Abstract
Current global research aims to explore the key role of diet and understand the benefits of a balanced diet. Furthermore, many authors have pointed to the added value of by-products as a solution to make food production chains more environmentally and economically sustainable. By-products emerge as an alternative matrix to fermentation, and the fermentation process has the potential to transform by-products into value-added products through an efficient and sustainable process. During fermentation, besides the consumption of molecules to grow, microbial enzymes act on several phytochemical compounds, creating new derivative compounds that affect the flavour and function of fermented beverages. As an alternative for consumers with lactose intolerance or vegan or vegetarian diets, new beverages produced from plant by-products and probiotic bacteria hold great promise for the global functional food market. Several challenges were overcome in developing these new products from by-products, namely the availability and quality/standardization of raw materials, adapted microbial starter cultures for fermentation, and optimization of production processes to maximize consumer acceptance and product yield. This review provides an overview of recent research/developments in the field of new fermented beverages from by-products, and aspects related to their functionality, beyond the challenges of these new beverages.
1. Introduction
The largest amount of food waste is produced in the fruit and vegetables sector. According to the Food and Agriculture Organization of the United Nations [1], every year, approximately 88 million tons of food waste are generated in the European Union, the cost of which is estimated at 143 billion euros. Food waste is not only an ethical or economic problem but also has significant consequences for the depletion of natural resources [2]. In addition, according to future projections, the world population will reach 8 billion by 2030 and exceed 9 billion by 2050, creating a demand for high-quality food [3]. To ensure that balanced foods are available in sufficient quantity and variety, the food industry must move towards a more sustainable model in which by-products can be converted very efficiently into high-value ingredients and/or products. Nonetheless, food systems are complex and depend on many economic, cultural and environmental factors [4].
A large amount of by-products and wastes are generated from fruit, vegetable, and cereal manufacturing industries, with a great economic potential, and are highly detrimental to the environment [5]. Thus, the circular bioeconomy aims to reduce the use of non-renewable resources while increasing the value of by-products that contribute to economic efficiency and growth [6,7,8]. Furthermore, the nutritional value of by-products can be high, often higher than their edible parts. In addition, these by-products may also contain bioactive compounds with greater antioxidant activity than the pulp, as their phytochemical profiles differ from other parts of the fruit [9]. Thus, by-products of the production of vegetables and fruits still contain biologically significant components such as fibres, vitamins, carotenes, antioxidants, and organic acids, which are crucial for maintaining human health [10,11,12]. In addition, the utilization of by-products contributes to the improvement of the food production chain’s economic and environmental sustainability, reducing the economic and environmental issues brought on by waste disposal in the food industry [13,14].
In recent years, there has been an increased investment in personal health and well-being. Modern lifestyles have problems such as increased consumption of foodstuffs with poor nutritional value, stressful timetables and insufficient exercise, all of which are detrimental to individual health [15]. Thus, there is increased consumer interest in functional foods which are those that provide health benefits to the consumer in addition to basic nutrition and can reduce the risk of certain diseases [13]. However, they must be sensorily acceptable and with reasonable prices to be accepted by many consumers [2]. Foods with natural bioactive compounds (e.g., dietary fibre), food with added bioactive substances (e.g., probiotics) and/or food ingredients added to conventional foods (e.g., prebiotics) are examples of functional foods [16]. Probiotics are defined as live microorganisms that have favourable effects on a consumer’s health when given in adequate quantities, while a prebiotic is a substrate that is selectively consumed by host microorganisms that improve health [17,18]. Furthermore, the present global study is focused on developing the crucial role that food and beverages in general play in preserving quality of life as well as in the prevention and treatment of chronic diseases. The consumption of natural products is rising as consumers become more aware of the value of high-quality beverages that offer health benefits [13,19].
In addition, around of worldwide, the requirement for alternatives to dairy products with high functional value, as well as fresh, nutritious, healthful and appetizing foods and drinks, are also increasing among consumers. Furthermore, there are popular movements toward vegetarianism and veganism, as well as the spread of lactose intolerance and milk protein allergy, which are accelerating [16]. Therefore, more and more non-dairy beverages are receiving increasing attention due to the presence of phytochemicals and their nutritional value [20].
Overall, the fruit and vegetable by-products are a rich resource that can be used to create a variety of products and provide numerous health benefits and these can be successfully incorporated into several foods, of which plant-based fermented products stand out [13]. In particular, fermented plant beverages are gaining popularity for their health-promoting properties, including protection against digestive disorders [20]. These products often use probiotic strains, making them an important functional food that, depending on the substrate used, can provide unique and specific health benefits [21].
Considering that fermented beverages are seen as a healthy alternative to soft beverages, alternative raw materials for fermentation are needed [22]. Additionally, to promote zero-waste technology and reduce manufacturing costs, numerous options for the utilization of by-products could be considered; thus, the production of plant-based fermented beverages to valorise the by-products is an interesting approach. One of the ways to produce beverages from by-products is the extraction of the liquid and further optimisation with key ingredients for the fermentation process, which can be visualized in Figure 1; however, the approach to produce fermented beverages can follow different methods.
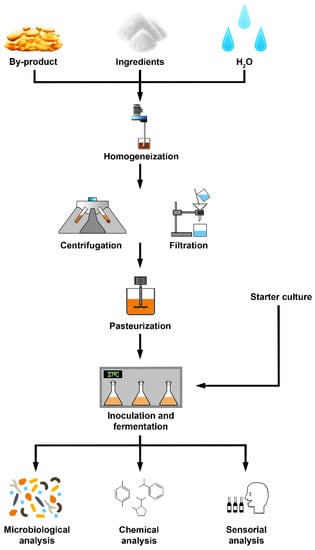
Figure 1.
Representative scheme of the development of a plant-based fermented beverage.
The fermentation process has been used for centuries in human food production and consumption. Fermented foods have long been an important component of the human diet, accounting for over one-third of global food consumption [22]. Fermentation has long been utilized as a safe preservation method that utilizes as few resources as possible and the use of lactic acid bacteria (LAB) for fermentation is known for its great capacity to enhance the functional, nutritional and sensory characteristics of plant and animal foods [23].
The process of fermentation consists in the mechanism by which microorganisms obtain energy from a carbon source, by which the edible and non-edible components of food undergo chemical and enzymatic processes, which give rise to certain metabolites as a result of the breaking of bonds between complex molecules and its transformation into simpler molecules [22]. A product obtained from a fermentation process, presents a nutritional improvement, such as the increase in the digestibility and bioavailability of nutrients as well as their antioxidant activity [24,25]. Regarding sensory characteristics, this process adds new flavours and textures to food [20,22,24].
This review presents an updated overview of the current knowledge on the main agri-food industry by-products used in the development of new fermented beverages, focusing on nutritional content, sensory properties and the evaluation of fermented products, as well their commercial availability and a way forward.
