as a method to dilute the consumption of ergot alkaloids while simultaneously supplying isoflavones to the animal, counteracting the negative impacts. In this study and dataset, the low concentration of isoflavones administered to cattle affected by tall fescue toxicosis might have been insufficient to enhance the animals’ ADG. The immediate rumination observed after feeding with DDG supplements and isoflavones could have diminished the effects of isoflavones, inactivating part of the dose and preventing breakdown by rumen bacteria as efficiently as required. Moreover, it is unclear whether exposure of isoflavones to sunlight in individual pens during feeding might affect the dose involving red clover isoflavones.
Serum prolactin concentrations were consistently low due to the interaction of ergot alkaloids with dopamine receptors, indicating that the animals were persistently experiencing symptoms from tall fescue toxicosis. Additionally, bromocriptine administration every three days contributed to the sustained low serum prolactin. It has been suggested that isoflavones do not interact with dopamine receptors, thereby not affecting prolactin concentrations. Further, it is possible that bromocriptine may have overwhelmed the capacity of isoflavones to mitigate toxicosis symptoms.
The data in Figure 1 and Figure 2 describe the analysis of rumen microbial communities, revealing that there were no significant differences observed between the various doses of isoflavones administered. Both alpha and beta diversity metrics clearly illustrated a high degree of similarity among these microbial communities, indicating that the variations in isoflavone doses did not substantially alter the composition of these bacterial communities. This suggests that the low dose of isoflavones administered to the animals, along with the duration of the experimental timeline, may not have been enough to induce noticeable changes in the microbial populations within the rumen. Therefore, further research is essential to explore the potential impacts of different isoflavone doses over an extended period, as this could deepen our understanding of how these compounds influence rumen microbiota and, ultimately, animal health and productivity.
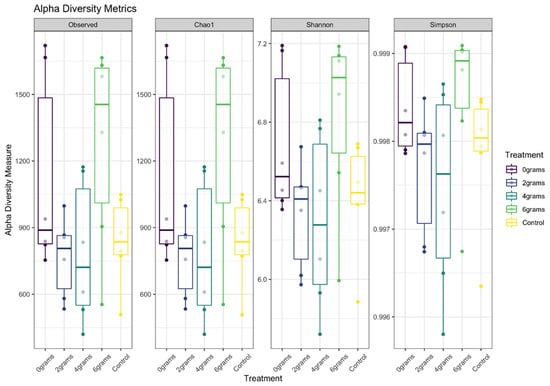
Figure 1.
Alpha diversity metrics of bacterial richness and evenness of ruminal bacterial communities among treatments in Angus cattle described by Observed, Chao1, Shannon, and Simpson indexes.
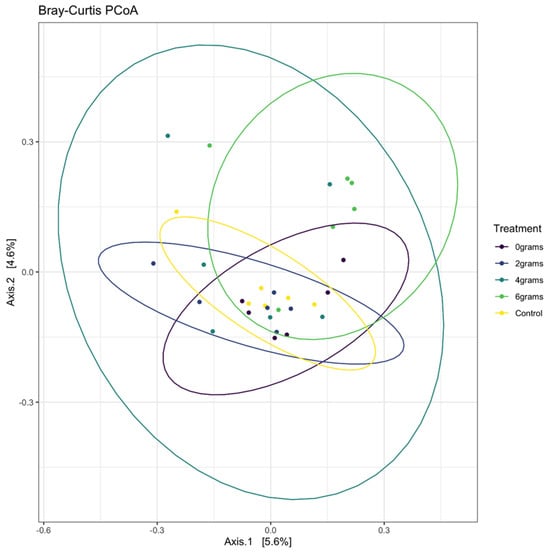
Figure 2.
Bray–Curtis PCoA grouped by dose treatment in Angus cattle. Points and ellipses of the same color represent taxa within treatment groups. Clustering indicates similarities of taxa among treatment groups.
3. Methods
All experimental procedures with animals were conducted according to the guidelines set by the University of Tennessee Institutional Animal Care and Use Committee. These experiments were conducted during late spring and early summer at the University of Tennessee, East Tennessee Research and Education Center (ETREC) Holston Unit, Knoxville, Tennessee (35°57′43″ N 83°51′29″ W).
