DLLμE/GC-MS as a Powerful Analytical Approach to Establish the Volatilomic Composition of Different Whiskeys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Whiskeys
2.3. Dispersive Liquid–Liquid Microextraction Procedure
2.4. Instrumental Analysis
2.4.1. Gas Chromatography-Mass Spectrometry (GC-MS) Conditions
2.4.2. Gas Chromatography-Flame Ionization Detector (GC-FID) Conditions
2.5. Analytical Method Validation
2.6. Statistical Analysis
3. Results and Discussions
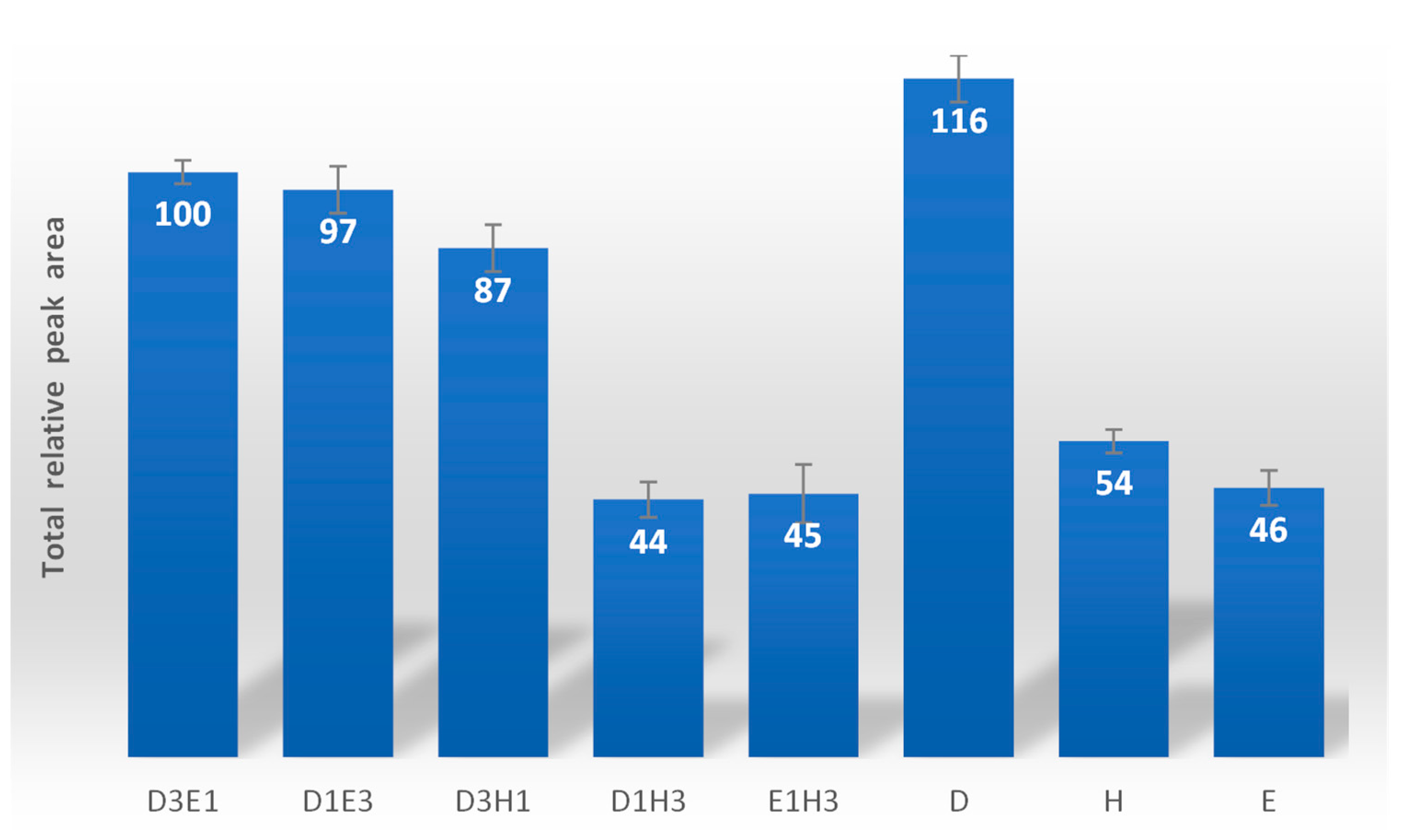
3.1. Selection of Extractor Solvent Using DLLμE/GC-MS
3.2. Method Validation
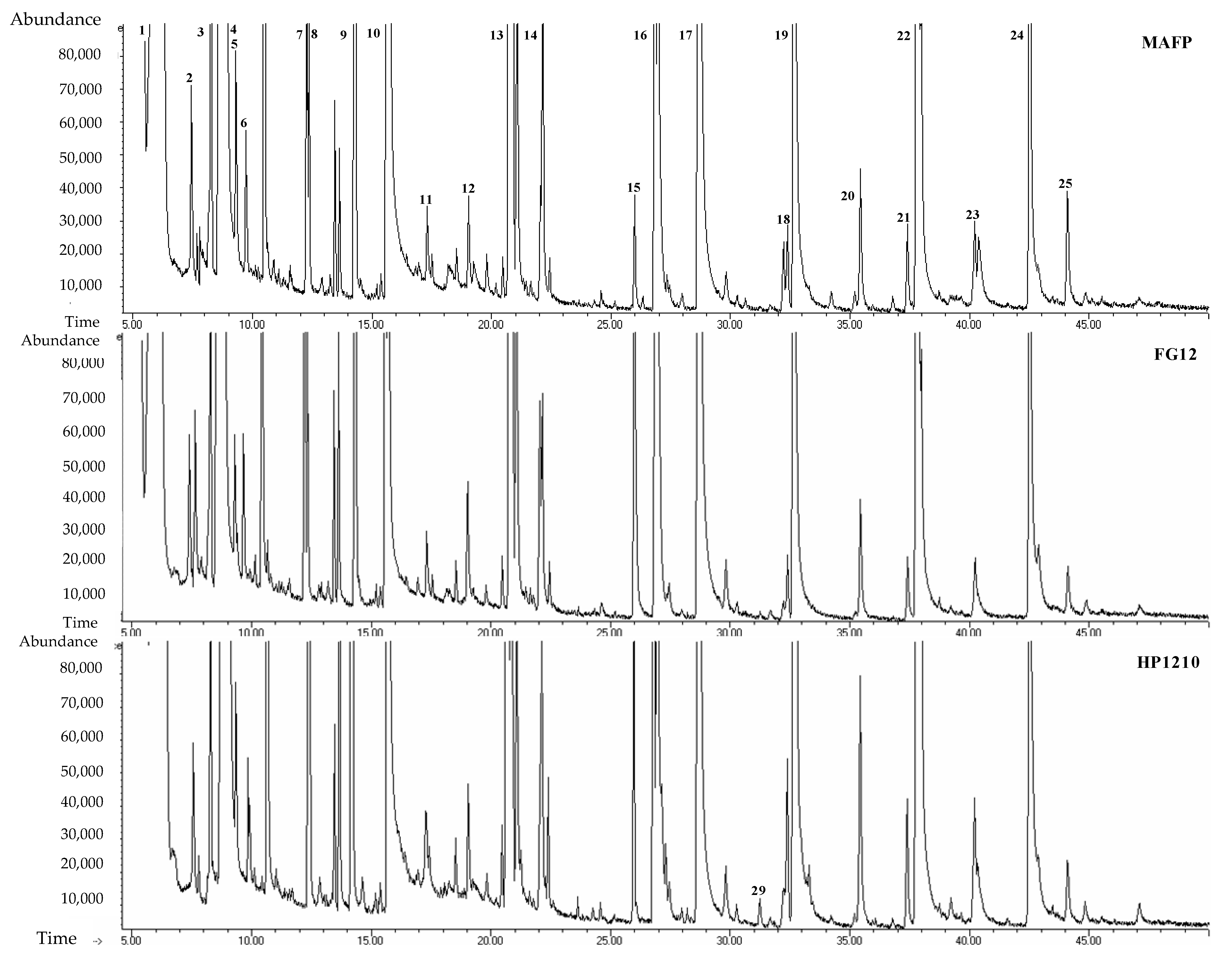
3.3. Analysis of Volatilomic Fingerprint of Whiskeys by DLLμE/GC-FID
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camara, J.S.; Marques, J.C.C.; Perestrelo, R.M.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M.; Ren, J.J.; Liu, H.Y.; Hao, Y.H.; et al. A Narrative Review of Sulfur Compounds in Whisk(e)Y. J. Chromatogr. A 2020, 6, 377–397. [Google Scholar]
- Caldeira, M.; Rodrigues, F.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Comparison of Two Extraction Methods for Evaluation of Volatile Constituents Patterns in Commercial Whiskeys. Elucidation of the Main Odour-Active Compounds. Talanta 2007, 74, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.S.; Marques, J.C.C.; Perestrelo, R.M.M.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M. Comparative Study of the Whisky Aroma Profile Based on Headspace Solid Phase Microextraction Using Different Fibre Coatings. J. Chromatogr. A 2007, 1150, 198–207. [Google Scholar] [CrossRef]
- Ferracane, A.; Manousi, N.; Tranchida, P.Q.; Zachariadis, G.A.; Mondello, L.; Rosenberg, E. Exploring the Volatile Profile of Whiskey Samples Using Solid-Phase Microextraction Arrow and Comprehensive Two-Dimensional Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2022, 1676, 463241. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Influence of the Production Process on the Key Aroma Compounds of Rum: From Molasses to the Spirit. J. Agric. Food Chem. 2016, 64, 9041–9053. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Blanco Gomis, D.; Mangas Alonso, J.J. Influence of Distillation System, Oak Wood Type, and Aging Time on Composition of Cider Brandy in Phenolic and Furanic Compounds. J. Agric. Food Chem. 2003, 51, 7969–7973. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Holland, R.; Crow, V.L. Esters and Their Biosynthesis in Fermented Dairy Products: A Review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization Technologies and Support Materials Suitable in Alcohol Beverages Production: A Review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Flamini, R.; Panighel, A.; De Marchi, F. Mass Spectrometry in the Study of Wood Compounds Released in the Barrel-Aged Wine and Spirits. Mass Spectrom. Rev. 2021, e21754. [Google Scholar] [CrossRef]
- Guerrero-Chanivet, M.; Valcarcel-Munoz, M.J.; Garcia-Moreno, M.V.; Guillen-Sanchez, D.A. Characterization of the Aromatic and Phenolic Profile of Five Different Wood Chips Used for Ageing Spirits and Wines. Foods 2020, 9, 1613. [Google Scholar] [CrossRef]
- Gollihue, J.; Richmond, M.; Wheatley, H.; Pook, V.G.; Nair, M.; Kagan, I.A.; DeBolt, S. Liberation of Recalcitrant Cell Wall Sugars from Oak Barrels into Bourbon Whiskey during Aging. Sci. Rep. 2018, 8, 15899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roullier-Gall, C.; Signoret, J.; Coelho, C.; Hemmler, D.; Kajdan, M.; Lucio, M.; Schafer, B.; Gougeon, R.D.; Schmitt-Kopplin, P. Influence of Regionality and Maturation Time on the Chemical Fingerprint of Whisky. Food Chem. 2020, 323, 126748. [Google Scholar] [CrossRef] [PubMed]
- Roullier-Gall, C.; Signoret, J.; Hemmler, D.; Witting, M.A.; Kanawati, B.; Schafer, B.; Gougeon, R.D.; Schmitt-Kopplin, P. Usage of FT-ICR-MS Metabolomics for Characterizing the Chemical Signatures of Barrel-Aged Whisky. Front. Chem. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Winstel, D.; Gautier, E.; Marchal, A. Role of Oak Coumarins in the Taste of Wines and Spirits: Identification, Quantitation, and Sensory Contribution through Perceptive Interactions. J. Agric. Food Chem. 2020, 68, 7434–7443. [Google Scholar] [CrossRef]
- del Toro del Toro, N.; Fong Casas, F.; Ayan Rial, J.; Caridad Portuondo González, M.; Crespo Sariol, H.; Navarro Campa, J.; Yperman, J.; Vandamme, D.; Carleer, R. Boltzmann-Based Empirical Model to Calculate Volume Loss during Spirit Ageing. Beverages 2019, 5, 60. [Google Scholar] [CrossRef]
- Kyraleou, M.; Herb, D.; O’Reilly, G.; Conway, N.; Bryan, T.; Kilcawley, K.N. The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit. Foods 2021, 10, 443. [Google Scholar] [CrossRef]
- Baltajiev, R.; Fartzov, K.; Dimov, S. The Effects of Oak Wood in Chardonnay Wine Production and Maturation, as Determined by High Performance Liquid Chromatography. J. Wine Res. 2007, 5, 147–151. [Google Scholar] [CrossRef]
