The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Dicarboxymethyl Cellulose Synthesis
2.2.1. Synthesis of Sodium Chloromalonate (NaCMA)
2.2.2. Synthesis of Dicarboxymethyl Cellulose
2.2.3. Degree of Substitution Determination
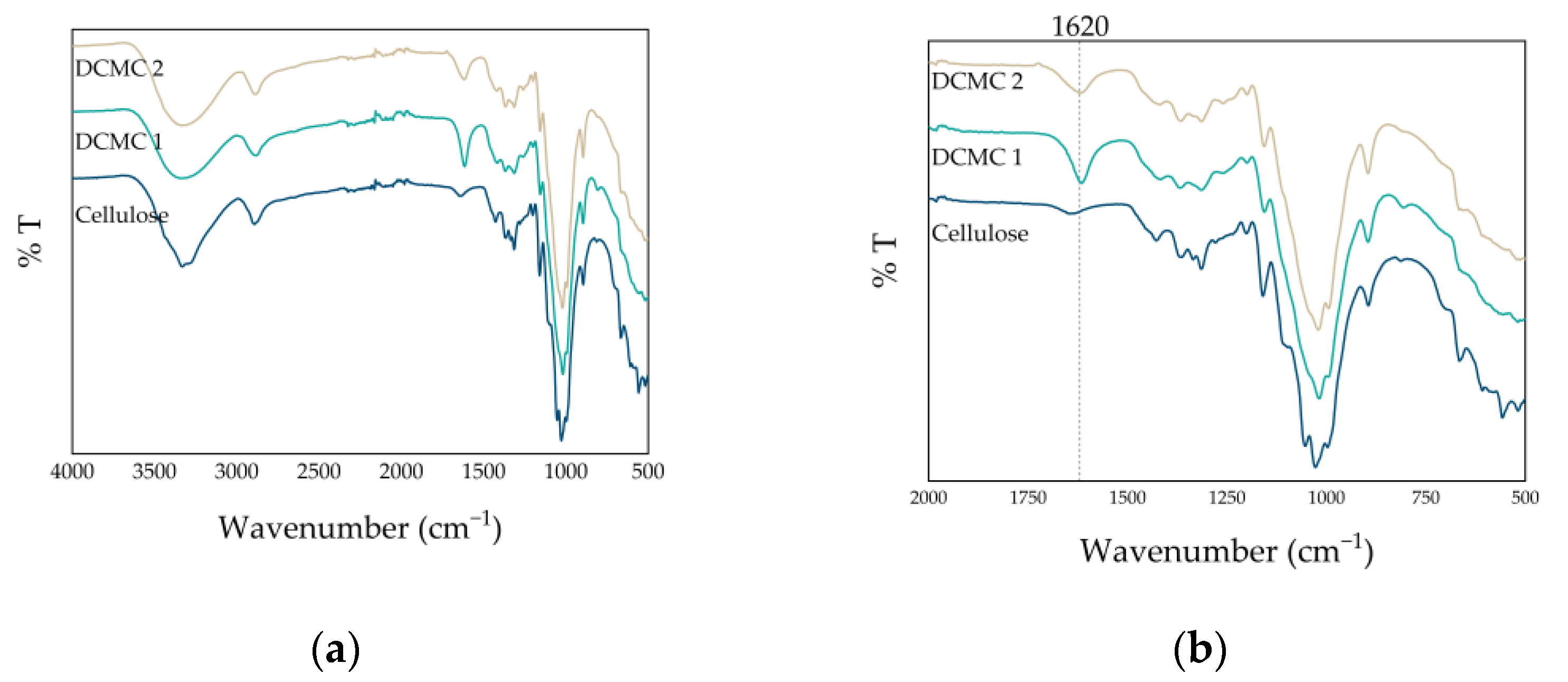
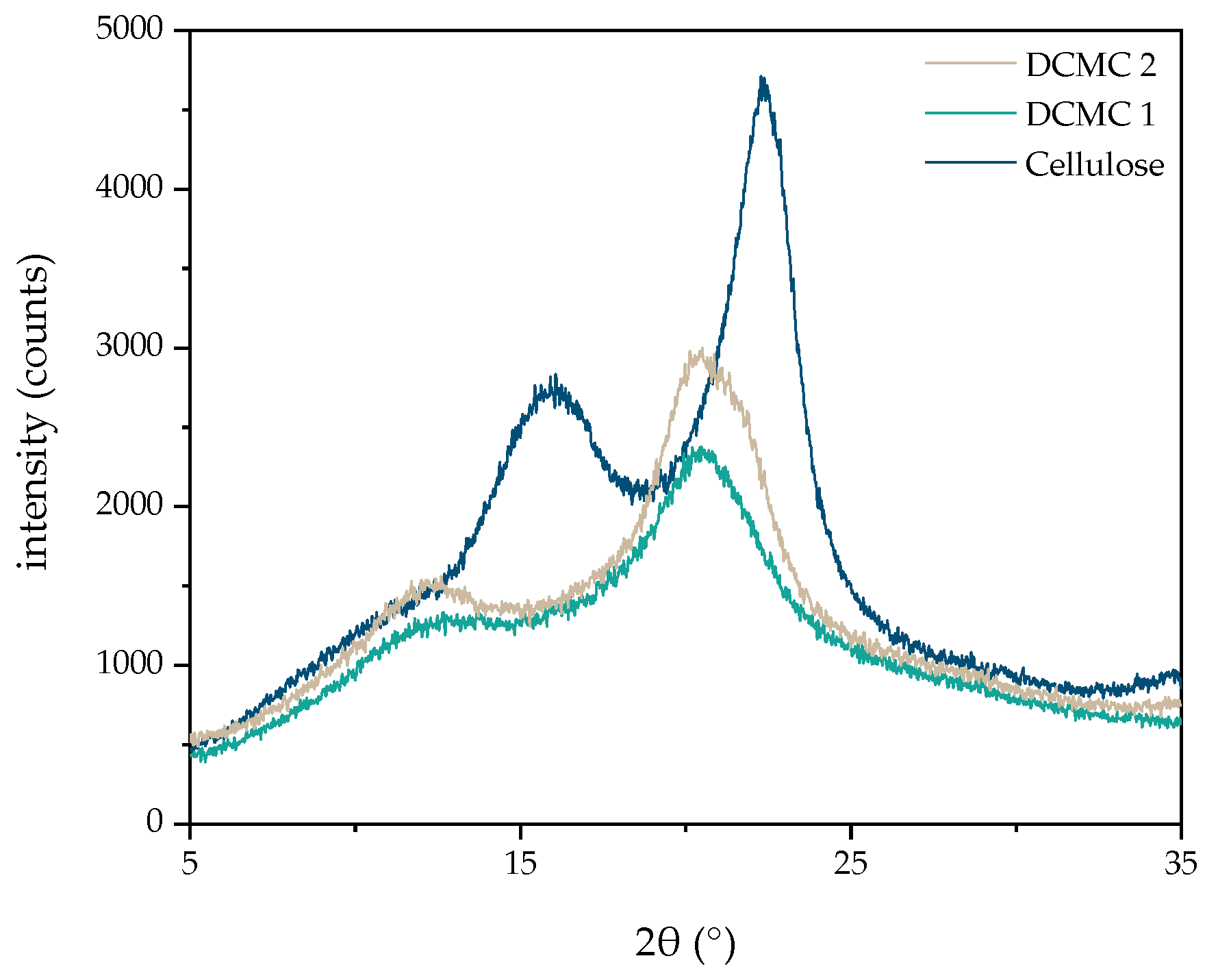
2.2.4. Structural Characterization of Dicarboxymethyl Cellulose
2.3. Wine Fining Trials
2.3.1. Wine Characterization
2.3.2. Adsorption of Wine Proteins
2.3.3. Heat Stability Test
2.3.4. Protein Quantification
2.4. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Dicarboxymethyl Cellulose Sodium Salt
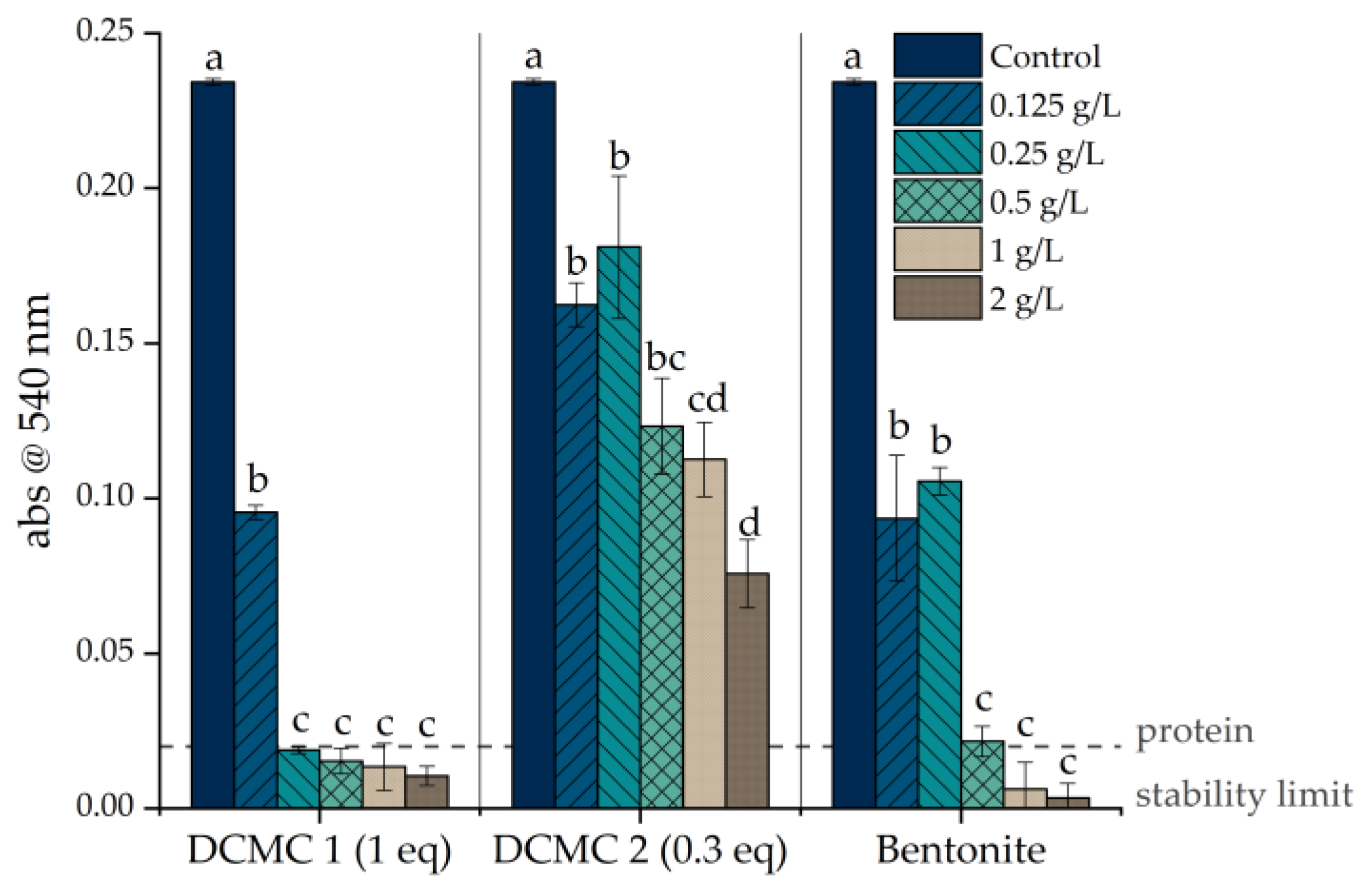
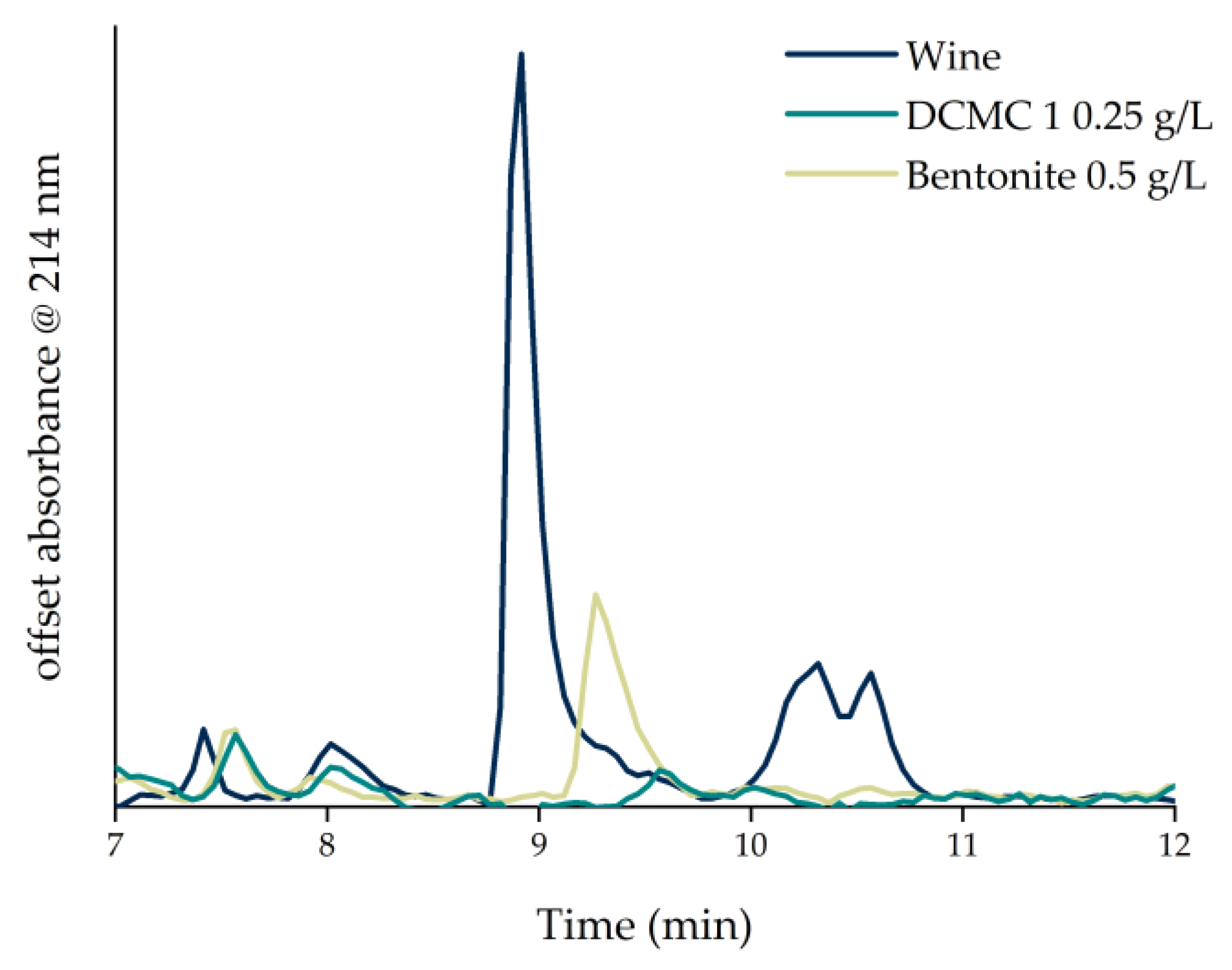
3.2. Wine Fining Trials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- McRae, J.M.; Barricklow, V.; Pocock, K.F.; Smith, P.A. Predicting protein haze formation in white wines. Aust. J. Grape Wine R. 2018, 24, 504–511. [Google Scholar] [CrossRef]
- Romanini, E.; McRae, J.M.; Bilogrevic, E.; Colangelo, D.; Gabrielli, M.; Lambri, M. Use of grape seeds to reduce haze formation in white wines. Food Chem. 2021, 341, 128250. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Mierczynski, P.; Maniukiewicz, W.; Visalakshan, R.M.; Vasilev, K.; Smith, P.A. Magnetic separation technology: Functional group efficiency in the removal of haze-forming proteins from wines. Food Chem. 2019, 275, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- Sommer, S.; Tondini, F. Sustainable Replacement Strategies for Bentonite in Wine Using Alternative Protein Fining Agents. Sustainability 2021, 13, 1860. [Google Scholar] [CrossRef]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef]
- Sui, Y.; McRae, J.M.; Wollan, D.; Muhlack, R.A.; Godden, P.; Wilkinson, K.L. Use of ultrafiltration and proteolytic enzymes as alternative approaches for protein stabilisation of white wine. Aust. J. Grape Wine Res. 2021, 27, 234–245. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef] [Green Version]
- Gago, D.; Chagas, R.; Ferreira, L.M.; Velizarov, S.; Coelhoso, I. A Novel Cellulose-Based Polymer for Efficient Removal of Methylene Blue. Membranes 2020, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Pérez, M.P.; Bautista-Ortín, A.B.; Durant, V.; Gómez-Plaza, E. Evaluating Alternatives to Cold Stabilization in Wineries: The Use of Carboxymethyl Cellulose, Potassium Polyaspartate, Electrodialysis and Ion Exchange Resins. Foods 2020, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- Obreque-Slier, E.; Espinola-Espinola, V.; Lopez-Solis, R. Wine pH Prevails over Buffering Capacity of Human Saliva. J. Agric. Food Chem. 2016, 64, 8154–8159. [Google Scholar] [CrossRef]