3.1. Experiment Design and Trial Methodology
The experiment was composed of 30 purebred Angus cattle (24 steers and six heifers), 6–8 months of age, and 264 ± 30 kg on average at the beginning of the 21-day trial [9]. The 30 cattle were born and weaned at the ETREC Holston Unit and grazing tall fescue before the study began. This study implemented a completely randomized design with repeated measures. Five treatments comprised 0 g, 2 g, 4 g, and 6 g isoflavone dosing and a control group. Animals were randomly assigned in groups of six animals per treatment, balanced by body weight and sex. The cattle undergoing isoflavone administration consisted of 24 cattle placed on a tall fescue pasture (~6.87 hectares) and the control group of 6 cattle placed on an annual ryegrass pasture (~1.69 hectares). Before the beginning of the trial, the 24 cattle not belonging to the control group went through a 7 d acclimation period to DDG) at 0.5 kg/hd/d DDG supplement, which was to be used as a carrier for the isoflavones (DDG nutritional value: Crude Protein: 25%; Crude Fat: 7%; Crude Fiber: 10%; DM basis; AgCentral Farmers Coop, Greenback, TN, USA). DDG was administered without isoflavones in individual pens at the working facility. The control group was not given access to DDG. Each day for the 21 d trial, the 24 cattle under isoflavone treatment were moved from the tall fescue pasture to individual pens at the working facility to receive the DDG supplement.
Control group grazing annual ryegrass was only moved from the pasture to the working facility weekly for sample collection, along with the cattle undergoing isoflavones treatment. For each treatment group, a total of 0 g, 2 g, 4 g, or 6 g of isoflavone powder was previously measured and placed in individual bags according to the respective dose, which was based on minimal supplementation estimates from previous work [9,11]. At the working facility, isoflavones were mixed with DDG, where the cattle received the 0.5 kg/hd/d DDG supplement as a carrier for the isoflavones. The working facility had nine individual pens where cattle were placed to receive the isoflavone treatment; after every group of nine animals, buckets were washed with water to eliminate residues from the previous animal. The source of red clover-origin isoflavones utilized in this study was the powdered Promensil product (PharmaCare Laboratories, Warriewood, NSW, Australia). Every three days throughout the trial, the 24 cattle on isoflavone treatment were also administered bromocriptine mesylate (Santa Cruz Biotechnology; Dallas, TX, USA; a dopaminergic receptor agonist derived from ergocryptine) at 0.1 mg/kg BW to induce tall fescue toxicosis symptoms in cattle. Bromocriptine was reconstituted as working stock in 95% ethanol and diluted with 0.9% Sodium Chloride, so around 40% of ethanol was given in a single intramuscular injection consisting of 42% of the stock solution (bromocriptine + ethanol) and 58% of Sodium Chloride. The control group did not receive bromocriptine mesylate.
3.2. Sampling
Individual rectal temperatures were recorded each day of the trials (GLA Agricultural Electronics M900, San Luis Obispo, CA, USA). Weekly, approximately 9 mL of blood was collected via coccygeal venipuncture (Corvac, Sherwood Medical., St. Louis, MO, USA), and body weight measurements were collected. Blood flow and vasculature measurements were collected weekly using color Doppler ultrasound (GE Logiq E Vet, Chicago, IL, USA) images of the median coccygeal artery. On days 21 and 28 of the trials, approximately 100 mL of rumen content was collected via orogastric tubing [18]. Rumen samples were stored at −80 °C until further processing. Blood samples were cooled and centrifuged at 2000× g and 4 °C for 20 min. The serum was separated and stored at −80 °C until further processing and analysis.
3.3. Pasture Management
The ~6.87-hectare field where the 24 cattle grazed during the experiments was prepared approximately three months before the studies began. The field was sprayed with herbicide to eliminate other plants growing beside tall fescue, specifically clovers. Approximately two weeks after spraying, the field was overseeded with tall fescue (KY-31) to obtain an adequate stand of the forage in the field. The ~1.69-hectare field where the six cattle grazed annual ryegrass (Control group) was also prepared before the studies began. The field was sprayed with a broad-spectrum herbicide to eliminate the plant species already existing in the pasture, and approximately two weeks after spraying, annual ryegrass was planted based on recommendations for an adequate forage stand.