- Monica Lee, K.Y.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Origins of Flavour in Whiskies and a Revised Flavour Wheel: A Review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar] [CrossRef]
- Caldeira, M.; Rodrigues, F.; Perestrelo, R.; Marques, J.C.; Câmara, J.S.; Alves, M.A.; Marques, J.C.; Fitzgerald, G.; James, K.J.; MacNamara, K.; et al. Changes in Volatile Composition of Madeira Wines during Their Oxidative Ageing. Anal. Chim. Acta 2003, 563, 188–197. [Google Scholar]
- Liu, M.; Zeng, Z.; Tian, Y. Elimination of Matrix Effects for Headspace Solid-Phase Microextraction of Important Volatile Compounds in Red Wine Using a Novel Coating. Anal. Chim. Acta 2005, 540, 341–353. [Google Scholar] [CrossRef]
- Karásek, P.; Planeta, J.; Varad’ová Ostrá, E.; Mikesová, M.; Goliás, J.; Roth, M.; Vejrosta, J. Direct Continuous Supercritical Fluid Extraction as a Novel Method of Wine Analysis. Comparison with Conventional Indirect Extraction and Implications for Wine Variety Identification. J. Chromatogr. A 2003, 1002, 13–23. [Google Scholar] [CrossRef]
- Ren, J.J.; Liu, H.Y.; Hao, Y.H.; He, P.G.; Fang, Y.Z. Determination of Resveratrol in Red Wine by Solid Phase Extraction-Flow Injection Chemiluminescence Method. Chin. Chem. Lett. 2007, 18, 985–988. [Google Scholar] [CrossRef]
- Fitzgerald, G.; James, K.J.; MacNamara, K.; Stack, M.A. Characterisation of Whiskeys Using Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2000, 896, 351–359. [Google Scholar] [CrossRef]
- Kúskús, P.M.; Czabaj, S.; Jerkovíc, I.J. Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers. Molecules 2022, 27, 4558. [Google Scholar] [CrossRef]
- Angioni, A.; Pintore, G.A.M.; Caboni, P. Determination of Wine Aroma Compounds by Dehydration Followed by GC/MS. J. AOAC Int. 2012, 95, 813–819. [Google Scholar] [CrossRef]
- Owens, J.E.; Zimmerman, L.B.; Gardner, M.A.; Lowe, L.E. Analysis of Whiskey by Dispersive Liquid−Liquid Microextraction Coupled with Gas Chromatography/Mass Spectrometry: An Upper Division Analytical Chemistry Experiment Guided by Green Chemistry. J. Chem. Educ. 2015, 93, 186–192. [Google Scholar] [CrossRef]
- Fontana, A.; Rodríguez, I.; Cela, R. Dispersive Liquid–Liquid Microextraction and Gas Chromatography Accurate Mass Spectrometry for Extraction and Non-Targeted Profiling of Volatile and Semi-Volatile Compounds in Grape Marc Distillates. J. Chromatogr. A 2018, 1546, 36–45. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 20 May 2022).
