Abstract
Consumer demands for new sensory experiences have driven the research of unconventional yeasts in beer. While much research exists on the use of various common Saccharomyces cerevisiae strains as well as non-Saccharomyces yeasts, there exists a gap in knowledge regarding other non-cerevisiae Saccharomyces species in the fermentation of beer, in addition to S. pastorianus. Here, five distinct species of Saccharomyces from the UC Davis Phaff Yeast Culture Collection, as well as one interspecies hybrid from Fermentis, were chosen to ferment 40 L pilot-scale beers. S. kudriavzevii, S. mikatae, S. paradoxus, S. bayanus, and S. uvarum yeasts were used to ferment wort in duplicate pairs, with one fermenter in each pair receiving 10 g/L dry-hop during fermentation. Analytical measurements were made each day of fermentation and compared to controls of SafAle™ US-05 and SafLager™ W 34/70 for commercial brewing parameters of interest. Finished beers were also analyzed for aroma, taste, and mouthfeel to determine the flavor of each yeast as it pertains to brewing potential. All beers exhibited spicy characteristics, likely from the presence of phenols; dry-hopping increased fruit notes while also increasing perceived bitterness and astringency. All of the species in this study displayed great brewing potential, and might be an ideal addition to beer depending on a brewery’s desire to experiment with flavor and willingness to bring a new yeast into their production environment.
Keywords:
non-conventional yeasts; Saccharomyces; fermentation; beer; dry-hopping; brewing potential 1. Introduction
Increasingly, changing demands by beer drinkers in search of new sensory experiences are driving research into novel fermentations [1,2,3,4]. Much of this research has utilized non-Saccharomyces yeast strains [5,6,7,8,9,10,11,12], which can be attributed to the rise in popularity of mixed-fermentation beers [13,14,15]. This pursuit of distinctive aromas and flavors has similarly driven the increased use of non-cerevisiae Saccharomyces species in the alcoholic fermentation of all beverages [16,17,18,19,20,21,22]. While much of this work has been focused on wine fermentations, the most widely used non-cerevisiae species in beer production is S. pastorianus, which has been used the world over in the production of lagers for centuries [20,23,24,25,26].
In addition to novel yeast-derived flavors, brewers are increasingly turning to dry-hopping to enhance their consumers’ sensory experience. Historically, this procedure of adding hops (Humulus lupulus) cones to beer when fermentation is active or finished was performed to provide microbial stability in packaging and during transport [27,28]. Relatively more recently, with the rise of craft and micro brewers, dry-hopping with pellets or advanced hop products [29] has become a common tactic used by brewers desiring to add interesting flavors and aromas to their beer [30].
All Saccharomyces yeast species that have been found to produce ethanol from carbohydrate sugar sources have been classified as part of the Saccharomyces sensu stricto (Sss) complex [31,32,33]. While the Sss currently contains ten distinct species, only eight have been linked to alcoholic beverage fermentation (Figure 1). S. cerevisiae and S. pastorianus have long been known for their use in alcoholic beverage production, but the Sss contains several non-conventional species. S. kudriavzevii, S. mikatae, S. paradoxus, S. bayanus, and S. uvarum are species that have already shown potential for alcoholic beverages, and have been identified in fermentations of wine, tepache, cider, chicha, palm wine, umqombothi, and other beverages around the world [19,34,35,36,37,38,39]. Many of these fermented beverages, however, contain mixed cultures of yeasts and sometimes bacteria, in addition to naturally formed interspecies hybrids between two or more different Saccharomyces species [24,40]. To date, none of these species have been evaluated in monoculture ale fermentations in a beer brewing context, but their efficacy has been previously reviewed [41]. Additionally, none of these yeasts have been studied for their use in the production of dry-hopped beer.
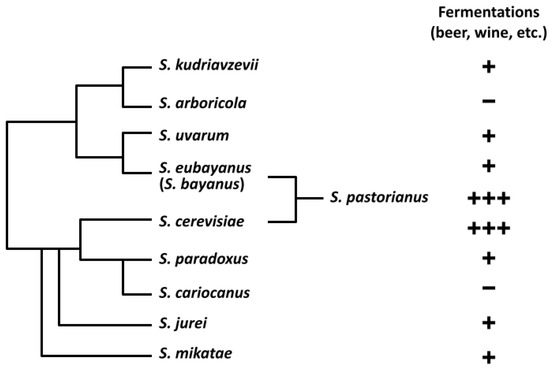
Figure 1.
Sss phylogeny and extent of use in alcoholic beverage fermentations. Saccharomyces bayanus is listed in parenthesis to indicate it was derived from multiple hybridization events [42]. S. pastorianus is shown as a genetic hybrid of S. eubayanus and S. cerevisiae [21]. Use in fermented beverages is indicated with plus signs (+) for current commercial use, with S. cerevisiae and S. pastorianus exhibiting the most ubiquitous use in beer, and negative signs (−) for no known use. S. cariocanus is known to be harboring just four translocated chromosomes different than S. paradoxus [43]. S. jurei has very recently been proven to have brewing potential [44]. Figure previously used in 2020 review by Bruner and Fox [41] of yeasts’ use in alcoholic beverage fermentations.
First isolated from oak trees of western Europe, S. kudriavzevii is a wild-type yeast that has been sequenced to contribute 23–96% of its genome to hybrids with S. cerevisiae [16,19,45]. While no commercial examples of its use in beer fermentation exist, S. kudriavzevii has been isolated from mixed cultures of farmhouse ciders in France and draft beer systems in Germany to New Zealand [46,47]. Due to its propensity to hybridize, this yeast has even been found as part of the genetic makeup in Belgian Trappist ale strains from Chimay, Westmalle, and Orval [48]. S. kudriavzevii is a cryophilic species and is currently used to ferment wines at lower temperatures (10 to 15 °C) in Europe and Australia [19,49]. Because it thrives at low temperatures and may have aromas similar to Belgian beers, S. kudriavzevii has potential for use in the production of hoppy lager beers in the brewing industry.
S. paradoxus has been found in African umqombothi [38] and white wine fermentations previously [50], but has only been studied for its beer brewing potential (at 15 °C) very recently, since the inception of this research [22]. S. paradoxus was one of the first species isolated as a member of the Sss in addition to S. pastorianus and S. cerevisiae and is typically found in tree sap of Northeastern Europe [51]. Being a wild-type yeast species suggests that S. paradoxus may produce interesting volatile aroma compounds at warmer (18 to 24 °C) ale temperatures [52].
