Fondillón Wine Adulteration by Addition of Other Monastrell Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Wine Samples
2.3. Sugars and Organic Acids
2.4. Antioxidant Activity (AA), Total Polyphenol Index (TPI), and Chromatic Characteristics
2.4.1. Antioxidant Activity (AA)
2.4.2. Total Polyphenol Index (TPI)
2.4.3. Chromatic Characteristics
2.5. Minerals
2.6. Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sugars and Organic Acids
3.2. Antioxidant Activity (AA), Total Polyphenol Index (TPI), and Chromatic Characteristics
3.3. Minerals
- Natural source: This is related to the grape variety, its maturity at harvest, and the type of soil and weather conditions during the growth process.
- Anthropogenic source: This refers to the (external) impurities of the environment that the wine acquires while the grape is growing or during the different winemaking processes (e.g., bottling, aging, etc.).
- Oenological source: This refers to the different steps of wine production.
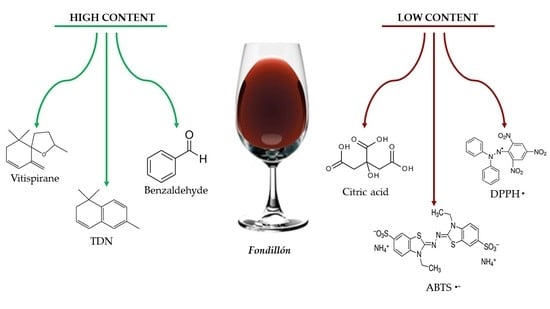
3.4. Volatile Compounds
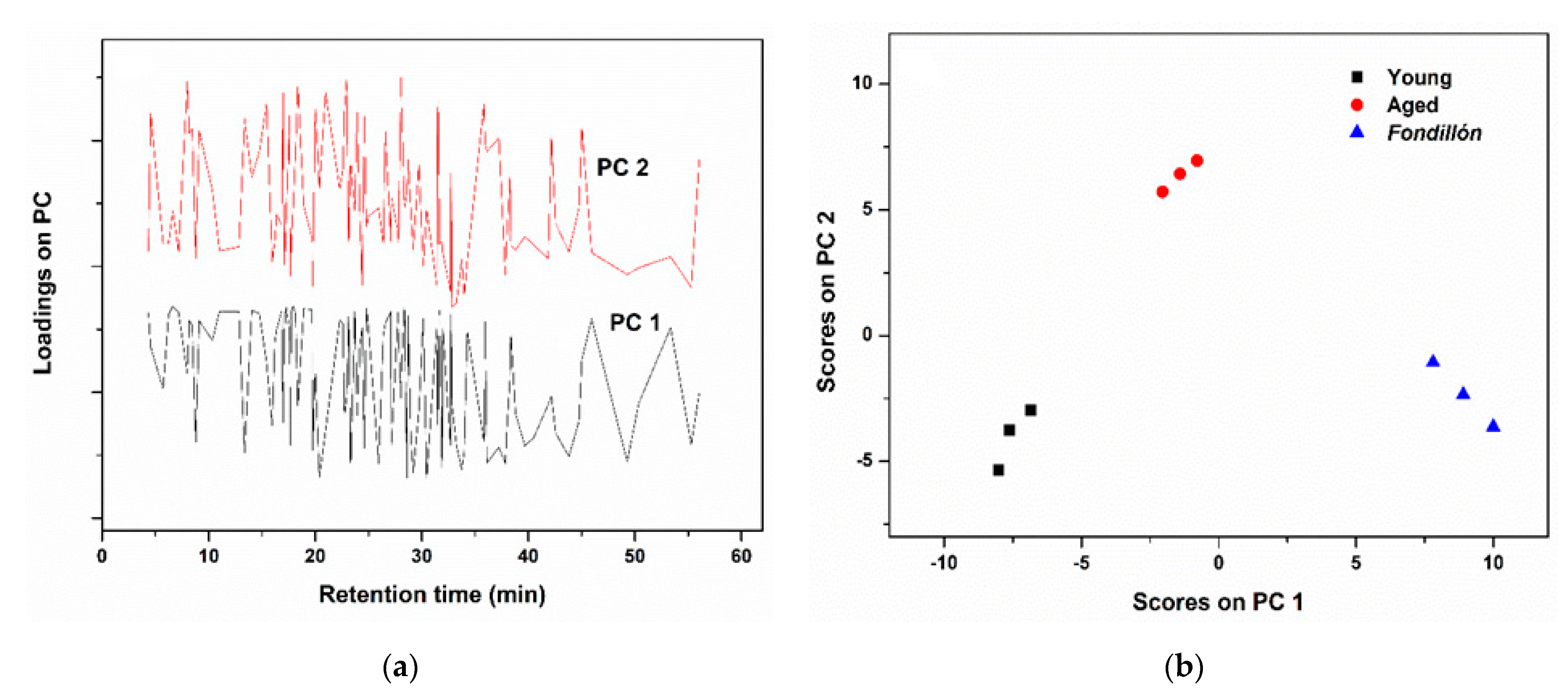
3.5. Principal Component Analysis (PCA)
4. Conclusions
- ✓
- Relatively high concentrations of Cu (~10 μg L−1), Mn (~110 μg L−1), Zn (~40 μg L−1), and Na (~4.5 g L−1);
- ✓
- Relatively high concentrations of fructose (~6.0 g L−1) and acetic acid (>1.0 g L−1);
- ✓
- Relatively high concentrations of the following volatile compounds: 1,1-diethoxyethane (~10 mg L−1), furfural (~0.6 mg L−1), benzaldehyde (~0.3 mg L−1), vitispirane (~0.3 mg L−1), and TDN (~0.3 mg L−1);
- ✓
- Relatively low concentrations of the following volatile compounds: ethyl octanoate (≤2.5 mg L−1) and ethyl decanoate (≤2.0 mg L−1).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Pasvanka, K.; Tzachristas, A.; Proestos, C. Quality tools in wine traceability and authenticity. In Quality Control in the Beverage Industry: Volume 17: The Science of Beverages; Woodhead Publishing: Duxford, UK, 2019; pp. 289–334. [Google Scholar]
- Preti, R. Progress in beverages authentication by the application of analytical techniques and chemometrics. In Quality Control in the Beverage Industry: Volume 17: The Science of Beverages; Woodhead Publishing: Duxford, UK, 2019; pp. 85–121. [Google Scholar]
- Sáenz-Navajas, M.P.; Jeffery, D.W. Perspectives on Wines of Provenance: Sensory Typicality, Quality, and Authenticity. ACS Food Sci. Technol. 2021, 1, 986–992. [Google Scholar] [CrossRef]