- Spínola, V.; Perestrelo, R.; Câmara, J.S.; Castilho, P.C. Establishment of Monstera Deliciosa Fruit Volatile Metabolomic Profile at Different Ripening Stages Using Solid-Phase Microextraction Combined with Gas Chromatography–Mass Spectrometry. Food Res. Int. 2015, 67, 409–417. [Google Scholar] [CrossRef]
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Câmara, J.S. Characterization of Volatile Substances in Apples from Rosaceae Family by Headspace Solid-Phase Microextraction Followed by GC-QMS. J. Sep. Sci. 2009, 32, 1875–1888. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Reis, D.; Silva, P.; Perestrelo, R.; C mara, J.S. Residue Analysis of Insecticides in Potatoes by QuEChERS-DSPE/UHPLC-PDA. Foods 2020, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Oliveira do Nascimento, H.; Zillene Franklin da Silva, M.; Beserra Alexandre, J.; Bastos Vidal, C.; Vieira Carvalho, T.; Ferreira do Nascimento, R. New HS-SPME-GC-BID Method for the Determination of Volatile Constituents in Distilled Beverages. Microchem. J. 2022, 181, 107669. [Google Scholar] [CrossRef]
- Perestrelo, R.; Barros, A.S.S.; Câmara, J.S.S.; Rocha, S.M.M. In-Depth Search Focused on Furans, Lactones, Volatile Phenols, and Acetals as Potential Age Markers of Madeira Wines by Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry Combined with Solid Phase Microextraction. J. Agric. Food Chem. 2011, 59, 3186–3204. [Google Scholar] [CrossRef] [PubMed]
- Phetxumphou, K.; Miller, G.; Ashmore, P.L.; Collins, T.; Lahne, J. Mashbill and Barrel Aging Effects on the Sensory and Chemometric Profiles of American Whiskeys. J. Inst. Brew. 2020, 126, 194–205. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Chemical composition analysis and authentication of whisky. J Sci Food Agric. 2015, 95, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Somogyi, A.; Thomas, C.; Zhang, H.; Cheng, Z.; Xu, S.; Miller, C.; Spivey, D.; Blake, C.; Smith, C.; et al. Analysis of Barrel-Aged Kentucky Bourbon Whiskey by Ultrahigh Resolution Mass Spectrometry. Food Anal. Methods 2020, 13, 2301–2311. [Google Scholar] [CrossRef]
- Heller, M.; Vitali, L.; Oliveira, M.A.; Costa, A.C.; Micke, G.A. A rapid sample screening method for authenticity control of whiskey using capillary electrophoresis with online preconcentration. J. Agric. Food Chem. 2011, 59, 6882–6888. [Google Scholar] [CrossRef]
- Savchuk, S.A.; Palacio, C.; Gil, A.; Tagliaro, F.; Kuznetsov, R.M.; Brito, A.; Appolonova, S.A. Determination of the chemical composition of alcoholic beverages by gas chromatography-mass spectrometry. J. Food Proc. Preserv. 2020, 44, e14676. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M.; Pastor, A.; Huerta, T. Yeast Starter Cultures Affecting Wine Fermentation and Volatiles. Food Res. Int. 2001, 34, 307–314. [Google Scholar] [CrossRef]