Saccharomyces mikatae is a wild yeast that contributes to genetic hybrids from interspecies hybridization events with S. cerevisiae and S. paradoxus [53], and was first isolated from soil and decaying leaves in Japan [43]. S. mikatae was shown to form a biofilm on the surface of liquid media (pellicle) after twenty-five days at 20 °C, similar to wild-type strains [43]. It produced fruity, banana, floral, and sweet perfume aromas in white wine, and ferment slowly, perhaps all due to its diversion from the S. cerevisiae parent genome [54,55]. Both S. paradoxus and S. mikatae offer unique characteristics that might be of interest to craft brewers creating beer at ale fermentation temperatures.
Saccharomyces bayanus was previously thought to be the parent of the lager strain, S. pastorianus [21,47,56], but the hybridization event that produced lager brewing yeast is now proven to have occurred between S. cerevisiae and S. eubayanus [25,34,57,58]. S. bayanus has been characterized as its own species within the Sss, but in order delineate it from S. eubayanus and S. uvarum, it is commonly referred to as S. bayanus var. bayanus [21,42]. Genetic analysis of organisms in beer fermentations have identified S. bayanus as part of blended cultures due to its chromosomal similarity to S. pastorianus [26], but it is most common as a solitary species in wine fermentations [58].
A close relative, Saccharomyces uvarum, was once was thought to be a variant of S. bayanus, but has since been confirmed as a distinct species [59]. S. uvarum has been found to be part of the mixed culture of spontaneously fermented wines [36], as well as an interspecies hybrid known in some Norwegian kveik strains [17]. Both S. bayanus and S. uvarum exhibit increased levels of isoamyl acetate in wine and brandy [60,61], and might contribute similar flavor to beer.
Some commercial yeast suppliers are leveraging the power of interspecies hybrids through research and development (R&D) to create distinctive sensory experiences, including a S. cerevisiae × S. bayanus hybrid produced by Fermentis-LeSaffre (Marcq-en-Baroeul, France, EU; fermentis.com/en/) known as SafŒno™ HDT18 [62]. This interspecies hybrid has been created through a LeSaffre R&D program to select a yeast strain that exhibits increased expression of aromatic terpenes. New research has identified these terpene compounds as some of the most impactful on dry-hopped beer aroma [30,63] through biotransformation with glycosides and alcohols to produce unique aroma characteristics [64]. While this yeast was developed for wine fermentations, it may be of great interest to brewers making dry-hopped beers, and was therefore selected for this study.
While there is much research regarding the use of some of these species in a laboratory scale or wine fermentation, work remains for their efficacy and commercial use in the production of beer. Additionally, little to no sensorial analysis exists on the use of any of these Saccharomyces spp. in the fermentation of beer, most notably at ale fermentation temperatures (18–20 °C) or in dry-hopped beers. The aim of this study is to assess the brewing potential of the non-conventional non-cerevisiae Saccharomyces species outlined above by assessing fermentation dynamics and performance, yeast abundance and viability post-fermentation for serial re-pitching, as well as the flavor characteristics of the resultant beer. Beers in this study will be run as both dry-hopped and standard fermentations due to the pervasiveness of dry-hopping in the American craft brewing industry. While the most widely used non-cerevisiae Saccharomyces species is S. pastorianus, this species will only be used as an analytical control in this study as much research already exists on its brewing potential.
2. Materials and Methods
2.1. Experimental Beers
A total of eight all-malt pilot-scale brews were performed on the 1.8 hL Anheuser-Busch Research Pilot Brewery at the University of California, Davis. Brewing parameters, as well as the malt, hops, water chemistry, mashing regime, pH, boiling parameters, and knockout temperatures followed the same method as outlined in previous research [65]. The experimental beer recipe was similar to an American Pale Ale or Session IPA, with a target original gravity of 10°P and 20 IBU from Centennial (8.3% AA, Hopsteiner, New York, NY, USA) in the kettle, to yield a 4.2% (v/v) alcohol beer under standard ale fermentation conditions. All parameters were chosen to mimic typical production conditions in an American craft brewery. Wort from each 180 L brew was split evenly by volume between four 56 L fermenters, to fill each with approximately 40 L of cooled wort, allowing for each brew to be used for multiple distinct fermentations.
2.2. Yeasts
Five experimental Saccharomyces yeasts sourced from the University of California, Davis, Phaff Yeast Culture Collection (UC Davis, Davis, CA, USA; phaffcollection.ucdavis.edu) included the type strains of S. kudriavzevii, S. mikatae, S. paradoxus, S. bayanus, and S. uvarum. Additionally, the analytical control S. cerevisiae and S. pastorianus species and one experimental S. cerevisiae × S. bayanus hybrid were provided by Fermentis (LeSaffre, Marcq-en-Baroeul, France) (Table 1). Yeasts from the Phaff Collection were revived from cryogenic storage and streaked onto potato dextrose agar (PDA) plates and incubated for 2 days at 30 °C before being moved to room temperature storage until propagation. Yeasts from Fermentis were provided as an active dry yeast with the emulsifier E491 (sorbitan monostearate) and stored at 4 °C until propagation.

Table 1.
Non-conventional non-cerevisiae Saccharomyces and control yeasts used in the fermentations of the experimental beer. Yeasts were sourced from either the Phaff Yeast Culture Collection at the University of California, Davis (UCD), or from Fermentis LeSaffre of Marcq-en-Baroeul, France (Saf). Type strain as defined in MycoBank (mycobank.org), origin, isolation, flocculation, and attenuation, as defined in the scientific or product literature. SafAle™ US-05 and SafLager™ W 34/70 are included as analytical controls.
All yeasts were propagated according to the same procedure to ensure consistency throughout this study. Due to time constraints with research brewing at the UC Davis facility, only one yeast was chosen on which to perform three biological replicates to ferment from three separate brews: S. cerevisiae SafAle™ US-05. Yeasts were propagated in wort consisting of 10.0% w/v (10.0°P, 1.040 Specific Gravity) dried pilsner malt extract (Briess CBW® Pilsen Light; Chilton, WI, USA) in deionized water with 20 ppm CaCl2 salts, targeting 5.2 pH, and 0.10% w/v yeast nutrient (Kerry Yeastex® 82; Beloit, WI, USA). Wort was boiled for ten minutes and sterilized via autoclave before being sterile filtered to remove protein and trub particulate. All transfers of yeast and wort were performed in a laminar flow hood or positive pressure room. Yeast colonies were transferred from PDA plate or package of active dry yeast via sterile inoculation loop to propagation wort and propagated stepwise over the course of 11 days following the methods outlined in previous research [65] as well as Figure 2. All growth took placeat room temperature on a platform orbital shaker (Innova™ 2000, New Brunswick Scientific; Edison, NJ, USA) set to 150 rpm. Yeast cell counts and viability testing with methylene blue were performed on all propagations and fermentations according to standard methods [66].