3.4. Ergot-Alkaloids Content and Nutrient Composition
A total of 18 fresh tall fescue samples were randomly harvested using a knife at ~5 cm above the soil surface. Each sample was divided into half for ergot alkaloids and nutrient composition analyses. The plant material was immediately transported to the laboratory. One half was placed in a refrigerator for approximately 3 h to cool down and then stored at −20 °C until further processing for ergot alkaloid content. The remaining half of the samples were placed in individual paper bags for species identification, obtaining a 100% tall fescue in all samples. After species identification, samples in paper bags were placed in an oven to dry the plant material and conduct Near Infrared Reflectance Spectroscopy (NIRS). Samples for ergot alkaloids analysis were freeze-dried and ground to pass through a 1 mm screen. Quantities of ergot alkaloids in fresh tall fescue were determined using HPLC with fluorescence detection [4,9,11]. See the online repository dataset for analyses.
3.5. Isoflavone Analysis
A powdered isoflavone supplement extracted from red clover was used to administer isoflavones for the respective treatment groups (Promensil, PharmaCare Laboratories, Warriewood, NSW, Australia). Quantifying isoflavones powder in Promensil, including biochanin A, formononetin, genistein, and daidzein, was performed similarly to [9,11] using LC-MS for detection.
Isoflavone extracts were prepared by adding 7 mL of 85% methanol in 0.5% acetic acid to ground samples in 50 mL conical polypropylene tubes. Samples were vortexed briefly and sonicated for 30 min at room temperature. Three milliliters of deionized water were added to each sample before vortexing and centrifuging for 8 min at 2200× g. The supernatant was filtered through a 0.45 μm GHP membrane syringe filter. Extracts were diluted, and flavone was added as an internal standard. One portion of each sample was analyzed as extracted, and a second portion was heated at 85 °C for five hours to hydrolyze isoflavone malonyl glucosides to the corresponding isoflavone glucosides; the difference between hydrolyzed and un-hydrolyzed portions determined concentrations of biochanin a-malonyl-glucoside and formononetin-malonyl-glucoside. LC-MS was used to analyze isoflavone extracts on a Waters Acquity UPLC coupled to a Waters Synapt G2 (q-ToF) high-resolution mass spectrometer. Chromatographic separation was obtained using a Waters BEH C18 UPLC column (1.7 μm, 2.1 mm × 150 mm). The mobile phase employed a mixture of water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B) in a linear gradient from 20% B to 80% B at a flow rate of 0.35 mL/min. The high-resolution mass spectrometer was operated in positive ion electrospray mode with a resolving power of ~14,000 and scanned from 100 to 1000 Da in 0.3 s. Leucine enkephalin was used to provide a lock mass (m/z 554.2615). Quantifying isoflavones using QuanLynx software with a linear calibration curve and internal standard method [10,19,20]. See the online repository dataset for analyses.
3.6. Prolactin Analyses
Serum prolactin concentrations were obtained using the radioimmunoassay protocol established by Bernard et al. [21] with intra- and inter-assay coefficients of variation of 7.18% and 9.58%, respectively.
3.7. Bacterial DNA Extraction, PCR, Sequencing, and Sequence Processing
The procedure for DNA extractions was similar to that described by Yu and Morrison [22]. After the chemical and physical cell lysis and precipitation of nucleic acids, metagenomic DNA was purified with RNase and proteinase K treatment, followed by using QIAamp columns from the Qiagen DNA Stool Mini Kit (Qiagen, Hilden, Germany). Metagenomic DNA concentration was determined using a DeNovix DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA). Extractions were stored at −20 °C until sequencing library preparation. Bacterial 16S rRNA genes were amplified by PCR. The V4 region of the 16S hypervariable gene was targeted using the primers 515F and 806R as described by Parada (2016) and Apprill (2015) under the following conditions for the first step of PCR [23,24]: initial denaturation step of 95 °C for three minutes then 25 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 55 °C, and elongation for 30 s at 72 °C, followed by a final elongation step at 72 °C for 5 min. Gel electrophoresis was used to ensure correctly sized DNA was amplified, then DNA was purified using AMPure XP beads (Beckman Coulter, Inc., Brea, CA, USA) as per the manufacturer’s protocol. Unique indices (Nextera, Illumina Inc., San Diego, CA, USA) were added to the purified and amplified bacterial and archaeal 16S DNA via PCR using the following conditions: initial denaturation at 95 °C for 3 min followed by eight cycles with a denaturation step of 30 s at 95 °C, annealing at 55 °C for 30 s, and an elongation step at 72 °C for 30 s, followed by a final elongation step at 72 °C for 5 min. Amplified and barcoded DNA was again purified using AMPure XP beads as per the manufacturer’s protocol. The amplicons were quantified and quality-checked on a Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Bacterial and archaeal amplicons were sequenced on the Illumina MiSeq (Illumina Inc., San Diego, CA, USA) using a v2 flow cell, 2 × 250 kit per manufacturer protocol. Read filtering, denoising, dereplication, sample inference, chimera removal, merging of paired-end reads, and taxonomic classification were performed with packages’ phylogeny and ‘DADA2’ [25,26]. Read filtering was based on quality profiles for all samples, filtering for a quality score greater than or equal to 25. Filtering parameters were set to truncate (truncLen) at 245 for forward and 245 for reverse reads. The maximum expected errors (maxEE) were set to 2 for both forward and reverse reads. Taxonomic classification was performed at the genus level using the SILVA v138.1 database [26].