- Bauwens, M.; De Saint-Hubert, M.; Cleynhens, J.; Brams, L.; Devos, E.; Mottaghy, F.M.; Verbruggen, A. Radioiodinated phenylalkyl malonic acid derivatives as pH-sensitive SPECT tracers. PLoS ONE 2012, 7, e38428. [Google Scholar] [CrossRef]
- Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L. Trichloroisocyanuric Acid: A Versatile and Efficient Chlorinating and Oxidizing Reagent. Eur. J. Org. Chem. 2019, 2019, 3544–3552. [Google Scholar] [CrossRef]
- Mendonça, G.F.; Sindra, H.C.; De Almeida, L.S.; Esteves, P.M.; De Mattos, M.C.S. Trihaloisocyanuric acids as convenient reagents for regioselective halogenation of β-dicarbonyl compounds. Tetrahedron Lett. 2009, 50, 473–475. [Google Scholar] [CrossRef]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2021, 13, 81. [Google Scholar] [CrossRef]
- Chagas, R.; Gericke, M.; Ferreira, R.B.; Heinze, T.; Ferreira, L.M. Synthesis and characterization of dicarboxymethyl cellulose. Cellulose 2019, 27, 1965–1974. [Google Scholar] [CrossRef] [Green Version]
- Marangon, M.; Van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and Wine Proteins: Their Fractionation by Hydrophobic Interaction Chromatography and Identification by Chromatographic and Proteomic Analysis. J. Agric. Food. Chem. 2009, 57, 4415–4425. [Google Scholar] [CrossRef]
- Ferreira, L.; Chagas, R.; Ferreira, R.B.; Coelhoso, I.; Velizarov, S. Compound, Method of Production and Uses Thereof. Patent No. WO/2019/197884, 17 October 2019. [Google Scholar]