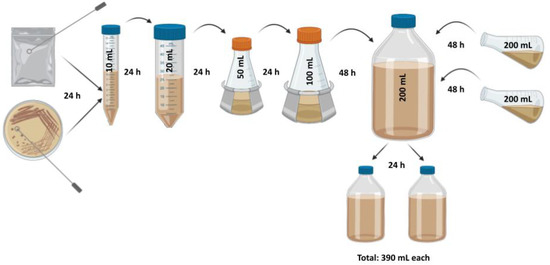
Figure 2.
Yeast propagation schematic following previous methods [65]. Yeasts were propagated to a final approximate total of 40.0 × 1010 cells in each bottle with a total of 390 mL of propagation wort, equivalent to the standard ale pitch rate of 1.0 × 106 cells per mL per °P [67] for each 40 L, 10°P pilot fermentation. Figure created on BioRender.com, not to scale.
2.3. Pilot-Scale Fermentations
Pilot fermentations were performed in 56.0 L glycol-cooled cylindroconical fermenters (JV Northwest; Canby, OR, USA) filled to 40.0 L and set to a standard ale temperature of 20.0 °C. Each unique Saccharomyces species (Table 1) was pitched to its own fermenter of wort in duplicate pairs, with the analytical control S. cerevisiae US-05 pair fermented in biological triplicate for quality assurance, totaling twenty distinct fermentations. One fermenter in each yeast pair received 10.0 g/L Centennial (8.3% AA, Hopsteiner, New York, NY, USA) T-90 hop pellets as a dry-hop when the measured gravity decreased to below 4.0°P or at seven days into fermentation, whichever occurred first, while the other was fermented traditionally. This amount of dry-hopping has become standard practice among craft breweries today, with many brewers far exceeding this amount at times [29,30,68,69]. End of fermentation or “terminal gravity” [70] was defined here as a change of less than 0.10°P gravity for two simultaneous days following dry-hop.
After fermentation was completed, all beer in all fermenters except the S. cerevisiae and S. pastorianus analytical controls were cold conditioned at 0.0 °C for two days to allow for natural clarification. The analytical controls were not conditioned for clarity because sensory analysis was not performed on these beers. Yeast and hops were removed from the bottom of the cylindroconical fermenter before the beer was transferred to a 19.6 L Sankey keg for carbonation. All were packaged from the kegs into CO2-purged 0.95 L (32 oz.) “Crowler™” cans (Ball Corporation; Westminster, CO, USA) and stored below 4.0 °C until sensory analysis and shipping.
2.4. Sample Collection and Preparation
Fermenting beers were aseptically sampled daily within a two-hour window of the time of knockout transfer of wort to fermenter. Using conical tubes, 50 mL of each sample was centrifuged (ThermoFisher Scientific; Waltham, MA, USA) at 20 °C and 3000× g RCF for five minutes. The clarified supernatant was then degassed for five minutes using the degas setting on a VWR B1500A-DTH 1.90 L ultrasonic cleaner (Radnor, PA, USA). Degassed samples were then decanted into the sample tubes of the Anton Paar (Graz, Austria, EU) auto-sampling carousel for immediate analysis. Samples were then measured for extract, gravity, alcohol [71], real degrees of fermentation (RDF), and calories using an Anton Paar Density Meter (DMA 5000 M) and alcolyzer (Alcolyzer Beer M) (Anton Paar USA Inc., Torrance, CA, USA). The DMA 5000 M has a repeatability within 0.000001 g/mL and the Alcolyzer Beer M has a repeatability within 0.03°P and 0.01 % v/v alcohol. pH was measured on a ThermoFisher benchtop pH meter (ThermoFisher Scientific, Waltham, MA, USA) that received a weekly three-point calibration.
2.5. Sensory Analysis
Each set of packaged beer from an individual fermentation was assigned a randomly selected three-digit code in order to ensure blind analysis of experimental samples. The willing members of the UC Davis Brewing and Malting Science laboratory team (n = 7) used a modified consensus method [72] with check-all-that-apply (CATA) [73] in two tastings to choose appropriate aroma descriptor terms from the DraughtLab Beer Flavor Map© (Figure S1) for the twelve beers being analyzed. S. cerevisiae and S. pastorianus controls fermentations were not included for sensory analysis due to their ubiquity in the brewing industry and known flavor potential for craft beer production. The lab members assessed beers served in 60 mL volumes in clear straight sided glasses, after being removed from cold storage (4.0 °C), under white light. Consensus panelists were instructed to cleanse their palates with water and unsalted crackers between each sample. The common aroma descriptors were parsed down to the twelve most recurrent amongst the experimental beers. Each of these twelve descriptors, the five accepted taste modalities, and three recurrent mouthfeel descriptors from the consensus panel were placed on a 9-point intensity scale for scoring by the local brewery panelists (Table 2).

Table 2.
Sample ballot given to brewery taste panels accompanying the beer for sensory analysis. Aroma attributes determined from consensus method with CATA performed by UC Davis Brewing Lab members.
Beers were cold transferred to local breweries within three weeks of packaging for sensory analysis with the descriptors previously determined via consensus. Trained beer sensory taste panels at Lagunitas Brewing Company (Petaluma, CA, USA), Deschutes Brewery (Bend, OR, USA), Russian River Brewing Company (Windsor, CA, USA), Sierra Nevada Brewing Company (Chico, CA, USA), Budweiser Brewery (Fairfield, CA, USA), and Sudwerk Brewing Company (Davis, CA, USA) used the descriptors determined previously by consensus method and rated each on a 9-point intensity scale from “none” to “extremely strong” [74,75]. Training, methods and frequency of sensory panels varied from brewery to brewery. However, it was minimally required that the panelists were able to accurately distinguish dry-hopped from non-hopped beer and identify German, Belgian, and American ale strain characteristics. Panelists from breweries were used due to the inability to train a sensory panel in person during the global COVID-19 pandemic. The total sample group to perform sensory analysis on the experimental beers consisted of 51 panelists (36 male and 15 female), ranging in age from 24 to 61. No panelists had medical reasons for not consuming alcohol.