3.8. Statistical Analyses
Physical data were analyzed using the PROC MIXED procedure on SAS 9.4 (SAS Inst. Inc., Cary, NC, USA) to determine the effects of the isoflavone dosing and frequency on rectal temperature, prolactin concentrations, average daily gain, and vasculature measurements. The PROC UNIVARIATE procedure was utilized to assess normality. All variables were approximately normally distributed and thus proceeded to be analyzed raw. The model included treatment × week as fixed effects and animal × treatment as random effects. The least-squares means were compared using Fisher’s LSD. Variables considered significant when tested at a p-value of <0.05, with tendencies declared at p-values between 0.05 and 0.10.
For rumen bacterial communities, analyses were conducted in the R environment. Alpha-diversity metrics were estimated using Chao1, Shannon, and Simpson indices. The normality of alpha diversity measurements was assessed using Shapiro–Wilks (W > 0.90), and differences among treatments were analyzed with a Kruskal–Wallis H-test. A Bray–Curtis principal coordinate analysis (PCoA) was used to estimate beta-diversity metrics. Differences among treatments for the PCoA were tested using a PERMANOVA with 999 permutations using the ‘vegan’ package [27]. Differential abundances in rumen bacterial communities among treatments were analyzed with ‘DESeq2’ [28], using a negative binomial model and a Wald test to address community differences. Multiple testing is addressed in ‘DESeq2’ using the Benjamini-Hochberg method for false discovery rates.
Author Contributions
Conceptualization, P.R.M. and K.J.M.; methodology, P.R.M., K.J.M., M.T.H., J.F.C.-L., F.N.S. and G.E.B.; formal analysis, J.F.C.-L. and M.T.H.; investigation, P.R.M., K.J.M., J.F.C.-L., F.N.S. and G.E.B.; writing—original draft preparation, J.F.C.-L. and M.T.H.; writing—review and editing, P.R.M., M.T.H., K.J.M., J.F.C.-L., F.N.S. and G.E.B.; project administration, P.R.M., K.J.M., J.F.C.-L., F.N.S. and G.E.B.; funding acquisition, P.R.M. and K.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PharmaCare Inc. (Warriewood, NSW, Australia).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in FigShare at DOI: 10.6084/m9.figshare.28278602.