| RI a | LRI b | Chemical Families | Linear Range (µg/L) | R2 | Slope | Intercept | LOD c (ng/L) | LOQ d (ng/L) | Trueness | Precision (RSD %) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rec. (%) ± % RSD | Intra−Day | Inter−Day | |||||||||
| Alcohols | |||||||||||
| 1045 | 1046 | Butan-2-ol | 1.00–120 | 0.997 | 0.21 | −1.04 | 21.4 | 71.4 | 90 ± 1 | 2.15 | 5.68 |
| 1056 | 1052 | Propan-1-ol | 1.00–190 | 0.996 | 0.04 | −0.58 | 25.6 | 85.2 | 86 ± 3 | 1.56 | 2.72 |
| 1187 | 1173 | Butan-1-ol | 0.03–19.6 | 0.998 | 0.08 | 0.00 | 0.56 | 1.86 | 97 ± 2 | 2.02 | 3.61 |
| 1246 | 1244 | 3-Methylbutan-1-ol | 20.0–600 | 0.999 | 0.25 | −1.63 | 28.8 | 96.0 | 74 ± 5 | 1.24 | 2.71 |
| 1380 | 1376 | Hexan-1-ol | 0.20–20.0 | 0.999 | 0.16 | 0.01 | 0.50 | 1.67 | 98 ± 3 | 3.75 | 4.70 |
| 1687 | 1686 | Methionol | 0.30–23.0 | 0.995 | 0.14 | 0.02 | 1.95 | 6.50 | 96 ± 5 | 8.76 | 14.6 |
| 1849 | 1848 | Benzyl alcohol | 0.04–37.0 | 0.997 | 0.33 | 0.19 | 1.44 | 4.83 | 90 ± 3 | 0.99 | 1.26 |
| 1950 | 1947 | 2-Phenylethanol | 0.50–120 | 0.996 | 0.16 | −1.23 | 35.2 | 117 | 99 ± 2 | 1.73 | 2.85 |
| 2110 | 2103 | Phenoxyethanol | 0.20–50.5 | 0.996 | 0.39 | −3.30 | 31.8 | 105 | 96 ± 1 | 2.03 | 4.13 |
| Esters | |||||||||||
| 1138 | 1137 | Isoamyl acetate | 0.10–24.0 | 0.999 | 0.13 | 0.01 | 0.65 | 2.14 | 89 ± 1 | 2.34 | 2.80 |
| 1223 | 1221 | Ethyl hexanoate | 0.10–200 | 0.999 | 0.34 | −2.46 | 8.64 | 28.8 | 98 ± 2 | 2.08 | 3.81 |
| 1319 | 1312 | Ethyl lactate | 0.10–60.0 | 0.996 | 0.10 | −2.08 | 5.56 | 18.5 | 97 ± 3 | 0.94 | 1.51 |
| 1453 | 1458 | Ethyl octanoate | 0.10–200 | 0.997 | 0.41 | −5.48 | 19.2 | 64.1 | 94 ± 1 | 0.67 | 1.56 |
| 1610 | 1610 | Ethyl decanoate | 0.10–200 | 0.998 | 0.58 | −0.04 | 1.20 | 4.00 | 97 ± 4 | 2.14 | 4.18 |
| 1662 | 1668 | Diethyl succinate | 0.10–21.0 | 0.999 | 0.42 | −0.03 | 0.63 | 2.08 | 99 ± 3 | 1.95 | 4.65 |
| 1855 | 1850 | Ethyl dodecanoate | 0.10–100 | 0.999 | 0.43 | −3.08 | 6.11 | 20.4 | 96 ± 3 | 2.69 | 3.89 |
| Acids | |||||||||||
| 1404 | 1408 | Acetic acid | 0.20–50.0 | 0.998 | 0.05 | −1.48 | 54.9 | 183 | 93 ± 6 | 4.83 | 7.66 |
| 1669 | 1666 | Butanoic acid | 0.30–50.0 | 0.997 | 0.01 | −0.47 | 63.1 | 210 | 89 ± 5 | 5.02 | 9.31 |
| 1815 | 1814 | Hexanoic acid | 0.20–100 | 0.998 | 0.28 | −3.66 | 39.6 | 132 | 87 ± 2 | 2.21 | 5.34 |