- Wongvitvichot, W.; Pithakratanayothin, S.; Wongkasemjit, S.; Chaisuwan, T. Fast and practical synthesis of carboxymethyl cellulose from office paper waste by ultrasonic-assisted technique at ambient temperature. Polym. Degrad. Stab. 2021, 184, 109473. [Google Scholar] [CrossRef]
- Obele, C.M.; Ibenta, M.E.; Chukwuneke, J.L.; Nwanonenyi, S.C. Carboxymethyl cellulose and cellulose nanocrystals from cassava stem as thickeners in reactive printing of cotton. Cellulose 2021, 28, 2615–2633. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fang, C.; Cheng, Y.; Tan, T.; Han, H. Synthesis and structure of carboxymethylcellulose with a high degree of substitution derived from waste disposable paper cups. Carbohydr. Polym. 2020, 237, 116040. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.-C.; Dogaru, B.-I.; Popescu, C.-M. Effect of Cellulose Nanocrystals Nanofiller on the Structure and Sorption Properties of Carboxymethyl Cellulose–Glycerol–Cellulose Nanocrystals Nanocomposite Systems. Materials 2020, 13, 2900. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Carboxymethyl Cellulose-Grafted Graphene Oxide/Polyethylene Glycol for Efficient Ni(II) Adsorption. J. Polym. Environ. 2021, 29, 859–870. [Google Scholar] [CrossRef]
- Bisla, V.; Rattan, G.; Singhal, S.; Kaushik, A. Green and novel adsorbent from rice straw extracted cellulose for efficient adsorption of Hg (II) ions in an aqueous medium. Int. J. Biol. Macromol. 2020, 161, 194–203. [Google Scholar] [CrossRef]