Acknowledgments
We thank Rebecca Payton for project assistance and prolactin analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kallenbach, R.L. Describing the dynamic: Measuring and assessing the value of plants in the pasture. Crop Sci. 2015, 55, 2531–2539. [Google Scholar] [CrossRef]
- Lusby, K.; McMurphy, W.; Strasia, C.; Smith, S.; Muntz, S. Effects of fescue endophyte and interseeded clovers on subsequent finishing performance of steers. J. Prod. Agric. 1990, 3, 103–105. [Google Scholar] [CrossRef]
- Wu, J.-H.; Li, Q.; Wu, M.-Y.; Guo, D.-J.; Chen, H.-L.; Chen, S.-L.; Seto, S.-W.; Au, A.L.; Poon, C.C.; Leung, G.P. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J. Nutr. Biochem. 2010, 21, 613–620. [Google Scholar] [CrossRef]
- Koontz, A.; Bush, L.; Klotz, J.; McLeod, K.; Schrick, F.; Harmon, D. Evaluation of a ruminally dosed tall fescue seed extract as a model for fescue toxicosis in steers. J. Anim. Sci. 2012, 90, 914–921. [Google Scholar] [CrossRef]
- Foote, A.; Kristensen, N.; Klotz, J.; Kim, D.; Koontz, A.; McLeod, K.; Bush, L.; Schrick, F.; Harmon, D. Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen. J. Anim. Sci. 2013, 91, 5366–5378. [Google Scholar] [CrossRef]
- Eisemann, J.H.; Huntington, G.B.; Williamson, M.; Hanna, M.; Poore, M. Physiological responses to known intake of ergot alkaloids by steers at environmental temperatures within or greater than their thermoneutral zone. Front. Chem. 2014, 2, 96. [Google Scholar] [CrossRef]
- Jia, Y.; Harmon, D.L.; Flythe, M.D.; Klotz, J.L. Interaction of isoflavones and endophyte-infected tall fescue seed extract on vasoactivity of bovine mesenteric vasculature. Front. Nutr. 2015, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.L. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 2015, 7, 2801–2821. [Google Scholar] [CrossRef]
- Melchior, E.A.; Smith, J.K.; Schneider, L.G.; Mulliniks, J.T.; Bates, G.E.; Flythe, M.D.; Klotz, J.L.; Ji, H.; Goodman, J.P.; Lee, A.R. Effects of endophyte-infected tall fescue seed and red clover isoflavones on rumen microbial populations and physiological parameters of beef cattle. Transl. Anim. Sci. 2019, 3, 315–328. [Google Scholar] [CrossRef]
- Flythe, M.; Kagan, I. Antimicrobial effect of red clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium, Clostridium sticklandii. Curr. Microbiol. 2010, 61, 125–131. [Google Scholar] [CrossRef]
- Aiken, G.E.; Flythe, M.D.; Kagan, I.A.; Ji, H.; Bush, L.P. Mitigation of ergot vasoconstriction by clover isoflavones in goats (Capra hircus). Front. Vet. Sci. 2016, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.E.; Flythe, M.D.; Kagan, I.A.; Aiken, G.E. Biochanin A (an isoflavone produced by red clover) promotes weight gain of steers grazed in mixed grass pastures and fed dried-distillers’ grains. Crop Sci. 2017, 57, 506–514. [Google Scholar] [CrossRef]
- Aiken, G.; Kirch, B.; Strickland, J.; Bush, L.; Looper, M.; Schrick, F. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers. J. Anim. Sci. 2007, 85, 2337–2345. [Google Scholar] [CrossRef]
- Strickland, J.R.; Aiken, G.E.; Klotz, J.L. Ergot alkaloid induced blood vessel dysfunction contributes to fescue toxicosis. Forage Grazinglands 2009, 7, 1–7. [Google Scholar] [CrossRef]
- Aiken, G.E.; Flythe, M.D. Vasoconstrictive responses by the carotid and auricular arteries in goats to ergot alkaloid exposure. Front. Chem. 2014, 2, 101. [Google Scholar] [CrossRef]
- Flythe, M.; Harrison, B.; Kagan, I.; Klotz, J.; Gellin, G.; Goff, B.; Aiken, G. Antimicrobial activity of red clover (Trifolium pratense L.) extract on caprine hyper ammonia-producing bacteria. Agric. Food Anal. Bacteriol. 2013, 3, 176–185. [Google Scholar]
- Guan, L.L.; Nkrumah, J.D.; Basarab, J.A.; Moore, S.S. Linkage of microbial ecology to phenotype: Correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 2008, 288, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kagan, I.A.; Flythe, M.D. Factors affecting the separation and bioactivity of red clover (trifoliumpratense) extracts assayed against clostridium sticklandii, a ruminal hyper ammonia-producing bacterium. Nat. Prod. Commun. 2012, 7, 1605–1608. [Google Scholar] [CrossRef]
- Kagan, I.A.; Flythe, M.D. Thin-layer chromatographic (TLC) separations and bioassays of plant extracts to identify antimicrobial compounds. J. Vis. Exp. JoVE 2014, 85, 51411. [Google Scholar]
- Bernard, J.; Chestnut, A.; Erickson, B.; Kelly, F. Effects of prepartum consumption of endophyte-infested tall fescue on serum prolactin and subsequent milk production of Holstein cows. J. Dairy Sci. 1993, 76, 1928–1933. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).