2. Fermented Beverages and Health Benefits
Several researchers have attempted the development of fermented beverages with health benefits in this direction [20]. Regarding beverages, their most useful characteristic is that they are highly suitable transporters to deliver and incorporate bioactive compounds and nutrients into the body. Additionally, the advantage is that refrigerated products can maintain their consumption viability for longer times [26,27]. Most known fermented beverages come from dairy by-products; however, due to the popular trend of vegetarians and vegans, as well as the accelerated appearance of lactose intolerance and allergy to cow’s milk proteins [16], there has been an increase in research and development focusing on the production of fermented beverages made from plant-based products and their health benefits [27,28]. However, the use of probiotic cultures in plant- based products presents a major challenge [29]. In addition, the food market is paying more and more attention to functional foods; thus, the by-products from the food industry are an important natural source of bioactive compounds and other high-value ingredients with significant health-enhancing properties [30]. Therefore, fermented beverages made from by-products can yield both greater amounts of health-beneficial bioactive compounds and bacterial probiotic strains [13,24,25]. In this way, recent studies have demonstrated that antioxidant substances, as well as phenolic and flavonoid molecules, help prevent cellular damage and cellular ageing in tissues, as well as life-threatening diseases such as numerous cancers, coronary heart disease and cerebrovascular disease [31,32,33,34]. Moreover, it is known that the biological activity of the final product can increase after the fermentation process. For example, previous studies have reported that the isoflavone profile of okara and soy beverages modifies during lactic acid fermentation, which enhances the bioactivity and bioavailability of isoflavone glycosides due to the conversion of isoflavone glycosides into their aglycones constituents [24,35,36].
Thus, fermented by-products, such as okara, acerola by-product, black tea residues and brewer’s spent grain present a greater amount of important nutrients, such as vitamins, amino acids, proteins and important bioactive compounds, such as phenolics, flavonoid compounds, as well as enhanced antioxidant activity, in comparison to their respective unfermented controls. This makes them invaluable resources to produce functional, healthy beverages employing the fermentation process, with benefits to both the environment, food processing industry processing costs and production and healthcare costs [24,37,38,39,40].
In addition to the nutrients and bioactive compounds, the intake of probiotic foods can be beneficial for many gastrointestinal diseases and, in addition, can have anti-pathogenic, anti-allergic, anti-angiogenic, anti-cancer and anti-inflammatory effects that affect other organs and also have anti-diabetic effects, anti-obesity effects or even effects on the brain and central nervous system [18,41]. Furthermore, the probiotic bacteria can improve the intestinal gut microbiota by replacing potentially pathogenic microorganisms within the intestinal gut with their species and strains, alongside others that benefit from symbiotic associations with them. These provide the human organism with health-improving metabolites and nutrients [25,27,30,42].
Nevertheless, there are some circumstances where fermented beverages fail to meet consumer sensorial expectations, due to factors such as pungent, unappealing smells, undesirable tastes or unpleasant textures. These can come from certain compounds, including those beneficial to health, which can have a negative influence on the overall sensorial characteristics [24,27]. Further research is required to properly adapt fermented beverages to the sensorial expectations of the consumer without diminishing their health-enhancing activity and their properties, and randomized controlled clinical trials can be used to emphasise this [20].
3. Fermentative Processes
Fermentative processes can be performed in batch or continuous and fed-batch systems. In the batch mode, the substrate and producing microorganisms are incorporated simultaneously in the process beginning and are not removed until the end of the fermentation [30]. In continuous and fed-batch modes, the producing microorganism may be immobilized and reutilized for multiple cycles, resulting in improved efficiency [30]. The microorganisms may grow on solid support (solid-state fermentations), but many industrial fermentations take place in liquid media, as required to produce plant-based beverages. Usually, the fermentation occurs in shake flasks since they are simple to use and it has been reported that for one-step fermentation, it improves mass transfer and reduces the potential inhibition by the substrate [43]. Fermentative processes also can be classified according to the microorganisms used to perform the fermentation. LAB are by far the most used, but other bacteria or fungi and yeasts have also been studied and used by researchers. The starter cultures used in fermented beverages may change from pure cultures, when for example a single probiotic LAB strain is used, to a complex symbiotic relationship, including different bacteria and yeasts [44].
3.1. Bacteria
Industrial food by-products and wastes are highly susceptive to spontaneous fermentation by the naturally occurring native microbiota in this kind of product. However, directed and controlled fermentations can be used to produce valorised final fermented products and LAB can play important roles in plant-derived waste fermentations [30]. According to Yu et al. [45], generalist LAB may be isolated from an extensive diversity of habitats but have different levels of performance, while specialist LAB are present in a limited range of habitats, and they are very well adapted to them.
With more than 30 genera and 300 species, LAB are a broad group of Gram-positive bacteria that have actively been used worldwide in food production due to their great potential to improve functional, technological, nutritional and sensory properties of food products; additionally, they are recognized to be healthy, and help improve the fermentative processes and the foods and feeds preservation [46]. They may act as native microflora or as starter cultures when added under controlled conditions. LAB are specialized in the bioconversion of carbohydrates, lipids, proteins, phenolics, some vitamins and minerals in organic acids such as lactic, acetic, propionic and butyric acids that reduce the pH of the fermented food, which will increase the product’s shelf life since the growth and inhibits the survival of many pathogenic bacteria [23,30,47]. Additionally, the bacteriocins (peptides), exopolysaccharides and volatiles produced as well as the direct competition of microbes also play an important role in antimicrobial activity in different food products. The bacteriocins also promote the nutritional stability of the final fermented food and some metabolites restrict the production of free radicals and reduce reactive oxygen species, increasing antioxidant activity in final products [16,23]
For the controlled fermentation of dairy products, the most common LAB cultures used are Streptococcus thermophilus, Lactococcus lactis, Leuconostoc spp. and Lactobacillus spp. However, the genera Pediococcus, Oenococcus and Weissella also play an important role for the development of plant-based fermented beverages [30,48]. The ideal LAB for a specific process of fermentation will be determined according to the characteristics of these industrial bacteria and of the plant-based material to be fermented. One aspect to keep in mind is LAB ability to produce acid lactic, which may be reintegrated into the food chain, improving protein digestibility and sensory qualities of fermented products [30]. Many times, co-culturing may be the best option. For example, L. plantarum and Pediococcus pentosaceus conjointly with monosodium glutamate were used to produce functional milk, increasing their content in bioactive metabolites, such as g-aminobutyric acid [49]. Kariyawasam et al. [50] combined Weissella cibaria and L. rhamnosus (adjunct cultures) with L. acidophilus, Bifidobacterium longum and Streptococcus thermophilus (starter culture) in cheese samples, resulting in the higher production of metabolites (lactic acid, acetic acid, bacteriocin) and antimicrobial activity. Co-culturing also was used in fruit by-products, soybean by-products and okara increasing the amount of organic acids and the bioconversion of polyphenols to antioxidants [23].
In addition to LAB, acetic acid bacteria (AAB) also may participate in the fermentation process of beverages. Fermentative bacteria such as Clostridium and Bacillus species, have been used to valorise food by-products and wastes with the main propose to obtain functional ingredients such as lactic acid, poly-g-glutamic acid and bioactive peptides, or other compounds of industrial interest such as glycosidases or caproate. For example, Bacillus coagulans, Bacillus amyloliquefaciens, Bacillus licheniformis and B. subtilis have been employed to ferment soy, rice, oak, fruit and sorghum products, while Clostridium cellulovorans and Clostridium beijerinckii strains have been used to fermented fruit wastes. These bacteria have been used alone or combined with other bacteria and/or fungi to produce additional flavour and health-promoting compounds [30,51,52].