| 2080 | 2083 | Octanoic acid | 0.20–100 | 0.997 | 0.34 | −9.05 | 34.7 | 116 | 89 ± 3 | 10.3 | 13.7 |
| 2273 | 2276 | Decanoic acid | 0.20–100 | 0.998 | 0.30 | −9.39 | 50.4 | 167 | 94 ± 4 | 9.85 | 12.9 |
| Carbonyl compounds | |||||||||||
| 741 | 744 | Acetaldehyde | 0.30–50.0 | 0.999 | 0.05 | 0.13 | 9.23 | 31.0 | 70 ± 4 | 4.98 | 5.45 |
| 2901 | 2907 | Syringaldehyde | 0.30–50.0 | 0.998 | 0.14 | 0.48 | 36.9 | 123 | 93 ± 8 | 7.66 | 9.44 |
| Furan compounds | |||||||||||
| 1444 | 1445 | Furfural | 0.30–50.0 | 0.998 | 0.15 | −1.19 | 23.8 | 79.4 | 86 ± 6 | 6.95 | 7.74 |
| 1565 | 1560 | 5-Methylfurfural | 0.30–50.0 | 0.998 | 0.12 | −0.03 | 1.10 | 3.67 | 99 ± 9 | 12.9 | 14.7 |
| Volatile phenol | |||||||||||
| 1880 | 1873 | Guaiacol | 0.10–30.0 | 0.993 | 0.35 | 0.34 | 4.23 | 12.3 | 91 ± 5 | 4.92 | 6.12 |
| Chemical Families | ID | Code | Concentration (µg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MANS | MA4Y | MAFP | HPDK | HP1210 | HP1840 | FGOR | FGF | FG12 | |||
| Alcohols | |||||||||||
| Butan-2-ol | R, MS | BUT2 | 13.1 ± 0.23 | 8.24 ± 0.11 | 8.71 ± 0.07 | 5.79 ± 0.04 | 6.78 ± 0.04 | 6.62 ± 0.03 | 8.52 ± 0.03 | 7.62 ± 0.04 | 9.19 ± 0.02 |
| Propan-1-ol | R, MS | PROP1 | 71.3 ± 0.02 | 73.1 ± 0.03 | 40.4 ± 0.01 | 92.1 ± 0.11 | 45.1 ± 0.06 | 47.0 ± 0.01 | 51.9 ± 0.05 | 43.9 ± 0.02 | 40.3 ± 0.02 |
| Butan-1-ol | R, MS | BUT1 | 0.45 ± 0.03 | 0.70 ± 0.05 | 10.3 ± 0.02 | 4.64 ± 0.03 | 9.44 ± 0.05 | 6.78 ± 0.00 | 4.85 ± 0.00 | 2.75 ± 0.00 | 8.75 ± 0.01 |
| Hexan-2-ol | MS | HEX2 | - | - | 0.09 ± 0.01 | - | - | - | 0.08 ± 0.00 | - | 0.46 ± 0.07 |
| 3-Methylbutan-1-ol | R, MS | 3M1B | 232 ± 0.06 | 256 ± 4.32 | 430 ± 5.25 | 239 ± 0.01 | 425 ± 5.89 | 433 ± 8.11 | 221 ± 1.42 | 235 ± 1.69 | 472 ± 2.56 |
| Pentan-1-ol | MS | PENT1 | - | - | 0.40 ± 0.02 | - | 0.23 ± 0.01 | - | 0.14 ± 0.01 | 0.23 ± 0.01 | 0.38 ± 0.06 |
| Hexan-1-ol | R, MS | HEX1 | 1.61 ± 0.02 | 4.99 ± 0.05 | 2.36 ± 0.01 | 2.75 ± 0.02 | 1.22 ± 0.04 | 1.58 ± 0.02 | 1.39 ± 0.01 | 0.76 ± 0.00 | 1.38 ± 0.19 |
| Methionol | R, MS | METH | - | - | - | 0.87 ± 0.01 | - | - | - | - | - |
| Benzyl alcohol | R, MS | BENA | - | 0.05 ± 0.01 | - | - | - | 0.12 ± 0.01 | - | - | 0.42 ± 0.03 |
| 2-Phenylethanol | R, MS | PHEN | 26.9 ± 0.48 | 15.7 ± 0.03 | 9.77 ± 0.07 | 22.9 ± 0.24 | 11.5 ± 0.05 | 11.1 ± 0.04 | 10.9 ± 0.02 | 9.69 ± 0.01 | 9.86 ± 0.02 |
| 2-Phenoxyethanol | R, MS | PHENO | 12.9 ± 0.23 | 15.3 ± 0.72 | 12.1 ± 0.16 | 15.5 ± 0.17 | 13.8 ± 0.28 | 14.3 ± 0.16 | 11.3 ± 0.13 | 9.93 ± 0.10 | 11.0 ± 0.09 |