- Pachova, V.; Ferrando, M.; Güell, C.; López, F. Protein adsorption onto metal oxide materials in white wine model systems. J. Food Sci. 2002, 67, 2118–2121. [Google Scholar] [CrossRef]
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine R. 2006, 12, 212–220. [Google Scholar] [CrossRef]
- Chagas, R.; Laia, C.A.T.; Ferreira, R.B.; Ferreira, L.M. Sulfur dioxide induced aggregation of wine thaumatin-like proteins: Role of disulfide bonds. Food Chem. 2018, 259, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. The complexity of protein haze formation in wines. Food Chem. 2009, 112, 169–177. [Google Scholar] [CrossRef]

| Sample | Molar Ratio AGU:NaCMA:NaOH 1 | mCellulose (g) | mNaCMA (g) | mNaOH (g) |
|---|---|---|---|---|
| DCMC 1 | 1:1:4 | 3.00 | 3.53 | 2.94 |
| DCMC 2 | 1:0.3:4 | 3.00 | 1.06 | 2.94 |
| Sample | NaCMA eq 1 | % Na | DS |
|---|---|---|---|
| DCMC 1 | 1 | 0.85 | 0.03 |
| DCMC 2 | 0.3 | 0.32 | 0.01 |
| Wavenumber (cm−1) | Assignment | Reference |
|---|---|---|
| 3300 | -OH stretching | [20,21] |
| 2890 | C-H stretching | [20,21] |
| 1620 | -COO- asymmetric stretching | [21] |
| 1429 | -COO- symmetric stretching | [21] |
| 1100 | -C-O-C | [20,21] |
| 1020 | -C-O-C | [20,21] |
| Sample | Dosage (g/L) | Heat Test (Δabs) | Protein Content (mg/L) | Protein Removal (%) | Results (S/U) 1 |