2.6. Statistical Analysis
Standard deviation values were determined for US-05 analytical data and viability measurements. Two-tailed statistical analysis (t-test) of fermentation data with corresponding p-values, as well as two-way analysis of variance (ANOVA) and coefficients of variance for sensory data were performed in Microsoft® Excel 2019, Version 2102 (Build 13801.20360) to determine the statistical difference between dry-hopped and traditional fermentations for all beers compared to each other, as well as compared to the analytical control.
3. Results and Discussion
3.1. Pilot Fermentations
Twenty fermentations were performed, with a mean original gravity (O.G.) of 10.2° Plato (±0.36) due to a higher brewhouse efficiency than expected for the recipe designed at 10.0°P (Figure S2). Fermentations were carried out at 20.0 °C, standard ale temperatures, and analytical parameters were measured on each day of fermentation. Results for the six experimental strains were compared with the two analytical control strains, S. cerevisiae US-05 (performed in triplicate) and S. pastorianus W 34/70. Vigorous and complete fermentations of the two analytical control species in this study suggest that an adequate yeast pitching rate, sufficient nutrients levels, and proper wort aeration procedures were utilized for all fermentations.
All fermentations reached terminal gravity within two weeks, with the exception of S. uvarum UCDFST 11-512, which took fifteen days for the non-hopped fermentation but only eleven days for the dry-hopped fermentation (Table 3). However, all average fermentation lengths were not shown to be statistically different between dry-hopped and non-hopped fermentations (p > 0.05). These fermentation lengths indicate all the yeasts studied here are viable candidates for production breweries that normally ferment lagers, but perhaps too long for breweries that normally produce ales. Conditioning time was not accounted for in this study as all fermentations were deemed terminal based on gravity determinations. This is opposed to commercial beer production settings that would instead deem beers terminal from the presence or absence of secondary metabolites such as diacetyl or acetaldehyde.

Table 3.
Terminal fermentation characteristics of Saccharomyces species and reference strains used to ferment all-malt wort at 40.0 L pilot scale under two different conditions: non-hopped or dry-hopped during fermentation. Measurements of original gravity (O.G.), final gravity (F.G.) as %w/w of apparent extract, alcohol by volume (ABV), real degree of fermentation (RDF), and calories (Cal) performed on Anton Paar Alcolyzer Beer M. Viability was performed from cells in suspension on non-hopped beers on day of terminal gravity, stained with methylene blue, as per standard procedure (69). Viability was not performed on dry-hopped beers due to interference from hops in suspension. Fermentation length as given in days to achieve final gravity. Strain listed as “Hybrid” is the HDT18 interspecies hybrid of S. bayanus x S. cerevisiae from LeSaffre R&D. US-05 analytical control is listed with standard deviation.
All yeasts measured for viability showed greater than 80.0% living cells at the end of fermentation, signifying a potential for serial re-pitching in a commercial setting. Viability was not measured on the two analytical control strains, US-05 and W 34/70, as their ability for propagation and serial re-pitching has been extensively studied [76,77,78]. Viability data for the interspecies hybrid S. bayanus × S. cerevisiae HD T18 were not available and should be further evaluated as it is not standard practice to re-pitch wine yeasts due to ethanol toxicity [79].
Differences between the average values for alcohol, calories, pH, and RDF when comparing dry-hopped and non-hopped fermentations for all yeast species were highly significant (p < 0.05). Dry-hopping has been shown to biochemically change the composition of wort during fermentation, allowing yeast access to a greater amount of fermentable sugars and subsequent additional fermentative capacity, a phenomenon known as hop creep [80,81,82,83,84]. A previous study was performed to determine if a yeast species or strain could be used to diminish the biochemical change of hop creep observed during fermentation with dry-hops [65]. Most of the novel yeasts shown here show no ability to mitigate the hop creep phenomenon in an effective manner, as all yeasts, with the exception of S. mikatae UCDFST 11-510, showed increases in RDF (Table 3) and alcohol (Figure 3) from the addition of dry-hops during fermentation.
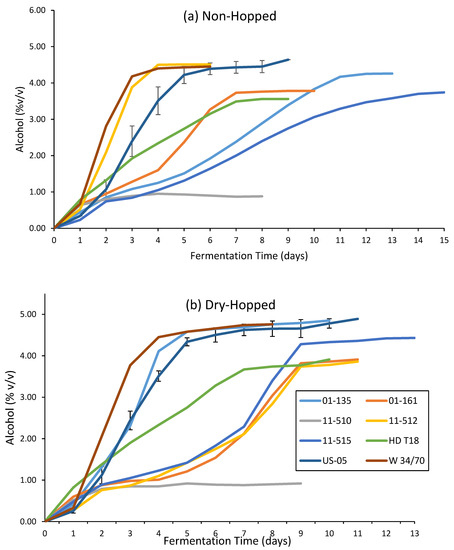
Figure 3.
Alcohol content by volume measured daily on the Anton Paar Alcolyzer Beer M, as reported for both (a) non-hopped and (b) dry-hopped fermentations of all yeasts in this study. Results for US-05 are reported as the mean of three biological replicates with error bars for standard deviation at each day of fermentation.
Fermentation dynamics were grouped more closely in the dry-hopped fermentations compared to the non-hopped treatment (Figure 3), with S. bayanus UCDFST 01-135 showing the most similar fermentation profile to both of the control strains, and S. kudriavzevii UCDFST 11-515, S. paradoxus UCDFST 01-161, and S. uvarum UCDFST 11-512 showing slower, yet steady fermentation. S. paradoxus UCDFST 01-161 showed decreased dynamics with the addition of dry-hops (Figure 3b), but was still a slower fermenter than the control strains in both treatments. The S. cerevisiae × S. bayanus hybrid HD T18 showed no change in dynamics with the addition of dry-hops, showing moderate and steady fermentative capacity, with a terminal RDF similar to S. paradoxus UCDFST 01-161 and S. uvarum UCDFST 11-512. The S. cerevisiae × S. bayanus hybrid HD T18 fermented to a lower relative alcohol content than these other strains due to it starting from a brew with the lowest O.G.