- Chandra, S.; Chapman, J.; Power, A.; Roberts, J.; Cozzolino, D. Origin and Regionality of Wines—The Role of Molecular Spectroscopy. Food Anal. Methods 2017, 10, 3947–3955. [Google Scholar] [CrossRef]
- Swinnen, G.M.a.J. The Political Economy of European Wine Regulations. J. Wine Econ. 2013, 8, 244–284. [Google Scholar] [CrossRef]
- Medina, B. Wine authenticity. In Food Authentication; Ashurst, P.R., Dennis, M.J., Eds.; Chapman & Hall: London, UK, 1996; pp. 60–107. [Google Scholar]
- Gonzálvez, A.; Llorens, A.; Cervera, M.L.; Armenta, S.; de la Guardia, M. Elemental fingerprint of wines from the protected designation of origin Valencia. Food Chem. 2009, 112, 26–34. [Google Scholar] [CrossRef]
- Office, E.A.-F. Over 1 Million Litres of Wine and Alcoholic Beverages Seized under OLAF-led Operation. Available online: https://anti-fraud.ec.europa.eu/media-corner/news/over-1-million-litres-wine-and-alcoholic-beverages-seized-under-olaf-led-operation-2021-07-22_en (accessed on 28 November 2022).
- Canizo, B.V.; Escudero, L.B.; Pellerano, R.G.; Wuilloud, R.G. Quality monitoring and authenticity assessment of wines: Analytical and chemometric methods. In Quality Control in the Beverage Industry: Volume 17: The Science of Beverages; Woodhead Publishing: Duxford, UK, 2019; pp. 335–384. [Google Scholar]
- Astray, G.; Martinez-Castillo, C.; Mejuto, J.C.; Simal-Gandara, J. Metal and metalloid profile as a fingerprint for traceability of wines under any Galician protected designation of origin. J. Food Compost. Anal. 2021, 102, 104043. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, F.; Gutiérrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Fang, Y.; Ma, T. Real wine or not? Protecting wine with traceability and authenticity for consumers: Chemical and technical basis, technique applications, challenge, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6783–6808. [Google Scholar] [CrossRef]
- Popîrdă, A.; Luchian, C.E.; Cotea, V.V.; Colibaba, L.C.; Scutarașu, E.C.; Toader, A.M. A review of representative methods used in wine authentication. Agriculture 2021, 11, 225. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Munera-Picazo, S.; Calín-Sánchez, Á.; Wojdyło, A.; Hernández, F.; Carbonell-Barrachina, Á.A. Bioactive compound composition of pomegranate fruits removed during thinning. J. Food Compost. Anal. 2015, 37, 11–19. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wines and Musts. OIV: Paris, France; vol. 2. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-ofanalysis/compendium-of-international-methods-of-analysisof-wines-and-musts-2-vol (accessed on 4 March 2023).