|---|---|---|---|---|---|
| Control | NA | 0.23 | 97.82 | NA | U |
| DCMC 1 | 0.125 | 0.10 | 27.68 | 72% | U |
| 0.25 | 0.02 | 24.79 | 75% | S | |
| 0.50 | 0.02 | 26.67 | 73% | S | |
| 1.00 | 0.01 | 24.50 | 75% | S | |
| 2.00 | 0.01 | 26.55 | 73% | S | |
| DCMC 2 | 0.125 | 0.16 | 34.93 | 64% | U |
| 0.25 | 0.18 | 38.21 | 61% | U | |
| 0.50 | 0.12 | 41.79 | 57% | U | |
| 1.00 | 0.11 | 42.03 | 57% | U | |
| 2.00 | 0.08 | 33.47 | 66% | U | |
| Bentonite | 0.125 | 0.09 | 28.42 | 71% | U |
| 0.25 | 0.11 | 22.70 | 77% | U | |
| 0.50 | 0.02 | 35.07 | 64% | S | |
| 1.00 | 0.01 | 31.92 | 67% | S | |
| 2.00 | 0.00 | 32.08 | 67% | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, D.; Chagas, R.; Ferreira, L.M. The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine. Beverages 2021, 7, 57. https://doi.org/10.3390/beverages7030057
Gago D, Chagas R, Ferreira LM. The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine. Beverages. 2021; 7(3):57. https://doi.org/10.3390/beverages7030057
Chicago/Turabian StyleGago, Diana, Ricardo Chagas, and Luísa M. Ferreira. 2021. "The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine" Beverages 7, no. 3: 57. https://doi.org/10.3390/beverages7030057
APA StyleGago, D., Chagas, R., & Ferreira, L. M. (2021). The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine. Beverages, 7(3), 57. https://doi.org/10.3390/beverages7030057