| Hexadecan-1-ol | R, MS | HEXAD | 0.38 ± 0.01 | 0.52 ± 0.02 | 0.43 ± 0.01 | 0.20 ± 0.03 | 0.18 ± 0.01 | 0.25 ± 0.01 | 0.62 ± 0.26 | 0.15 ± 0.01 | 0.06 ± 0.01 |
| Esters | |||||||||||
| Isoamyl acetate | R, MS | ISOAC | 8.70 ± 0.01 | 5.21 ± 0.02 | 0.32 ± 0.00 | 13.3 ± 0.08 | 8.38 ± 0.06 | 5.46 ± 0.11 | 5.07 ± 0.01 | 4.39 ± 0.02 | 12.9 ± 0.03 |
| Ethyl hexanoate | R, MS | EHEX | 129 ± 1.01 | 76.9 ± 1.80 | 53.5 ± 0.09 | 136 ± 1.05 | 83.8 ± 0.13 | 69.7 ± 0.13 | 58.3 ± 0.03 | 54.8 ± 0.02 | 55.3 ± 0.10 |
| Ethyl ortoformate | MS | EORT | 0.14 ± 0.01 | 0.55 ± 0.03 | 0.73 ± 0.04 | 0.43 ± 0.02 | 0.57 ± 0.04 | 0.67 ± 0.01 | 0.39 ± 0.05 | 0.22 ± 0.01 | 0.26 ± 0.04 |
| Ethyl lactate | R, MS | ELAC | 2.15 ± 0.05 | 4.30 ± 0.10 | 7.81 ± 0.01 | 22.7 ± 1.98 | 25.2 ± 0.06 | 35.1 ± 0.02 | 5.54 ± 0.00 | 8.92 ± 0.01 | 11.7 ± 0.95 |
| Ethyl octanoate | R, MS | EOCT | 60.5 ± 0.10 | 61.4 ± 0.04 | 60.3 ± 0.04 | 51.0 ± 0.13 | 54.6 ± 0.11 | 58.1 ± 0.06 | 28.3 ± 0.07 | 27.9 ± 0.00 | 34.2 ± 0.06 |
| Ethyl decanoate | R, MS | EDEC | 92.6 ± 0.00 | 93.2 ± 0.01 | 105 ± 0.22 | 94.7 ± 0.05 | 97.2 ± 1.37 | 115 ± 1.06 | 124 ± 0.01 | 127 ± 0.13 | 130 ± 0.22 |
| Diethyl succinate | R, MS | DSUC | 0.33 ± 0.01 | 1.09 ± 0.03 | 1.46 ± 0.02 | 0.29 ± 0.01 | 0.71 ± 0.04 | 1.30 ± 0.06 | 0.36 ± 0.00 | 0.28 ± 0.00 | 0.29 ± 0.00 |
| Ethyl 9-decenoate | MS | E9DEC | - | - | 0.11 ± 0.02 | - | 0.37 ± 0.11 | - | 0.14 ± 0.02 | - | 0.10 ± 0.01 |
| Phenylethyl acetate | MS | PHENAC | 0.17 ± 0.02 | 0.23 ± 0.04 | 0.35 ± 0.01 | 0.45 ± 0.02 | 0.92 ± 0.13 | 4.57 ± 0.03 | 0.36 ± 0.06 | 0.67 ± 0.01 | 2.02 ± 0.11 |
| Ethyl dodecanoate | R, MS | EDODE | 10.8 ± 1.40 | 37.5 ± 0.97 | 9.46 ± 0.17 | 33.8 ± 0.24 | 19.1 ± 0.48 | 18.6 ± 0.82 | 12.7 ± 0.12 | 5.60 ± 0.15 | 8.22 ± 0.30 |
| Ethyl tetradecanoate | MS | ETET | 0.08 ± 0.06 | 0.12 ± 0.01 | 0.32 ± 0.01 | 0.28 ± 0.05 | 0.60 ± 0.04 | 0.38 ± 0.03 | 0.44 ± 0.06 | 0.19 ± 0.01 | 0.22 ± 0.03 |
| Acids | |||||||||||
| Acetic acid | R, MS | AACE | 2.15 ± 0.02 | 3.09 ± 0.14 | 8.86 ± 1.05 | - | - | - | - | - | 5.41 ± 0.14 |
| Butanoic acid | R, MS | BUTA | 8.14 ± 0.04 | 17.7 ± 0.14 | 16.9 ± 0.11 | 15.7 ± 0.04 | 18.7 ± 0.08 | 19.1 ± 0.15 | 14.8 ± 0.01 | 14.0 ± 0.03 | 18.1 ± 0.21 |
| Hexanoic acid | R, MS | HEXA | 27.7 ± 0.03 | 42.8 ± 1.31 | 50.3 ± 1.43 | 39.2 ± 0.10 | 59.5 ± 1.45 | 61.5 ± 1.75 | 35.8 ± 0.64 | 27.5 ± 0.03 | 55.6 ± 2.43 |
| Octanoic acid | R, MS | OCTA | 99.7 ± 0.13 | 86.5 ± 0.09 | 54.3 ± 1.34 | 60.4 ± 0.09 | 21.9 ± 0.01 | 21.0 ± 0.00 | 42.6 ± 0.08 | 21.4 ± 0.00 | 21.3 ± 0.05 |