3.2. Fungi and Yeast
Fungi and yeasts grow easily in food by-products and wastes producing a wide range of extracellular enzymes, antibiotics, compounds and pigments such as glycosidase and lipase enzymes, lactic acid, functional carbohydrates, organic acids or carotenoids. In fact, this kind of fermentation process promotes the release of important compounds from the plant substrate and/or favour, with the biotransformation of the compounds originally present in the by-products or residues being widely used to obtain several food ingredients with additional nutritional and health-promoting properties [30]. As for the LAB selection, for fungal fermentation, it is also extremely important to keep in mind that the production of specific compounds is generally dependent on the proprieties of the by-product or waste materials to be fermented as well as the fungal species used during the fermentation process. These raw materials usually are rich in nondigestible fibres and oligosaccharides that may lead to improved technological, nutritional and functional properties in foods previously fermented [30,53,54]. For example, fermentations developed by Aspergillus and Trichoderma species produce arabinoxylo-oligosaccharides and xylose, depending on by-product or waste used (cellulosic rich vegetable wastes). Aspergillus spp. also may produce fructooligosaccharides (bagasse from sugar cane, banana peel and leaves) and isomaltulose, which have prebiotic properties (cane molasses), while Yarrowia lipolytica may produce citric and oleic acid (olive-mill wastewaters) and a combined fermentation by Aspergillus Pleurotus and Hericium improve polyphenols extraction, increasing antioxidant activities and fibre (cooked-maize residue) [30]. When fermentation is performed by fungi, the metabolic traits are so vast that a strategic fermentation should be developed to improve the production of compounds with high added value, valorising food by-products and wastes.
Among yeasts, Saccharomyces cerevisiae is the most studied one and it plays an important role in fermented beverages and food production due to its ability to metabolize sugars during the fermentative process. Nevertheless, recent research has revealed a huge yeast biodiversity that includes dozens of species, which in addition to conventional Saccharomyces cerevisiae, are often found within symbiotic communities [44,55]. For example, S. cerevisiae was used pure or in co-culture with Fructilactobacillus florum (lactic acid bacteria) to ferment apple pomace and wheat flour [56]. Non-Saccharomyces yeasts, such as Brettanomyces spp. have been explored for producing reduced alcohol beverages since they have several distinct properties that give them an advantage in unconventional fermentations. Lowton et al. [44] revised the biotechnological potential of the specie Brettanomyces claussenii, for the development of innovative fermented beverages using non-traditional substrates, such as by-products of agriculture (mainly the ones containing lactose). As for bacteria and fungi, the selection of yeast strains also should consider their characteristics related to the production of peptides with antimicrobial properties, flavour compounds, different enzymatic activities, functional characteristics such as probiotics that could have health-promoting effects, as well as the raw material proprieties [57].
4. Fermented Beverages from By-Products
Plant-based fermented beverages have grown in scale and quality in recent years since beverages are one of the best ways to provide bioactive compounds and nutrients to our bodies. These beverages are considered sustainable functional beverages, which usually results from the fermentation of raw materials by Lactobacillus or yeasts and are frequently used as alternative options for milk and other dairy-based foods by the population in general, but also by vegetarian and lactose intolerant population [27,44].
Additionally, the rapid growth of the world’s population has resulted in the fast industrialization of sectors such as food production and processing, leading to a huge production of food by-products and wastes, which are known to be a rich source of healthier important compounds. These products have global environmental, social and economic consequences since, for example for fruit processing industries, by-products can amount to up to 50% of the whole fruit and 40 to 50% of fruits and vegetables produced are discarded globally [58]. In the next sections the use of food by-products and wastes to produce nutritional and bio-functional fermented beverages will be discussed.
4.1. Fruit and Vegetable—Based Fermented Beverages
Fruits and vegetable by-products are the most used plant raw materials for developing fermented beverages, since they have the highest wastage rates of any food. In Table 1, the papers found for vegetal or fruit-based fermented beverages that resulted from by-products and waste use are summarised. Regarding vegetable by-products, Li et al. [38] studied the distinctive metabolites of Lactobacillus plantarum during the fermentation process using by-products from black tea production (including broken tea segments and tea stalk). These authors concluded that fermented beverages have positive and negative impacts on the metabolic products and the characteristics of beverages from black tea. Overall, the flavours and organoleptic properties of fermented beverages were improved, but a decrease in total nutrients and some bioactive components (polyphenols, L-theanine and various biogenic amines) were verified. The aim of the Konrade et al. [2] study was to analyse the properties of extruded cereals supplemented with plant by-products (apple, carrot and pumpkin by-product flour) and to obtain fermented drinks from production losses. The use of apple and pumpkin by-products resulted in the highest dry matter content; however, the sensory evaluation demonstrated that samples using apple by-product had more intense levels of flavour and acidity, whereas the colour was most pronounced in samples with pumpkin by-products. With this preliminary research, it was concluded that this kind of food loss (visually defective products) could be used to obtain value products, promoting non-waste technologies and lowering manufacturing costs.
Zhu et al. [59] fermented Chinese bayberry pomace using sequential fermentation with yeast, LAB and AAB to study the beverage quality. The authors conclude that combined fermentation could preserve the colour of the beverage, slow down the degradation of anthocyanins, promote the conversion of flavonoids into flavonol aglycones, increase the phenolic acid contents and, consequently, could improve the overall quality of fermented beverages. Hien et al. [60], Aguilar [61] and Gutiérrez-Sarmiento et al. [62] fermented pineapple by-products (crumbs, peels and shells, respectively) with Saccharomyces cerevisiae (two first works) or natural starters for tepache fermentation. In the first work, the authors optimized the production process, obtaining a fermented beverage with 5–6% of ethanol, a light-yellow colour bright and transparent, light sweetness and a light aroma of ethyl alcohol and it was pungent but very mild (dryness is about 11–14° Bx), and they concluded that the slow freezing of raw materials will decrease the number of microorganisms, increasing the amount of reducing sugar and the yield of juice recuperation. Aguilar [61] uses an ultrasound pre-treatment to improve the fermentation process and final product characteristics. Their microscopy analysis revealed that 5 min of ultrasounds caused favourable modifications in the suspended matter that are important for the physical stability of beverages (colour uniformity, enhanced soluble solids during the initial stages of fermentation and helped the ethanol release from yeasts). Gutiérrez-Sarmiento et al. [62] studied microbial community structure, physicochemical characteristics and predictive functionalities of the Mexican tepache fermented beverage. This research showed that fermented beverages had 9.5 Brix degrees and an acidic pH. The ethanol, acetic and L-lactic acid concentrations increased significantly, while the total sugars decreased. The metabolic potential revealed that glycolysis and citrate cycle metabolism were more prevalent in the fungal community, whereas glycolysis, fructose and tricarboxylic acid metabolism were more common for the bacterial community.
Leonarski et al. [37] used acerola by-product as raw material to produce a kombucha-like beverage and bacterial cellulose and Vieira, Battistini et al. [25] and Vieira, Souz et al. [63] studied the influence of a fermented soy beverage enriched with acerola by-product on the gut microbiota. All authors used acerola by-products to develop their beverages. Leonarski et al. [37] observed that the amount of acerola by-product used affected the product formation. These authors obtained a similar ethanol concentration in all samples but verified that yeast metabolism was accelerated in the presence of higher concentrations of acerola by-product, leading to higher productions of acetic acid and bacterial cellulose; additionally, the physicochemical characteristics of cellulose produced were also positively affected when high concentrations of acerola by-product were used. Vieira, Battistini et al. [25] and Vieira, Souz et al. [63] identified that soy fermented beverages with probiotics and/or prebiotics resulted in different effects on the faecal microbiota from lean and obese individuals, since the symbiotic effect increased the cumulative production of acetic, lactic and formic acids for faecal microbiota from lean individuals, and of valeric and caproic acids for faecal microbiota from obese individuals after 48 h of fermentation in the TIM-2 in vitro model. Overall, the authors concluded that fermented soy beverages supplemented with acerola by-product and probiotic strains (L. acidophilus and B. longum) may be used as a potential symbiotic food due to the bifidogenic effect observed in both microbiotas, valorising at the same time this fruit by-product. Additionally, this fermented beverage may be considered as a good plant-based vehicle for the probiotic strains tested against gastrointestinal conditions simulated in vitro, mainly for B. longum, which showed the highest in vitro gastrointestinal resistance.