Of note is the strain UCDFST 11-510, S. mikatae, as it was an outlier from the group with the lowest RDF (Table 3) and final amount of alcohol produced, whether dry-hopped or not (Figure 3). UCDFST 11-510 recorded 99.0 ± 0.5% yeast viability in suspension at the end of fermentation, yet only 14.2% RDF in the non-hopped treatment. This indicates the strain is a potential candidate for low- or no-alcohol beer fermentations if brewing parameters are adjusted to get the final alcohol below 0.5% (v/v) and considerations are taken for microbial stability. Analysis of the sugars remaining in this beer may aid in determining which carbohydrates this S. mikatae strain was able to assimilate during fermentation and should be performed with future research. Additionally, this species has been shown to form a pellicle on top of fermenting beer after twenty-five days at 20 °C [43], suggesting that it may ferment comparably slowly as wild-type yeasts, such as Brettanomyces or Hanseniaspora spp. Further research regarding S. mikatae in fermentation for the production of low- and no-alcohol beers should be performed.
3.2. Sensory Analysis
The flavor of the beers from these fermentations was investigated for aroma, taste, and mouthfeel in order to further qualify the brewing potential of these non-conventional Saccharomyces yeasts. S. cerevisiae and S. pastorianus controls fermentations were not included for sensory analysis due to their ubiquity in the brewing industry and known flavor potential for craft beer production. Modified consensus method with CATA from the UC Davis Brewing and Malting Science lab members yielded twelve aroma and three mouthfeel descriptors that were deemed most discriminant and non-redundant from the Beer Flavor Map© as provided by DraughtLab©. The most commonly agreed upon descriptors included Cereal, Earthy, Spicy, Grassy, Citrus, Tropical, Stone Fruit, Stale, Vegetal, Solvent, Rotten, and Metallic for aroma, with additional descriptors within each aroma category outlined above (Table 2). DraughtLab© sensory software (version 3.18.0, DraughtLab, LLC: Webster, NY, USA, 2021) was used to confirm that statistically significant differences were observed for all of the consensus CATA terms after accounting for both panelist and replication effects. Body, Alcohol, and Astringency were selected as the most common mouthfeel descriptors.
From the panelists at participating breweries, all beers showed increases in bitterness and astringency from the high level of dry-hopping (Figure 4), suggesting that beer clarification prior to packaging may have been necessary to fully distinguish the effects of the hops without particulates in suspension effecting flavor. The base beer was also of relatively low alcohol (0.9 to 4.5% abv) and bitterness (20 calculated IBU) content, which could contribute to perceived bitterness from the increase in humulinones from dry-hopping a low IBU beer [85], or perceived astringency from the increase in polyphenol content [80]. Dry-hopping increased the fruit (Citrus, Tropical, and Stone Fruit) perception on all beers as expected from the Centennial cultivar used here [86], with the exception of Stone Fruit in UCDFST 01-161 S. paradoxus.
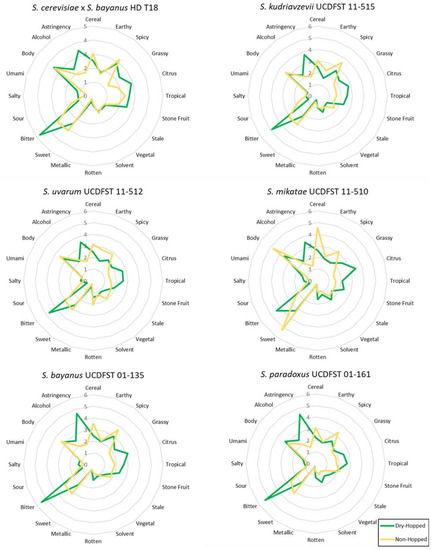
Figure 4.
Radar charts of attributes for each experimental yeast fermentation in this study. Dry-hopped treatments are shown in green, while non-hopped are shown in yellow. (n = 51, with 36 male and 15 female).
All experimental beers displayed Spicy aromas, likely from the expression of phenols, but genetic testing for the POF phenotype should be performed to confirm [87]. Interestingly, these Spicy aromas were perceived lower in the dry-hopped beers, in contrast to expectations, as resinous and spicy characteristics are also noted as aroma characteristics of Centennial hops. On average, many of the unique attributes perceived in the beers fermented with these yeasts can be generally considered as off-flavors in beer (Solvent, Metallic, Vegetal, Rotten, or Stale). Trained panelists perceived these descriptors in very low amounts, with no off-flavor characteristic achieving an average greater than 2 on the 9-point intensity scale. All attribute averages did not exceed a 6 on the 9 point scale and were thus displayed as such in Figure 4 for ease of analysis.
Other descriptors were written in on the ballot (Table 2) by the trained panelists at breweries. Beers made with S. uvarum UCDFST 11-512 commonly had notes of diacetyl in the dry-hopped treatment and sulfur in the non-hopped fermentation. Beers made with S. kudriavzevii UCDFST 11-515 were described as having distinct phenolic and sulfur characteristics in the non-hopped treatment. The non-hopped beer fermented with S. mikatae UCDFST 11-510 was perceived as being wort-like, likely due to its low attenuation. Write-in descriptors are presented here only if more than 10% of panelists (n = 5) reported a given characteristic.
4. Conclusions
Fermentation dynamics and yeast viabilities here suggest that appropriate pitching rate, adequate nutrients, and proper wort aeration from the brewhouse were achieved on all brews and fermentations. All yeasts reached terminal gravity in under two weeks, with the exception of S. uvarum UCDFST 11-512, which took fifteen days for the non-hopped fermentation. These dynamics make all the yeasts studied viable candidates for production breweries; conditioning time should be accounted for but were not studied here. All fermentations in this study were deemed terminal based on gravity as opposed to metabolite production like in most production breweries, so further analysis and brewer-specific standards are required before recommendations of full-scale production. All yeasts displayed high potential for re-pitching in a commercial setting with high viabilities at the end of fermentation in the non-hopped fermentations. These high numbers are promising, but viability should be assessed during fermentation and prior to re-pitch in order to ensure adequate cell count for vigorous growth in a commercial setting.
With the exception of S. mikatae UCDFST 11-510, all yeasts displayed increased RDF and alcohol with the addition of dry-hops during fermentation, as was expected due to hop creep. Further research should be pursued in the use of S. mikatae UCDFST 11-510 and other strains of this species for the production of low- and no-alcohol beers and its possible resistance to hop creep. Strong phenolic characteristics were perceived in the flavor of beers fermented with all non-conventional yeasts, but dry-hopping, in this case with Centennial, decreased this aroma while increasing all fruit aromas, as well as bitterness and astringency. No flavors that are generally associated with poor fermentation scored high among trained sensory panels. Comparisons to the standard control beer yeast fermentations should have been performed in sensory analysis as well, but experimental time constraints did not allow this. Previous research has shown these yeasts’ ability to co-ferment with standard S. cerevisiae, and flavor analysis should also be performed on these potential combinations. All of these species displayed great brewing potential given a brewery’s desire to experiment with flavor and willingness to bring in a new yeast.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/beverages7030068/s1, Figure S1: The Beer Flavor Map©, as provided by DraughtLab©, which outlines the flavor descriptors common to beer and was used to determine terms for consensus method and subsequent descriptive analysis. Figure S2: The average of the standard brew day analytical parameters documented in this experiment, with error bars representing standard deviation.