- Carbonell-Barrachina, A.A.; Calín-Sánchez, A.; Bagatar, B.; Hernández, F.; Legua, P.; Martínez-Font, R.; Melgarejo, P. Potential of Spanish sour-sweet pomegranates (cultivar C25) for the juice industry. Food Sci. Technol. Int. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- Iris, F.F.; Benzie, a.J.J.S. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.M.; Garau, M.C.; Negre, A.; March, J.; Martorell, A. Composición Fenólica y Actividad Antioxidante de Variedades Minoritarias de Vid de las Islas Baleares. In Proceedings of the VII Foro Mundial del Vino, Logroño, La Rioja, Spain, 12–14 May 2010; pp. 12–14. [Google Scholar]
- OIV. Compendium of International Methods of Wine and Must Analysis, 2022nd ed.; OIV: Paris, France, 2022; Volume 1. [Google Scholar]
- Glories, Y. The color of red wines. Connaiss. Vignevini 1984, 18, 195–217. [Google Scholar]
- Issa-Issa, H.; Hernández, F.; Lipan, L.; López-Lluch, D.; Carbonell-Barrachina, Á.A. Quality, nutritional, volatile and sensory profiles and consumer acceptance of Fondillón, a sustainable european protected wine. Agronomy 2021, 11, 1701. [Google Scholar] [CrossRef]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Ionete, R.E.; Stefanescu, I.; Artem, V.; Bala, C. Classification of red wines using suitable markers coupled with multivariate statistic analysis. Food Chem. 2016, 192, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel-Muñoz, M.J.; Guerrero-Chanivet, M.; Rodríguez-Dodero, M.C.; García-Moreno, M.V.; Guillén-Sánchez, D.A. Analytical and Chemometric Characterization of Fino and Amontillado Sherries during Aging in Criaderas y Solera System. Molecules 2022, 27, 365. [Google Scholar] [CrossRef]
- Cliff, M.; Pickering, G. Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J. Wine Res. 2006, 17, 45–52. [Google Scholar] [CrossRef]
- Caldeira, I.; Santos, R.; Ricardo-Da-Silva, J.M.; Anjos, O.; Mira, H.; Belchior, A.P.; Canas, S. Kinetics of odorant compounds in wine brandies aged in different systems. Food Chem. 2016, 211, 937–946. [Google Scholar] [CrossRef]
- de Villiers, A.; Alberts, P.; Tredoux, A.G.J.; Nieuwoudt, H.H. Analytical techniques for wine analysis: An African perspective; a review. Anal. Chim. Acta 2012, 730, 2–23. [Google Scholar] [CrossRef]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compost. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Del Alamo, M.; Bernal, J.L.; Del Nozal, M.J.; Gómez-Cordovés, C. Red wine aging in oak barrels: Evolution of the monosaccharides content. Food Chem. 2000, 71, 189–193. [Google Scholar] [CrossRef]
- Echeverry, C.; Ferreira, M.; Reyes-Parada, M.; Abin-Carriquiry, J.A.; Blasina, F.; González-Neves, G.; Dajas, F. Changes in antioxidant capacity of Tannat red wines during early maturation. J. Food. Eng. 2005, 69, 147–154. [Google Scholar] [CrossRef]
- Roginsky, V.; De Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Barsukova, T.; Adams, D.O. The antioxidant activity of Californian red wines does not correlate with wine age. J. Sci. Food Agric. 2006, 86, 834–840. [Google Scholar] [CrossRef]
- Rivero-Pérez, M.D.; González-Sanjosé, M.L.; Ortega-Herás, M.; Muñiz, P. Antioxidant potential of single-variety red wines aged in the barrel and in the bottle. Food Chem. 2008, 111, 957–964. [Google Scholar] [CrossRef]
- Pfahl, L.; Catarino, S.; Fontes, N.; Graça, A.; Ricardo-Da-silva, J. Effect of barrel-to-barrel variation on color and phenolic composition of a red wine. Foods 2021, 10, 1669. [Google Scholar] [CrossRef]