| Decanoic acid | R, MS | DECA | 50.7 ± 0.75 | 50.5 ± 1.73 | 58.9 ± 2.84 | 67.8 ± 1.09 | 71.1 ± 2.11 | 71.9 ± 2.02 | 55.6 ± 1.42 | 39.4 ± 0.63 | 59.8 ± 2.80 |
| Dodecanoic acid | MS | DODA | - | - | 3.24 ± 2.45 | - | 4.87 ±1.47 | - | 1.77 ± 0.62 | - | 3.16 ± 0.46 |
| Carbonyl compounds | |||||||||||
| Acetaldehyde | R, MS | ACET | 16.7 ± 0.11 | 32.4 ± 0.06 | 26.1 ± 0.08 | 11.1 ± 0.10 | 24.7 ± 0.08 | 25.9 ± 0.06 | 11.3 ± 0.06 | 21.7 ± 0.04 | 20.2 ± 0.02 |
| Benzaldehyde | MS | BENZ | - | - | 0.26 ± 0.02 | - | - | - | - | - | 3.75 ± 0.56 |
| Syringaldehyde | R, MS | SYR | - | 11.6 ± 0.01 | 5.91 ± 0.46 | - | 4.42 ± 0.16 | 1.35 ± 0.08 | 0.70 ± 0.09 | 31.1 ± 0.58 | 4.47 ± 0.16 |
| Furanic compounds | |||||||||||
| 2-Furfural | R, MS | 2FUR | 20.2 ± 0.06 | 37.2 ± 1.26 | 16.8 ± 0.03 | 18.4 ± 0.03 | 14.5 ± 0.03 | 17.4 ± 0.04 | 6.88 ± 0.03 | 2.65 ± 0.02 | 12.4 ± 0.01 |
| 5-Methylfurfural | R, MS | 5M2F | - | 1.99 ± 0.02 | 0.75 ± 0.00 | - | 0.76 ± 0.01 | 0.93 ± 0.01 | 0.53 ± 0.00 | 0.43 ± 0.00 | 0.62 ± 0.00 |
| Volatile phenols | |||||||||||
| Phenol | MS | PHEN | - | - | - | - | 0.12 ± 0.01 | - | - | - | - |
| Vanillin | MS | VAN | 0.42 ± 0.04 | 0.54 ± 0.02 | 0.58 ± 0.08 | 0.14 ± 0.01 | 0.32 ± 0.06 | 0.27 ± 0.04 | 0.14 ± 0.07 | 0.13 ± 0.01 | 0.18 ± 0.03 |
| Others | |||||||||||
| Cyclodecane | MS | CDEC | 3.53 ± 0.46 | 1.15 ± 0.16 | 0.12 ± 0.01 | 1.03 ± 0.02 | 1.42 ± 0.14 | 0.15 ± 0.03 | 0.53 ± 0.08 | 0.47 ± 0.04 | 0.28 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perestrelo, R.; Caldeira, M.; Rodrigues, F.; Pereira, J.A.M.; Câmara, J.S. DLLμE/GC-MS as a Powerful Analytical Approach to Establish the Volatilomic Composition of Different Whiskeys. Beverages 2022, 8, 53. https://doi.org/10.3390/beverages8030053
Perestrelo R, Caldeira M, Rodrigues F, Pereira JAM, Câmara JS. DLLμE/GC-MS as a Powerful Analytical Approach to Establish the Volatilomic Composition of Different Whiskeys. Beverages. 2022; 8(3):53. https://doi.org/10.3390/beverages8030053
Chicago/Turabian StylePerestrelo, Rosa, Michael Caldeira, Freddy Rodrigues, Jorge A. M. Pereira, and José S. Câmara. 2022. "DLLμE/GC-MS as a Powerful Analytical Approach to Establish the Volatilomic Composition of Different Whiskeys" Beverages 8, no. 3: 53. https://doi.org/10.3390/beverages8030053
APA StylePerestrelo, R., Caldeira, M., Rodrigues, F., Pereira, J. A. M., & Câmara, J. S. (2022). DLLμE/GC-MS as a Powerful Analytical Approach to Establish the Volatilomic Composition of Different Whiskeys. Beverages, 8(3), 53. https://doi.org/10.3390/beverages8030053