Author Contributions
J.B. conceived this study, performed the bulk of the research, gathered and transcribed data, and wrote the original manuscript. A.M. assisted in the brewing of beer and brew day sample collection, data curation with figure manipulation and statistics, and assisted with the final editing of the manuscript. G.F. supervised the work, offered insight, and assisted with final editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the UC Davis Food Science and Technology Department in the form of a graduate fellowship, as well as funds from the H.A. Jastro-Shields Research Award, Margrit Mondavi Graduate Fellowship, and Michael J. Lewis Endowment.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of California, Davis (IRB ID: 1757809-1 on 2 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Full data set available upon request to authors at emails listed above.
Acknowledgments
Much gratitude to Anne Flesch and Kevin Lane of Fermentis, as well as Kyria Boundy-Mills and Irnayuli Sitepu from the UC Davis Phaff Collection for advice in yeast selection and revival of cryogenically stored yeast. Appreciation to Lindsey Barr and Victoria Chaplin at DraughtLab for providing access to their amazing sensory software. Thanks to Jean-Xavier Guinard and Lindsey Barr for helping formulate the way to run this sensory analysis during a global COVID pandemic. Many thanks to Joy Wilson of Lagunitas Brewing, Amanda Benson of Deschutes Brewery, Vinnie Cilurzo of Russian River Brewing, Katrina Kettler of Budweiser Fairfield, Andrew Reyes of Sierra Nevada Brewing, and Alex Patil of Sudwerk Brewing for helping coordinate sensory panels for these beers.
Conflicts of Interest
The authors declare no conflict of interest as all research was performed and funded while the authors were students or professors at University of California, Davis in Davis, CA, USA.
References
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.D.C.; Padilha, F.F. Beer molecules and its sensory and biological properties: A review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Tian, J. Determination of several flavours in beer with headspace sampling-gas chromatography. Food Chem. 2010, 123, 1318–1321. [Google Scholar] [CrossRef]
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Dykstra, J. The Beer Connoisseur; Café Media: Atlanta, GA, USA, 2020; pp. 18–29. [Google Scholar]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Application of non-Saccharomyces yeasts isolated from kombucha in the production of alcohol-free beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef]
- Bellut, K.; Arendt, E.K. Chance and challenge: Non-Saccharomyces yeasts in nonalcoholic and low alcohol beer brewing—A review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Gibson, B.; Geertman, J.-M.A.; Hittinger, C.T.; Krogerus, K.; Libkind, D.; Louis, E.J.; Magalhães, F.; Sampaio, J. New yeasts—New brews: Modern approaches to brewing yeast design and development. FEMS Yeast Res. 2017, 17, fox038. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. Novel brewing yeast hybrids: Creation and application. Appl. Microbiol. Biotechnol. 2017, 101, 65–78. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Gamero, A.; Dijkstra, A.; Smit, B.; De Jong, C. Aromatic potential of diverse non-conventional yeast species for winemaking and brewing. Fermentation 2020, 6, 50. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Tyakht, A.; Kopeliovich, A.; Klimenko, N.; Efimova, D.; Dovidchenko, N.; Odintsova, V.; Kleimenov, M.; Toshchakov, S.; Popova, A.; Khomyakova, M.; et al. Characteristics of bacterial and yeast microbiomes in spontaneous and mixed-fermentation beer and cider. Food Microbiol. 2021, 94, 103658. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, A.; Zara, G.; Legras, J.-L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel starters for old processes: Use of Saccharomyces cerevisiae strains isolated from artisanal sourdough for craft beer production at a brewery scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- Peris, D.; Lopes, C.A.; Belloch, C.; Querol, A.; Barrio, E. Comparative genomics among Saccharomyces cerevisiae × Saccharomyces kudriavzevii natural hybrid strains isolated from wine and beer reveals different origins. BMC Genom. 2012, 13, 407. [Google Scholar] [CrossRef]
- Krogerus, K.; Preiss, R.; Gibson, B.; Krogerus, K.; Preiss, R.; Gibson, B. A Unique Saccharomyces cerevisiae × Saccharomyces uvarum hybrid isolated from Norwegian farmhouse beer: Characterization and reconstruction. Front. Microbiol. 2018, 9, 2253. [Google Scholar] [CrossRef] [PubMed]
- Mulero-Cerezo, J.; Briz-Redón, A.; Serrano-Aroca, A. Saccharomyces Cerevisiae Var. Boulardii: Valuable probiotic starter for craft beer production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Peris, D.; Pérez-Torrado, R.; Hittinger, C.T.; Barrio, E.; Querol, A. On the origins and industrial applications of Saccharomyces cerevisiae × Saccharomyces kudriavzeviihybrids. Yeast 2018, 35, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Kielland-Brandt, M.C.; Hansen, J. Lager brewing yeast. In Comparative Genomics; Sunnerhagen, P., Piskur, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 145–164. [Google Scholar]
- Rainieri, S.; Kodama, Y.; Kaneko, Y.; Mikata, K.; Nakao, Y.; Ashikari, T. Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl. Environ. Microbiol. 2006, 72, 3968–3974. [Google Scholar] [CrossRef] [PubMed]
- Nikulin, J.; Vidgren, V.; Krogerus, K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing potential of the wild yeast species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020, 246, 2283–2297. [Google Scholar] [CrossRef]
- Gibson, B.; Liti, G. Saccharomyces pastorianus: Genomic insights inspiring innovation for industry. Yeast 2015, 32, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Kanamori, T.; Itoh, T.; Kodama, Y.; Rainieri, S.; Nakamura, N.; Shimonaga, T.; Hattori, M.; Ashikari, T. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 2009, 16, 115–129. [Google Scholar] [CrossRef]
- Bing, J.; Han, P.-J.; Liu, W.-Q.; Wang, Q.-M.; Bai, F.-Y. Evidence for a Far East Asian origin of lager beer yeast. Curr. Biol. 2014, 24, R380–R381. [Google Scholar] [CrossRef] [PubMed]
- Wendland, J. Lager yeast comes of age. Eukaryot. Cell 2014, 13, 1256–1265. [Google Scholar] [CrossRef]
- Brown, H.; Morris, G. On certain functions of hops used in the dry-hopping of beers. Trans. Inst. Brew 1893, 6, 94–106. [Google Scholar]
- Moritz, E.R.; Morris, G.H. A Text-Book of the Science of Brewing; Spon: London, UK, 1891. [Google Scholar]
- LaFontaine, S.R.; Shellhammer, T.H. How hoppy beer production has redefined hop quality and a discussion of agricultural and processing strategies to promote it. MBAA TQ 2019, 56, 1–12. [Google Scholar] [CrossRef]