- Chira, K.; Jourdes, M.; Teissedre, P.L. Cabernet sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. Eur. Food Res. Technol. 2012, 234, 253–261. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Escott, C.; Suárez Lepe, J.A. Evolution of the Phenolic Fraction and Aromatic Profile of Red Wines Aged in Oak Barrels. ACS Omega 2020, 5, 7235–7243. [Google Scholar] [CrossRef]
- Kment, P.; Mihaljevič, M.; Ettler, V.; Šebek, O.; Strnad, L.; Rohlová, L. Differentiation of Czech wines using multielement composition—A comparison with vineyard soil. Food Chem. 2005, 91, 157–165. [Google Scholar] [CrossRef]
- Cozzolino, D. Elemental composition in grapes and wine: Role, analytical methods and their use. In Handbook of Mineral Elements in Food; Wiley Blckwell: Chichester, UK, 2015; pp. 473–487. [Google Scholar]
- Pohl, P. What do metals tell us about wine? TrAC Trends Analyt Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Frías, S.; Conde, J.E.; Rodríguez-Bencomo, J.J.; García-Montelongo, F.; Pérez-Trujillo, J.P. Classification of commercial wines from the Canary Islands (Spain) by chemometric techniques using metallic contents. Talanta 2003, 59, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Jarauta, I.; Cacho, J.; Ferreira, V. Concurrent phenomena contributing to the formation of the aroma of wine during aging in oak wood: An analytical study. J. Agric. Food Chem. 2005, 53, 4166–4177. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Barros, A.S.; Câmara, J.S.; Rocha, S.M. In-depth search focused on furans, lactones, volatile phenols, and acetals as potential age markers of Madeira wines by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction. J. Agric. Food Chem. 2011, 59, 3186–3204. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Alañón, M.E.; Calvo, E.; Cejudo, M.J.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Volatile compounds as markers of ageing in Tempranillo red wines from La Mancha D.O. stored in oak wood barrels. J. Chromatogr. 2011, 1218, 4910–4917. [Google Scholar] [CrossRef]
- Issa-Issa, H.; Guclu, G.; Noguera-Artiaga, L.; López-Lluch, D.; Poveda, R.; Kelebek, H.; Selli, S.; Carbonell-Barrachina, Á.A. Aroma-active compounds, sensory profile, and phenolic composition of Fondillón. Food Chem. 2020, 316, 126353. [Google Scholar] [CrossRef]
- Winterhalter, P.; Gök, R. TDN and β-damascenone: Two important carotenoid metabolites in wine. ACS Symp. Ser. 2013, 1134, 125–137. [Google Scholar] [CrossRef]
- Marais, J.; van Wyk, C.J.; Rapp, A. Effect of Sunlight and Shade on N orisoprenoid Levels in Maturing Weisser Riesling and Chenin blanc Grapes and Weisser Riesling Wines. Outh African J. Enol. Vitic. 1992, 13, 23–32. [Google Scholar] [CrossRef]
- Khairallah, R.; Reynolds, A.G.; Bowen, A.J. Harvest date effects on aroma compounds in aged Riesling icewines. J. Sci. Food Agric. 2016, 96, 4398–4409. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Flavors & Fragrances; Sigma-Aldrich: Saint Louis, MO, USA, 2012. [Google Scholar]


| Wine Type | Organic Acids | Sugars | |||||
|---|---|---|---|---|---|---|---|
| Citric | Tartaric | Malic | Lactic | Acetic | Glucose | Fructose | |
| (g L−1) | |||||||
| ANOVA | |||||||
| *** | *** | *** | * | *** | *** | *** | |
| Tukey Multiple Range Test ‡ | |||||||
| Young | 2.50 b | 5.15 b | 6.95 b | 4.73 ab | 0.87 b | 5.82 b | 4.10 b |