Liu et al. [27] studied the development of an alcoholic beverage from spent coffee grounds hydrolysates fermented with Torulaspora delbrueckii and Pichia kluyveri. The addition of yeast extracts improved the growth of both yeasts, especially P. kluyveri, resulting in higher ethanol production and did not impact on most of the alkaloid production but a reduction was observed in chlorogenic and caffeic acids content when P. kluyveri was used and more odour-active chemicals were produced, including acetate esters and 2-phenylethyl alcohol. Yeast extract had more impact on the fermentation performed by P. kluyveri. Álvarez et al. [64] extracted pectic polysaccharides from Opuntia ficus-indica peels by acid hydrolysis or ultrasound-assisted extraction and used them to develop a symbiotic beverage elaborated with apple juice and sugar cane using tibicos as a starter culture, mimicking an apple-based “Tepache” beverage. The use of ultrasound improves galacturonic acid presence in extracts, and red prickly pear shows high levels of galacturonic acid, yields and methoxylation degrees. The fermented beverage obtained exhibited functional characteristics since an increase of beneficial microorganisms defined as probiotics was observed (i.e., yeasts and bifidobacteria). The beverage was microbiologically and mechanically stable for 42 days at 7 °C.

Table 1.
Vegetal or fruit-based fermented beverages from by-products and wastes.
Table 1.
Vegetal or fruit-based fermented beverages from by-products and wastes.
| Source | Fermentative Microorganism | Conditions | Main Conclusions/Analysis | References |
|---|---|---|---|---|
| Broken black tea segments and stalks | Lactobacillus plantarum | Bacterial suspension: 1% Temperature: 37 °C Time: 24, 48, 72 and 96 h | Prolonged fermentation process increased flavonoid and phenolic compound amounts, and decreased nutrient, polyphenol and other bioactive compounds’ amounts. Overall improvement of the tea’s characteristic sensory/flavour acceptability. | [38] |
| Extruded cereals enriched with plant by-products | Saccharomyces cerevisiae Leuconostoc mesentericus | Substrate: 10 g sugar Temperature: 27 °C Time: 9 h pH: 4.24–4.35 | Combination of flours with 15% plant by-product content improved overall quality of fermented beverages derived from rye-oat extruded cereal rejects—both visual and sensory characteristics. | [2] |
| Chinese bayberry pomace | LAC AAB Yeasts | Sequential fermentations: 1—0.02% (w/w) live yeast 24 h at 25 °C 2—0.6% (w/w) LAB 48 h at 30 °C 3—0.1% (w/w) AAB 3 days at 30 °C | Combined fermentation with yeasts, LAB and AAB improved beverage quality, regarding appearance/colour, antioxidant activity and phenolic compound contents. | [59] |
| Pineapple crumbs by-product | Saccharomyces cerevisiae | Yeast suspension: 0.01% Temperature: 26–30 °C Time: 2 days pH: 4.2 22° Bx | Improved sensory results from the fermented beverage, particularly when submitted to slow freezing. Slow freezing at 10–12 °C was also effective in removing microorganisms from the fermented juice | [60] |
| Pineapple peels | Saccharomyces cerevisiae | Substrate: sugar solution (15% w/w) Temperature: 24 °C Time: 72 h pH: 3.3–3.5 12.5–12.7° Bx | Ultrasound treatment helped with the release of cell tissue contents, including the release of yeast cell contents, resulting in an overall improvement to the fermented beverage. However, the fermentation process can also be negatively affected, depending on intensity and timing of the ultrasound treatment. | [61] |
| Pineapple shells | Natural starters for tepache fermentation | Substrate: 10% (w/v) brown sugar Temperature: 30 °C Time: 24, 48 and 72 h pH: 5 | It was identified that Gibberella, Zygosaccharomyces, Talaromyces, Epicoccum, Kabatiella and Saccharomyces genera represented the fungal fungi. Lactobacillus, Leuconostoc, Acetobacter and Lactococcus genera, Leuconostcaceae and Streptococcaceae families represent the bacterial community. This starter culture converted the sugar in alcohol, lactic and acetic acid, which give the characteristic flavour, taste and aroma to tepache. Lactic, alcoholic and acetic fermentation achieved their highest points at 72 h, allowing to obtain a tepache beverage with the proper physio-chemical properties. | [62] |
| Acerola by-product | Komagataeibacter Rhaeticus Bacillus megaterium Bacillus aryabhattai Bacillus flexus Bacillus simplex Brettanomyces bruxellensis Zygosaccharomyces Bisporus | Bacterial suspension: 10% (v/v) Substrate: 70 g/L glucose/fructose 1:1 Temperature: 30 °C Time: 3, 6, 9, 12 and 15 days | Increase in content of bioactive compounds such as phenols and vitamin C from the by-product, and in microbiological metabolic activity with the introduction of the glucose/fructose substrate to the existing bioactive compounds, allowing for faster by-product fermentation. | [37] |
| Acerola by-product | Lactobacillus acidophilus Bifidobacterium longum Streptococcus thermophilus | Substrate: Sucrose (50 g/L) and dextrose (10 g/L) Temperature: 37 °C pH: 5.5 | The fermented soy beverage containing acerola by-product and inoculated with symbiotic probiotic/prebiotic microorganisms, improved the gut microbiota of lean and obese individuals. The change was more noticeable with obese individuals, whose gut microbiota approached the lean individual profile. This fermented beverage showed to be a good way to improve probiotic strains intake, mainly for B. longum, which was the probiotic that showed the highest in vitro gastrointestinal resistance. | [25,63] |
| Spent coffee grounds | Torulaspora delbrueckii Pichia kluyveri | Yeast suspension: 1% (v/v) Temperature: 20 °C Time: 14 days pH: 5 8.14–10.17° Bx | Yeast extract improved the growth of P. kluyveri but not the T. delbrueckii. Yeasts enhance the production of succinic and lactic acids and the production of more odour-active compounds such as short-chain esters. | [65] |
| Prickly pear (green and red) | LAC AAB Bifidobacteria Yeasts | Substrate: 60% saccharose Temperature: 25 °C Time: 108 h | Ultrasound extraction on the fermented beverage improved degree of methoxylation, but overall compound yield diminished in comparison to conventional extraction. Increase of beneficial microorganisms as probiotics was also reported when the beverages were stored for 3 weeks. | [64] |
4.2. Dairy-Based
The claim for dairy and dairy-based products has increased worldwide, leading to a large-scale production plants, which consequently increases the pollution level due to the increasing water utilization and emission of effluents and wastes into the environment [66]. Thus, this by-product or waste valorisation is also crucial. In Table 2, the sources, fermentative microorganisms, and fermentative conditions are summarised, as well as the main conclusions of research works where the dairy-based fermented beverages were produced from by-products and wastes.