- LaFontaine, S.R.; Shellhammer, T.H. Investigating the factors impacting aroma, flavor, and stability in dry-hopped beers. MBAA TQ 2019, 56, 13–23. [Google Scholar] [CrossRef]
- Mortimer, R.K. Evolution and variation of the yeast (Saccharomyces) genome. Genome Res. 2000, 10, 403–409. [Google Scholar] [CrossRef]
- Sicard, D.; Legras, J.-L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. Comptes Rendus Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Borneman, A.R.; Pretorius, I. Genomic insights into the Saccharomyces sensu stricto complex. Genetics 2015, 199, 281–291. [Google Scholar] [CrossRef]
- Libkind, D.; Hittinger, C.T.; Valério, E.; Gonçalves, C.; Dover, J.; Johnston, M.; Gonçalves, P.; Sampaio, J. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Pérez-Través, L.; Sangorrín, M.P.; Barrio, E.; Querol, A.; Lopes, C.A. Saccharomyces uvarum is responsible for the traditional fermentation of apple CHICHA in Patagonia. FEMS Yeast Res. 2016, 17, fow109. [Google Scholar] [CrossRef]
- Demuyter, C.; Lollier, M.; Legras, J.-L.; Le Jeune, C. Predominance of Saccharomyces uvarum during spontaneous alcoholic fermentation, for three consecutive years, in an Alsatian winery. J. Appl. Microbiol. 2004, 97, 1140–1148. [Google Scholar] [CrossRef]
- Cordente, T.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.S.; Korshunova, I.V.; Jespersen, L.; Naumov, G.I. Molecular genetic identification of sensu stricto strains from African sorghum beer. FEMS Yeast Res. 2003, 3, 177–184. [Google Scholar] [CrossRef]
- Mateo, J.; Jimenez, M.; Huerta, T.; Pastor, A. Contribution of different yeasts isolated from musts of monastrell grapes to the aroma of wine. Int. J. Food Microbiol. 1991, 14, 153–160. [Google Scholar] [CrossRef]
- Bisson, L.F. Yeast hybrids in winemaking. Catal. Discov. Pract. 2016, 1, 27–34. [Google Scholar] [CrossRef][Green Version]
- Bruner, J.; Fox, G. Novel non-Cerevisiae Saccharomyces yeast species used in beer and alcoholic beverage fermentations. Fermentation 2020, 6, 116. [Google Scholar] [CrossRef]
- Nguyen, H.-V.; Legras, J.-L.; Neuvéglise, C.; Gaillardin, C. Deciphering the hybridisation history leading to the lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380T. PLoS ONE 2011, 6, e25821. [Google Scholar] [CrossRef]
- Naumov, G.I.; James, S.A.; Naumova, E.S.; Louis, E.; Roberts, I.N. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int. J. Syst. Evol. Microbiol. 2000, 50, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, M.; Michel, M.; Kunz, O.; Kuusisto, T.; Magalhães, F.; Krogerus, K.; Gibson, B. Unique brewing-relevant properties of a strain of Saccharomyces jurei isolated from ash (Fraxinus excelsior). Front. Microbiol. 2021, 12, 681. [Google Scholar] [CrossRef]
- Erny, C.; Raoult, P.; Alais, A.; Butterlin, G.; Delobel, P.; Matei, F.; Casaregola, S.; Legras, J.L. Ecological success of a group of Saccharomyces cerevisiae/Saccharomyces kudriavzevii hybrids in the Northern European wine-making environment. Appl. Environ. Microbiol. 2012, 78, 3256–3265. [Google Scholar] [CrossRef]
- Masneuf, I.; Hansen, J.; Groth, C.; Piskur, J.; Dubourdieu, D. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl. Environ. Microbiol. 1998, 64, 3887–3892. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.; Hansen, J.; Piškur, J. A natural chimeric yeast containing genetic material from three species. Int. J. Syst. Evol. Microbiol. 1999, 49, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Gonzaález, S.S.; Barrio, E.; Querol, A. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl. Environ. Microbiol. 2008, 74, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.P.; Gonçalves, P. Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus. Appl. Environ. Microbiol. 2008, 74, 2144–2152. [Google Scholar] [CrossRef]
- Orlic, S.; Redžepović, S.; Jeromel, A.; Herjavec, S.; Iacumin, L. Influence of indigenous Saccharomyces paradoxus strains on Chardonnay wine fermentation aroma. Int. J. Food Sci. Technol. 2007, 42, 95–101. [Google Scholar] [CrossRef]
- Martini, A.V.; Martini, A. A proposal for correct nomenclature of the domesticated species of the genus Saccharomyces. In Biotechnology Applications in Beverage Production; Cantarelli, C., Lanzarini, G., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 1–16. [Google Scholar]
- Majdak, A.; Herjavec, S.; Orlic, S.; Redzepovic, S.; Mirosevic, N. Comparison of wine aroma compounds produced by Saccharomyces paradoxus and Saccharomyces cerevisiae Strains. Food Technol. Biotechnol. 2002, 40, 103–109. [Google Scholar]
- Dunn, B.; Richter, C.; Kvitek, D.J.; Pugh, T.; Sherlock, G. Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res. 2012, 22, 908–924. [Google Scholar] [CrossRef]
- Bellon, J.R.; Schmid, F.; Capone, D.L.; Dunn, B.; Chambers, P.J. Introducing a new breed of wine yeast: Interspecific hybridisation between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS ONE 2013, 8, e62053. [Google Scholar] [CrossRef]
- Bellon, J.; Schmidt, S.; Solomon, M. Case study: Development of Saccharomyces cerevisiae × Saccharomyces mikatae wine yeast hybrids and their potential to deliver alternative wine styles. AWRI Technol. Rev. 2019, 241, 6–11. [Google Scholar]
- Naumova, E.S.; Naumov, G.I.; Masneuf-Pomarède, I.; Aigle, M.; Dubourdieu, D. Molecular genetic study of introgression between Saccharomyces bayanus and S. cerevisiae. Yeast 2005, 22, 1099–1115. [Google Scholar] [CrossRef]
- Fay, J.C.; Liu, P.; Ong, G.T.; Dunham, M.J.; Cromie, G.A.; Jeffery, E.W.; Ludlow, C.L.; Dudley, A.M. A polyploid admixed origin of beer yeasts derived from European and Asian wine populations. PLoS Biol. 2019, 17, e3000147. [Google Scholar] [CrossRef]
- De Almeida, P.M.C. Microbe Domestication and the Identification of the Wild Genetic Stock of Wine Yeasts. Master’s Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- Nguyen, H.-V.; Gaillardin, C. Evolutionary relationships between the former species Saccharomyces uvarum and the hybrids Saccharomyces bayanus and Saccharomyces pastorianus; reinstatement of Saccharomyces uvarum (Beijerinck) as a distinct species. FEMS Yeast Res. 2005, 5, 471–483. [Google Scholar] [CrossRef]
- Torrado, R.P.; González, S.S.; Combina, M.; Barrio, E.; Querol, A. Molecular and enological characterization of a natural Saccharomyces uvarum and Saccharomyces cerevisiae hybrid. Int. J. Food Microbiol. 2015, 204, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Januszek, M.; Satora, P.; Wajda, L.; Tarko, T. Saccharomyces bayanus enhances volatile profile of apple brandies. Molecules 2020, 25, 3127. [Google Scholar] [CrossRef] [PubMed]
- SAFŒNOTM HD T18. Available online: https://fermentis.com/en/fermentation-solutions/you-create-wine/safoeno-hd-t18/ (accessed on 6 April 2021).