| Aged | 3.72 a | 6.95 a | 9.78 a | 5.44 a | 0.63 c | 6.89 b | 3.88 b |
| Fondillón | 0.84 c | 2.52 c | 1.93 c | 4.42 b | 1.27 a | 7.12 a | 6.01 a |
| Wine Type | ABTS•+ | FRAP | DPPH• | Total Phenolic Index | Color Intensity | Tonality | Color Density | Y ¶ | R ¶ | B ¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| (mmol Trolox L−1) | (mg AG/100 mL Wine) | (%) | ||||||||
| ANOVA | ||||||||||
| *** | *** | *** | *** | *** | *** | *** | *** | ** | ** | |
| Tukey Multiple Range Test ‡ | ||||||||||
| Young | 2.30 a | 7.42 b | 2.18 b | 285 a | 9.13 a | 0.60 b | 7.88 a | 32.4 b | 53.9 a | 13.7 a |
| Aged | 2.35 a | 13.9 a | 2.43 a | 289 a | 8.70 a | 0.60 b | 7.48 a | 32.4 b | 53.5 a | 14.1 a |
| Fondillón | 1.28 b | 3.52 c | 1.49 c | 221 b | 3.92 b | 1.87 a | 3.62 b | 60.1 a | 32.2 b | 7.73 b |
| Wine Type | Ca | Mg | Na | K | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|
| (mg L−1) | (μg L−1) | |||||||
| ANOVA | ||||||||
| ** | *** | *** | ** | *** | ** | *** | *** | |
| Tukey Multiple Range Test ‡ | ||||||||
| Young | 8.88 a | 14.2 c | 2.87 c | 148 a | 30 c | Traces b | 60 c | 10 c |
| Aged | 8.36 b | 16.1 a | 3.79 b | 125 b | 170 a | Traces b | 60 b | 20 b |
| Fondillón | 7.93 c | 15.3 b | 4.94 a | 144 a | 140 b | 10 a | 110 a | 40 a |
| Code | Volatile Compounds | Young | Aged | Fondillón |
|---|---|---|---|---|
| (mg L−1) | ||||
| V1 | 1,1-diethoxyethane | nd | nd | 0.13 |
| V6 | Furfural | nd | 0.09 | 0.60 |
| V13 | 1,1-diethoxy-2-methylpropane | nd | nd | 0.02 |
| V14 | Benzaldehyde | nd | 0.01 | 0.28 |
| V15 | 1-(1-ethoxyethoxy)-pentane | nd | nd | 0.03 |
| V28 | Isoamyl butyrate | nd | nd | 0.04 |
| V30 | Guaiacol | nd | nd | 0.01 |
| V31 | Ethyl sorbate | nd | nd | 0.13 |
| V33 | Nonanal | nd | nd | 0.11 |
| V39 | Diethyl butanedioate | 1.86 | 1.90 | 2.80 |
| V53 | 4-Ethylguaiacol | nd | nd | 0.02 |
| V55 | Vitispirane | 0.12 | 0.22 | 0.37 |
| V57 | trans-Whiskey lactone | 0.02 | 0.05 | 0.06 |
| V60 | cis-Whiskey lactone | 0.11 | 0.09 | 0.17 |
| V64 | Eugenol | nd | 0.01 | 0.01 |
| V65 | TDN | 0.00 | 0.04 | 0.35 |
| Wine Type | PCs | Variance (%) | |||
|---|---|---|---|---|---|
| This PC | Cumulative | RMSEC | RMSECV | ||
| Young/Aged—Fondillón | 1 | 50.45 | 50.45 | 0.48 | 3.64 |
| 2 | 23.60 | 74.01 | |||
| Fondillón—Aged | 1 | 33.12 | 33.12 | 0.62 | 2.16 |
| 2 | 26.78 | 59.90 | |||
| Fondillón—Young | 1 | 40.29 | 40.29 | 0.65 | 1.84 |
| 2 | 16.10 | 56.39 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa-Issa, H.; Hernández, F.; López-Lluch, D.; Uysal, R.S.; Carbonell-Barrachina, Á.A. Fondillón Wine Adulteration by Addition of Other Monastrell Wines. Beverages 2023, 9, 28. https://doi.org/10.3390/beverages9010028
Issa-Issa H, Hernández F, López-Lluch D, Uysal RS, Carbonell-Barrachina ÁA. Fondillón Wine Adulteration by Addition of Other Monastrell Wines. Beverages. 2023; 9(1):28. https://doi.org/10.3390/beverages9010028
Chicago/Turabian StyleIssa-Issa, Hanán, Francisca Hernández, David López-Lluch, Reyhan Selin Uysal, and Ángel A. Carbonell-Barrachina. 2023. "Fondillón Wine Adulteration by Addition of Other Monastrell Wines" Beverages 9, no. 1: 28. https://doi.org/10.3390/beverages9010028
APA StyleIssa-Issa, H., Hernández, F., López-Lluch, D., Uysal, R. S., & Carbonell-Barrachina, Á. A. (2023). Fondillón Wine Adulteration by Addition of Other Monastrell Wines. Beverages, 9(1), 28. https://doi.org/10.3390/beverages9010028