Bahnas et al. [67] developed novel fermented products using by-products from the dairy industry containing papaya pulp and stevia leaves. The authors prepare fermented probiotic cheese whey and milk permeate-based beverages and did not observe significant differences between both beverages regarding pH and titratable acidity. These kinds of fermented beverages were evaluated with a higher overall score but both beverages were characterized with good sensorial characteristics and presented a high probiotic viable count, during 10 days of cooled storage. Chua and Liu [68] and Zhu et al. [69] fermented whey tofu, which is a resulting by-product from tofu manufacturers industry. Chua and Liu [68] studied the impact of amino acid supplementation on growth kinetics and flavour by Torulaspora delbrueckii in soy (tofu) whey alcoholic beverage fermentation. The addition of amino acids caused a quicker sugar consumption and inferior levels of residual sugar; nonetheless, it did not lead to greater ethanol production. Specifically, isoleucine led to an initial yeast growth rate that was smaller, as well as to a slower sugar utilization during the first 4 days, being the least preferred amino acid by this yeast. The use of amino acids resulted in a larger production of higher alcohols and their corresponding alcohol-derived esters and generally improves the sugar use and increases the flavour of the resultant whey beverage. The work conducted by Zhu et al. [70] was different. These authors optimized the lactic acid fermentation process to develop a tofu whey beverage containing high-isoflavone aglycones. The combination of L. rhamnosus and L. paracasei (1:1) favoured the enrichment of bioactive isoflavone aglycones, the percentage composition (daidzein, genistein) and the viable cell counts increased after the fermentation of tofu whey, and the pH value declined to 4.48 and the titratable acidity was 0.28%. The odour compounds, namely the aldehydes and alcohols, are usually metabolized at lower amounts after fermentation, making that product a functional probiotic drink enhanced with isoflavone aglycones. Begum et al. [71] developed a probiotic beverage using Chhana whey and watermelon juice that was optimized based on physicochemical and sensory characteristics of different ratios whey/juice used. A ratio of 75:25 of whey: juice fermented for 5 h at 37 °C was verified as the highest sensory score for overall acceptability. Additionally, the authors also concluded that the beverage can be preserved for 21 days at 4 °C, with greater acceptability with increasing probiotic bacterial count. Pega et al. [72] studied the impact of high-pressure processing (HPP) on quality and microbiological properties of a fermented drink produced from sweet whey. HPP was used after beverage fermentations, conserving the flavour and texture characteristics until 45 days without affecting chromatic parameters. This allowed to achieve a higher shelf-life than that obtained for traditional fermented beverages produced from milk. Plate counts for Streptococcus thermophilus and Lactobacillus delbrueckii after 200 MPa maintained optimal levels. Liutkevičius et al. [73] developed a fermented buttermilk-based beverage and studied its effect on young volunteers’ health issues. These authors verified that the anthropometric and body composition, arterial blood pressure, and pulse evaluation indicators of recipients did not change significantly when the beverage was consumed. The preferred quality parameters (synaeresis, viscosity, sensory properties and acceptability) were achieved for a buttermilk beverage containing 0.3% of milk protein concentrate.

Table 2.
Dairy-based fermented beverages from by-products and wastes.
Table 2.
Dairy-based fermented beverages from by-products and wastes.
| Source | Fermentative Microorganism | Conditions | Main Conclusions/Analysis | References |
|---|---|---|---|---|
| Cheese whey and milk permeate | Lactobacillus acidophilus Lactobacillus paracasei | Substrate: 10% papaya pulp and 1.0% stevia leaves extract Temperature: 40 °C Time: 8 h | The fermented whey beverage with both papaya pulp and stevia leaves extracts in the composition, demonstrated improved sensory characteristics up to 10 days, especially when fermented by Lactobacillus paracasei. | [67] |
| Tofu whey | Lactobacillus paracasei Lactobacillus rhamnosus Lactobacillus plantarum Leuconostoc mesenteroides | Bacterial suspension: 2% (v/v) Temperature: 37 °C Time: 24 h pH: 3.9–5.0 | The mixture of Lactobacillus rhamnosus and Lactobacillus paracasei species had potential to hydrolyze isoflavone glucosides into aglycones. Volatile compounds demonstrated sensorial improvements in fermented whey since significant amounts of aldehydes and alcohols responsible for the undesirable tofu whey odours were oxidized during fermentation. Additionally, the best fermentative conditions found were 32 °C, pH 6 and 20 h, which conduced to the highest cell count levels. | [70] |
| Tofu whey | Torulaspora delbrueckii | Yeast suspension: 1% (v/v) Temperature: 20 °C Time: 10 days | Individual aminoacids (valine, leucine, isoleucine and phenylalanine) had different effects on the fermentation activity of T. delbrueckii. In general, their use led to quicker sugar consumption and smaller residual sugar; nonetheless, it did not lead to higher ethanol formation or high yeast growth. Amino acids had a small impact on organic acid formation, no effect on isoflavone glucoside hydrolysis and resulted in the greater production of higher alcohols and their corresponding alcohol-derived esters. | [68] |
| Chhana whey and watermelon juice | Lactobacillus acidophilus | Bacterial suspension: 1% Substrate: 10% sugar Temperature: 37 °C Time: 5 h | The beverage blends with 70% whey/30% watermelon (blend C) juice and 75% whey/25% watermelon juice (blend D) showed the highest scores for sensory characteristics. However, blend D showed better nutrient content than blend C. Blend D also demonstrated a shelf-time of 21 days at 4 ± 1 °C and only 2 days shelf-time at 30 ± 1 °C. | [71] |
| Sweet whey | Streptococcus thermophilus Lactobacillus delbrueckii | Formulation: 75–85% (w/w) sweet whey 8–13% (w/w) milk 6–8% (w/w) sucrose 1–3% (w/w) inulin 0.1–1% (w/w) guar gum Temperature: 42–43 °C Time: 1, 30 and 45 days pH: 4.7 | High-Pressure processing of sweet whey prevented post acidification after the fermentation process and, consequently, higher quality sensorial attributes. The method also significantly reduced cell viability of the existing bacterial strains post-fermentation processing, allowing a shelf-life extension of the fermented beverage. | [72] |
| Buttermilk | Lactococcus lactis Leuconostoc mesenteroides | Starter culture: 0.025% Temperature: 24 °C pH: 4.5–4.6 | Buttermilk with 0.3% milk protein concentrate presented the most positive response towards fermentation. Synaeresis, viscosity, overall sensory and health properties showed improvements in comparison to the non-enriched version. | [73] |
4.3. Legume and Cereal-Based
Cereals are an important food source in the human diet and, consequently, large amounts of by-products are generated throughout the chain of production. Cereal by-products are rich in nutrients consisting of the germ and fibrous bran, which are produced during dry and wet milling of grains, brewers’ spent grain from the brewing industry or other by-products obtained from bread-making and starch production [74]. In Table 3 the papers found for cereal-based fermented beverages that resulted from by-products and wastes are summarised. Pasqualone et al. [75] studied the impact of processing variables on the physicochemical properties and flavour of a traditional beverage produced using wheat bran. The addition of natural starter, temperature increase and thermal treatment presented a favourable impact on total phenolic compounds, ferulic acid, antioxidant activity and brown index. Volatiles such as alcohols, carboxylic acids and esters also increased at higher fermentation temperature. Tan et al. [40] used submerged fermentation with Bacillus subtilis to produce an innovative nutritional beverage from brewers’ spent grains. Their results revealed that seven essential amino acids and citric acid cycle intermediates as well as antioxidant activity and total phenolic content increased, while all carbohydrates decreased in the beverages after fermentation, proving the potential of this nutritious beverage. Voss et al. [24,35] and Mok et al. [39] fermented okara, a soy by-product to develop a new beverage. In the first work, the authors studied the impact of fructose and fructooligosaccharides supplementation upon the fermentation of hydrolysed okara and its effects on bioactive compounds. They proved that Bifdobacterum animalis and/or Lactobacillus rhamnosus were effective in the fermentation of okara and the specific bacteria growth rate and acid production during fermentation were dependent mainly on the carbon source (fructose and FOS addition) present but the strain used also had an impact. Additionally, the concentrations of isoflavone aglycones were greater in fermented okara (enhancement of isoflavones’ bioavailability). Voss et al. [24] concluded that okara beverages fermented with probiotic bacteria maintained the viable cells through storage. The aglycones isoflavones increased during the okara fermentation and consequently can improve the functional benefits from the beverages for the consumers. Moreover, fermented beverages from okara hydrolysates presented an increase of the antioxidant and ACE inhibitory activities during the in vitro gastrointestinal digestion. Mok et al. [39] concluded that fermentation enhanced the level of soluble dietary fibre and the bioaccessibility of total phenolic content, amino acids, fatty acids and vitamin K2 MK-7. Similar to Voss et al. [24], these authors also verified that Bacillus subtilis remained viable after digestion. Additionally, the concentrations of acetic acid, propionic acid and butyric acid also were higher in fermented beverages. Their findings revealed that fermented okara has the potential to be used as a functional food ingredient.