- Lafontaine, S.; Caffrey, A.; Dailey, J.; Varnum, S.; Hale, A.; Eichler, B.; Dennenlöhr, J.; Schubert, C.; Knoke, L.; Lerno, L.; et al. Evaluation of variety, maturity, and farm on the concentrations of monoterpene diglycosides and hop volatile/nonvolatile composition in five Humulus lupulus cultivars. J. Agric. Food Chem. 2021, 69, 4356–4370. [Google Scholar] [CrossRef]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef]
- Bruner, J.; Marcus, A.; Fox, G. Dry-hop creep potential of various Saccharomyces yeast species and strains. Fermentation 2021, 7, 66. [Google Scholar] [CrossRef]
- Schisler, D.O. Comparison of revised yeast counting methods. J. Am. Soc. Brew. Chem. 1986, 44, 81–85. [Google Scholar] [CrossRef]
- Bamforth, C.W. Scientific Principles of Malting and Brewing; American Society of Brewing Chemists, Ed.; American Society of Brewing Chemists: St. Paul, MN, USA, 2006; p. 246. [Google Scholar]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Hauser, D.G.; Van Simaeys, K.R.; Lafontaine, S.R.; Shellhammer, T.H. A comparison of single-stage and two-stage dry-hopping regimes. J. Am. Soc. Brew. Chem. 2019, 77, 251–260. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001; p. 656. [Google Scholar]
- A Technical Committee. Alcohol. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food, 2nd ed.; Springer New York: New York, NY, USA, 2010; pp. 227–257. [Google Scholar]
- Varela, P.; Ares, G. (Eds.) Novel Techniques in Sensory Characterization and Consumer Profiling; CRC Press: Boca Raton, FL, USA, 2014; p. 408. [Google Scholar]
- Moskowitz, H.R. Intensity scales for pure tastes and for taste mixtures. Percept. Psychophys. 1971, 9, 51–56. [Google Scholar] [CrossRef]
- Moskowitz, H.R.; Sidel, J.L. Magnitude and hedonic scales of food acceptability. J. Food Sci. 1971, 36, 677–680. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Kennedy, A.I.; Hodgson, J.A.; Thurston, P.; Smart, K.A. Impact of serial repitching on lager brewing yeast quality. J. Am. Soc. Brew. Chem. 2003, 61, 1–9. [Google Scholar] [CrossRef]
- Kalayu, G. Serial re-pitching: Its effect on yeast physiology, fermentation performance, and product quality. Ann. Microbiol. 2019, 69, 787–796. [Google Scholar] [CrossRef]
- Large, C.R.L.; Hanson, N.A.; Tsouris, A.; Abou Saada, O.; Koonthongkaew, J.; Toyokawa, Y.; Schmidlin, T.; Moreno-Habel, D.A.; McConnellogue, H.; Preiss, R.; et al. Genomic stability and adaptation of beer brewing yeasts during serial repitching in the brewery. BioRxiv 2020, 166157. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Choi, Y.-R.; Lee, S.-Y.; Park, J.-T.; Shim, J.-H.; Park, K.-H.; Kim, J.-W. Screening wild yeast strains for alcohol fermentation from various fruits. Mycobiology 2011, 39, 33–39. [Google Scholar] [CrossRef]
- Olodokun, O.; Cowley, T.; James, S.; Smart, K.A. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Kirkpatrick, K.R.; Shellhammer, T.H. A cultivar-based screening of hops for dextrin degrading enzymatic potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. [Google Scholar] [CrossRef]
- Kirkendall, J.A.; Mitchell, C.A.; Chadwick, L.R. The freshening power of centennial hops. J. Am. Soc. Brew. Chem. 2018, 76, 178–184. [Google Scholar] [CrossRef]
- Bruner, J.; Williams, J.; Fox, G. Further exploration of hop creep variability with Humulus lupulus cultivars and proposed method for determination of secondary fermentation. MBAA TQ 2020, 57, 169–176. [Google Scholar] [CrossRef]
- Stokholm, A.; Lindsey, N.R.; Shellhammer, T.H. Evaluating a benchtop fermentation method for estimating dextrin degradation by hops’ diastatic enzymes during dry-hopping. Brew. Sci. 2020, 73, 140–148. [Google Scholar]
- Maye, J.P.; Smith, R.; Leker, J. Humulinone formation in hops and hop pellets and its implications for dry hopped beers. MBAA TQ 2016, 53, 23–27. [Google Scholar] [CrossRef]
- Hopsteiner. Hop Profiles—Centennial. Available online: https://www.hopsteiner.com/variety-data-sheets/Centennial/ (accessed on 12 April 2021).
- Mertens, S.; Steensels, J.; Gallone, B.; Souffriau, B.; Malcorps, P.; Verstrepen, K.J. Rapid screening method for Phenolic Off-Flavor (POF) production in yeast. J. Am. Soc. Brew. Chem. 2017, 75, 318–323. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).