Finally, De Francesco et al. [76] characterized 22 commercial Italian Grape Ale (IGA) beverages. This typical fermented beverage results from a merging of beer and wine concepts that combined the fermentation of barley malt wort and grape must blending for their development. IGAs beverages showed a complex and heterogeneous profile, mainly due the percentage of grape must that was used, the process stages of their addition (boiling, whirlpool, primary fermentation), different tank materials (steel, cement, wood), and the type of fermentation (bottom, top, spontaneous). Higher amounts of grape must lead to high ethanol concentration, low bitterness and low pH but it had a negative effect on foam stability. Additionally, wood tanks combined with spontaneous fermentation led to a sensory profile where the flavour is between wine and beer.

Table 3.
Cereal-based fermented beverages from by-products and wastes.
Table 3.
Cereal-based fermented beverages from by-products and wastes.
| Source | Fermentative Microorganism | Conditions | Main Conclusions/Analysis | References |
|---|---|---|---|---|
| Wheat bran and corn flour | Natural starters for spontaneous fermentation | Temperature: 4, 14 and 24 °C Time: 3 days | The parameters for the fermentation process can influence the physio-chemical/sensorial parameters of the fermented beverage. Lower fermentative temperatures (4 °C) reduce the intensity of less desired odours and increase total phenolic contents and antioxidant activity. Pasteurization post-treatment of fermented beverages further increases antioxidant activity. | [75] |
| Brewers spent grain | Bacillus subtilis | Temperature: 37 °C Time: 72 h 200 rpm | Submerged fermentation of BSG with Bacillus subtilis conduce to a decrease in sugars and significant increase in amino acids, antioxidant activity and total phenolic content, in comparison to the unfermented control beverage, resulting in an overall increase in nutritional value of the fermented beverage. | [40] |
| Okara | Bifidobacterium animalis Lactobacillus rhamnosus | Bacterial suspension: 1% Substrate: 3 or 6% (w/v) fructose and 2% (w/v) FOS Temperature: 37 °C pH: 4.55 ± 0.05 Time: 2, 4, 6, 8, 10, 12 and 24 h 9.65 ± 0.06° Bx 120 rpm | Okara samples supplemented with a carbon source showed a higher growth rate. The aglycone content and antioxidant activity show significant differences for okara fermented by different species, with the overall content of isoflavone aglycones increasing, the genistein presents the highest increase in the fermented okara by B. animalis. | [35] |
| Okara | Bifidobacterium animalis Lactobacillus rhamnosus | Bacterial suspension: 1% Substrate: 3 or 6% (w/v) fructose and 2% (w/v) FOS Temperature: 37 °C pH: 4.55 ± 0.05 Time: 0, 7, 14, 21, and 28 days 9.65 ± 0.06° Bx 120 rpm | Probiotic bacteria viability maintains throughout the entirety of the storage time. Significant increase of antioxidant activity, total phenolic content and various isoflavones (genistin and daidzin). Fermented beverage with in vitro ACE inhibitory activity. However, overall sensory characteristics of the fermented beverages are inferior in comparison to the unfermented version. | [24] |
| Okara | Bacillus subtilis | Inoculation: 106 CFU/g of B. subtilis/g okara Temperature: 37 °C Time: 72 h | Fermentation of okara increase soluble dietary fibre and nutrient yield, namely amino acids, fatty acids and bioactive compounds such as antioxidants and total phenolic content. Digestion of fermented okara also improves gut flora regulation. | [39] |
| Barley malt wort and grape must | Spontaneous fermentation S. cerevisiae | 22 commercial Italian Grape Ale samples were acquired on the Italian market | The step of the process in which the grape must is added (boiling, whirlpool, primary fermentation), the tank materials (steel, cement, wooden), and the fermentation type (bottom, top, spontaneous) are the main reasons that Italian Grape Ale shows a complex and heterogeneous profile. | [76] |
5. Fermented Beverages Challenges and Conclusions
Interest in functional beverages is growing, considering developed society’s increasing health awareness and marketing to health-conscious consumers. In addition, modern consumers are increasingly choosing foods that have favourable effects on their health, society and the environment. However, there is still much to understand regarding fermented beverages; advances in the discovery and characterization of probiotics (including yeast species) will enhance opportunities for health claims. Probiotics are intrinsically correlated to the health benefits of several food products, so it is reasonable to expect a similar effect when included in other types of new beverages coming to the market. Gut health is extremely important to human and animal well-being and our knowledge of probiotics, and their discovery must advance, as well as research for alternative probiotic delivery options.
On the other side, the processing of by-products is a valuable tool for the sustainable recovery of bioactive compounds in the implementation of innovative technologies. Furthermore, where the production of by-products from the food industry is inevitable, the best form of recovery is their reuse in the food chain. In general, plant raw materials have good potential for full utilization, because the resulting by-products are of high added value and can be sources of nutrients. In addition, processing technologies are key tools to remove properties harmful to consumer health, optimize the recovery of interesting compounds and valorise residues that would previously have been environmental problems in the food industry. Thus, according to several authors, the valorisation of food waste and by-products is a way to increase the food chains’ economic and environmental sustainability. Considering the above examples of beverages made from various by-products and their properties, it is possible to notice the existing potentials and how much remains to be explored in this context. In addition to adding value to beverages, the use of by-products can meet market demand for new products and reduce waste.
In this review, different approaches so far developed for the potential use of by-products in the production of fermented beverages with functional properties have been recorded. Nevertheless, the complete development of these new beverage formulations requires additional research, mainly in sensorial, physical and chemical characterization fields to develop a palatable flavour profile and a viable product.
Author Contributions
Conceptualization, bibliographic research and analysis, writing—original draft preparation: E.M.C.A., N.F.B.A. and G.B.V.; writing—review and editing: E.M.C.A. and G.B.V.; critical feedback: M.E.P.; project administration and funding acquisition: M.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds from FCT—Fundação para a Ciência e a Tecnologia, through project UID/Multi/50016/2021.
Data Availability Statement
Not available.
Acknowledgments
Acknowledgments are given to the Universidade Católica Portuguesa for providing the financial support to CBQF Associate Laboratory under the UID/Multi/50016/2021 FCT project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO—Food and Agriculture Organization of the United Nations. The State of Food and Agriculture—Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; p. 182. [Google Scholar]
- Konrade, D.; Lidums, I.; Klava, D.; Ence, E.; Kirse-Ozolina, A. Investigation of extruded cereals enriched with plant by-products and their use in fermented beverage production. Agron. Res. 2019, 17, 1346–1355. [Google Scholar] [CrossRef]
- European Commission Sustainable Food. Available online: https://ec.europa.eu/environment/archives/eussd/food.htm (accessed on 15 December 2022).
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Cernauskas, D.; Klupsaite, D.; Ruzauskas, M.; Alisauskaite, J.; Baltrusaitytė, A.; Dapsas, M.; et al. Antimicrobial, Antioxidant, Sensory Properties, and Emotions Induced for the Consumers of Nutraceutical Beverages Developed from Technological Functionalised Food Industry By-Products. Foods 2020, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qin, S.; Sirohi, R.; Ahluwalia, V.; Zhou, Y.; Sindhu, R.; Binod, P.; Rani, R.; Kumar, A.; Taherzadeh, M.J.; et al. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour. Technol. 2021, 332, 125181. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun, S.; Wong, J.W.C. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Mak, M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Sun, C. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Voss, G.B.; Rodríguez-Alcalá, L.M.; Valente, L.M.P.; Pintado, M.M. Impact of different thermal treatments and storage conditions on the stability of soybean byproduct (okara). J. Food Meas. Charact. 2018, 12, 1981–1996. [Google Scholar] [CrossRef]
- Campos, D.A.; Ribeiro, T.B.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M. Integral Valorization of Pineapple (Ananas comosus L.) By-Products through a Green Chemistry Approach towards Added Value Ingredients. Foods 2020, 9, 60. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Borgonovi, T.F.; Batista, C.L.F.M.; Lúcia, A.; Penna, B. Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidi fi cation and properties of probiotic fermented products. LWT—Food Sci. Technol. 2018, 98, 69–76. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S. Fruit and Vegetable-Based Beverages—Nutritional Properties and Health Benefits; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128166895. [Google Scholar]
- Devaki, C.S.; Premavalli, K.S. Fermented Vegetable Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128152713. [Google Scholar]
- Bansal, S.; Mangal, M.; Processing, O. Non-Dairy Based Probiotics: A Healthy Treat for Intestine Non-Dairy Based Probiotics: A Healthy Treat for Intestine. Crit. Rev. Food Sci. Nutr. 2016, 56, 1856–1867. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-waterhouse, D.; Waterhouse, G.I.N.; Cui, C. Food Science and Human Wellness Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Voss, G.B.; Monteiro, M.J.P.; Jauregi, P.; Valente, L.M.P.; Pintado, M.E. Functional characterisation and sensory evaluation of a novel synbiotic okara beverage. Food Chem. 2021, 340, 127793. [Google Scholar] [CrossRef]
- Vieira, A.D.S.; Battistini, C.; Bedani, R.; Saad, S.M.I. Acerola by-product may improve the in vitro gastrointestinal resistance of probiotic strains in a plant-based fermented beverage. LWT 2021, 141, 110858. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods: Commercial Trends, Research, and Health Implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Liu, J.; Li, C.; Li, J.; Zhu, D. Plant-Based Fermented Beverages and Key Emerging Processing Technologies. Food Rev. Int. 2022. [Google Scholar] [CrossRef]
- Gerliani, N.; Hammami, R.; Aïder, M. Production of functional beverage by using protein-carbohydrate extract obtained from soybean meal by electro-activation. LWT 2019, 113, 108259. [Google Scholar] [CrossRef]
- Voss, G.; Campos, D.; Pintado, M. Cereal Bars Added with Probiotics and Prebiotics. In Probiotics and Prebiotics in Foods: Challenges, Innovations, and Advances; Academic Press: Cambridge, MA, USA, 2021; pp. 201–217. [Google Scholar]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Voss, G.B.; Oliveira, A.L.S.; Alexandrea, E.M.d.C.; Pintado, M.E. Importance of polyphenols: Consumption and human health. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Academic Press: Cambridge, MA, USA, 2022; pp. 1–24. [Google Scholar]
- Voss, G.B.; Valente, L.M.; Pintado, M. Impact of fructose and fructooligosaccharides supplementation upon the fermentation of hydrolyzed okara and its impact upon bioactive components. SDRP J. Food Sci. Technol. 2018, 3, 460–472. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2018, 51, 148–155. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Zanella, E.; Stambuk, B.U.; de Oliveira, D.; Poletto, P. Production of kombucha-like beverage and bacterial cellulose by acerola byproduct as raw material. LWT 2021, 135, 110075. [Google Scholar] [CrossRef]
- Li, R.; Luo, W.; Liu, Y.; Chen, C.; Chen, S.; Yang, J.; Wu, P.; Lv, X.; Liu, Z.; Ni, L.; et al. The investigation on the characteristic metabolites of Lactobacillus plantarum RLL68 during fermentation of beverage from by-products of black tea manufacture. Curr. Res. Food Sci. 2022, 5, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Mok, W.K.; Tan, Y.X.; Chen, W.N. Evaluating the potential of Bacillus subtilis fermented okara as a functional food ingredient through in vitro digestion and fermentation. Food Biotechnol. 2021, 35, 136–157. [Google Scholar] [CrossRef]
- Tan, Y.X.; Mok, W.K.; Chen, W.N. Potential novel nutritional beverage using submerged fermentation with Bacillus subtilis WX-17 on brewers’ spent grains. Heliyon 2020, 6, e04155. [Google Scholar] [CrossRef]
- Brüssow, H. Probiotics and prebiotics in clinical tests: An update. F1000Research 2019, 8, 1157. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.; Rodrigues, L. One-step process for producing prebiotic arabino-xylooligosaccharides from Brewer’s spent grain employing Trichoderma species. Food Chem. 2019, 1, 86–94. [Google Scholar] [CrossRef]
- Lawton, M.R.; de Riancho, D.L.; Alcaine, S.D. Lactose utilization by Brettanomyces claussenii expands potential for valorization of dairy by-products to functional beverages through fermentation. Curr. Opin. Food Sci. 2021, 42, 93–101. [Google Scholar] [CrossRef]
- Yu, A.; Leveau, J.; Marco, M. Abundance, diversity, and plant-specific adaptations of plant-associated lactic acid bacteria. Environ. Microbiol. Rep. 2019, 12, 16–29. [Google Scholar] [CrossRef]
- Sedó Molina, G.E.; Shetty, R.; Xiao, H.; Wätjen, A.P.; Tovar, M.; Bang-Berthelsen, C.H. Development of a novel lactic acid bacteria starter culture approach: From insect microbiome to plant-based fermentations. LWT 2022, 167, 113797. [Google Scholar] [CrossRef]
- Girma, A.; Aemiro, A. Antibacterial Activity of Lactic Acid Bacteria Isolated from Fermented Ethiopian Traditional Dairy Products against Food Spoilage and Pathogenic Bacterial Strains. J. Food Qual. 2021, 2021, 9978561. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.L.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT 2021, 135, 110061. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Jeewanthi, R.K.C.; Lee, N.-K.; Paik, H.-D. Characterization of cottage cheese using Weissella cibaria D30: Physicochemical, antioxidant, and antilisterial properties. J. Dairy Sci. 2019, 102, 3887–3893. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Comparison of optimization approaches (response surface methodology and artificial neural network-genetic algorithm) for a novel mixed culture approach in soybean meal fermentation. J. Food Process Eng. 2019, 42, e13124. [Google Scholar] [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Direct IBE fermentation from mandarin orange wastes by combination of Clostridium cellulovorans and Clostridium beijerinckii. AMB Express 2019, 9, 1. [Google Scholar] [CrossRef]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation to Re-cycle Apple By-Products for Wheat Bread Fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef]
- Chebaibi, S.; Grandchamp-Leriche, M.; Clément, T.; Burgé, G.; Allais, F.; LAZIRI, F. Improvement of protein content and decrease of anti-nutritional factors in olive cake by solid-state fermentation: A way to valorize this industrial by-product in animal feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef]
- Hittinger, C.T.; Steele, J.L.; Ryder, D.S. Diverse yeasts for diverse fermented beverages and foods. Curr. Opin. Biotechnol. 2018, 49, 199–206. [Google Scholar] [CrossRef]
- Martău, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 742020. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Merín, M.G.; Belloch, C. Editorial: Biotechnological Applications of Yeasts in Beverages and Food: From Fermentation to Innovation. Front. Microbiol. 2022, 13, 970418. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.L.; Arruda, T.Y.P.; Morzelle, M.C.; Pereira, A.P.A.; Casarotti, S.N. Fruit by-products as potential prebiotics and promising functional ingredients to produce fermented milk. Food Res. Int. 2022, 161, 111841. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Ye, X.; Zhou, Z. Mixed fermentation of Chinese bayberry pomace using yeast, lactic acid bacteria and acetic acid bacteria: Effects on color, phenolics and antioxidant ingredients. LWT 2022, 163, 113503. [Google Scholar] [CrossRef]
- Hien, T.T.; Khang, V.C.; Van Muoi, N.; Truc, T.T. Production of a fermented beverage from pineapple (Ananas comosus) byproduct crumbs. Mater. Today Proc. 2022, 60, 2034–2042. [Google Scholar] [CrossRef]
- Aguilar, K. Evaluating ultrasound pre-treatment as a tool for improving the process of a fermented beverage made from pineapple by-products. Braz. J. Food Technol. 2022, 25, 1–13. [Google Scholar] [CrossRef]
- Gutiérrez-Sarmiento, W.; Peña-Ocaña, B.A.; Lam-Gutiérrez, A.; Guzmán-Albores, J.M.; Jasso-Chávez, R.; Ruíz-Valdiviezo, V.M. Microbial community structure, physicochemical characteristics and predictive functionalities of the Mexican tepache fermented beverage. Microbiol. Res. 2022, 260, 127045. [Google Scholar] [CrossRef]
- Vieira, A.D.S.; de Souza, C.B.; Padilha, M.; Zoetendal, E.G.; Smidt, H. Susana Marta Isay Saad Koen Venema 5 Impact of a fermented soy beverage supplemented with acerola by-product on the gut microbiota from lean and obese subjects using an in vitro model of the human colon. Appl. Microbiol. Biotechnol. 2021, 105, 3771–3785. [Google Scholar] [CrossRef]
- Álvarez, S.A.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Gallegos-Infante, J.A.; Pérez-Martínez, J.D.; Rosas-Flores, W. Functional fermented beverage made with apple, tibicos, and pectic polysaccharides from prickly pear (Opuntia ficus-indica L. Mill) peels. J. Food Process. Preserv. 2021, 45, e15745. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Liu, S.Q. The potential of spent coffee grounds hydrolysates fermented with Torulaspora delbrueckii and Pichia kluyveri for developing an alcoholic beverage: The yeasts growth and chemical compounds modulation by yeast extracts. Curr. Res. Food Sci. 2021, 4, 489–498. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gaffey, J.; Sharma, M.; Dewhurst, R.J.; Moreau, B.; Newbold, J.; Clark, W.; Thakur, V.K.; Gupta, V.K. Valorization of dairy waste and by-products through microbial bioprocesses. Bioresour. Technol. 2022, 346, 126444. [Google Scholar] [CrossRef]
- Bahnas, W.; Abbas, K.; Metry, W.; Elewa, N.H. A Novel Bio-Fermented Beverages from Dairy By-Products Based with Papaya Pulp and Stevia Leaves. J. Food Dairy Sci. 2019, 10, 467–472. [Google Scholar] [CrossRef]
- Chua, J.-Y.; Liu, S.-Q. Effect of single amino acid addition on growth kinetics and flavor modulation by Torulaspora delbrueckii in soy (tofu) whey alcoholic beverage fermentation. Food Res. Int. 2020, 135, 109283. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Zhu, X.; Wu, D.; Ma, T.; Tuo, Y.; Jiang, S.; Qian, F.; Mu, G. In vitro assessment of probiotic and functional properties of Bacillus coagulans T242. Food Biosci. 2020, 36, 100675. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Zhang, L. Optimization of lactic acid fermentation conditions for fermented tofu whey beverage with high-isoflavone aglycones. LWT 2019, 111, 211–217. [Google Scholar] [CrossRef]
- Begum, T.; Islam, M.Z.; Siddiki, M.S.R.; Habib, R.; Rashid, M. Preparation of Fermented Beverage from Whey-Based Watermelon (Citrullus lanatus) Juice. Asian J. Dairy Food Res. 2020, 38, 301–306. [Google Scholar] [CrossRef]
- Pega, J.; Denoya, G.I.; Castells, M.L.; Sarquis, S.; Aranibar, G.F.; Vaudagna, S.R.; Nanni, M. Effect of High-Pressure Processing on Quality and Microbiological Properties of a Fermented Beverage Manufactured from Sweet Whey Throughout Refrigerated Storage. Food Bioprocess Technol. 2018, 11, 1101–1110. [Google Scholar] [CrossRef]
- Liutkevičius, A.; Speičiene, V.; Alenčikiene, G.; Mieželiene, A.; Narkevičius, R.; Kaminskas, A.; Abaravičius, J.A.; Vitkus, D.; Jablonskiene, V.; Sekmokiene, D. Fermented buttermilk-based beverage: Impact on young volunteers’ health parameters. Czech J. Food Sci. 2016, 34, 143–148. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R.; Bran, W. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef]
- Pasqualone, A.; Summo, C.; Laddomada, B.; Mudura, E.; Coldea, T.E. Effect of processing variables on the physico-chemical characteristics and aroma of borş, a traditional beverage derived from wheat bran. Food Chem. 2018, 265, 242–252. [Google Scholar] [CrossRef]
- De Francesco, G.; Marconi, O.; Sileoni, V.; Perretti, G. Barley malt wort and grape must blending to produce a new kind of fermented beverage: A physicochemical composition and sensory survey of commercial products. J. Food Compos. Anal. 2021, 103, 104112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).