Absorbance Spectroscopy of Heads, Hearts and Tails Fractions in Fruit Spirits
Abstract
1. Introduction
2. Materials and Methods
2.1. Mash Fermentation
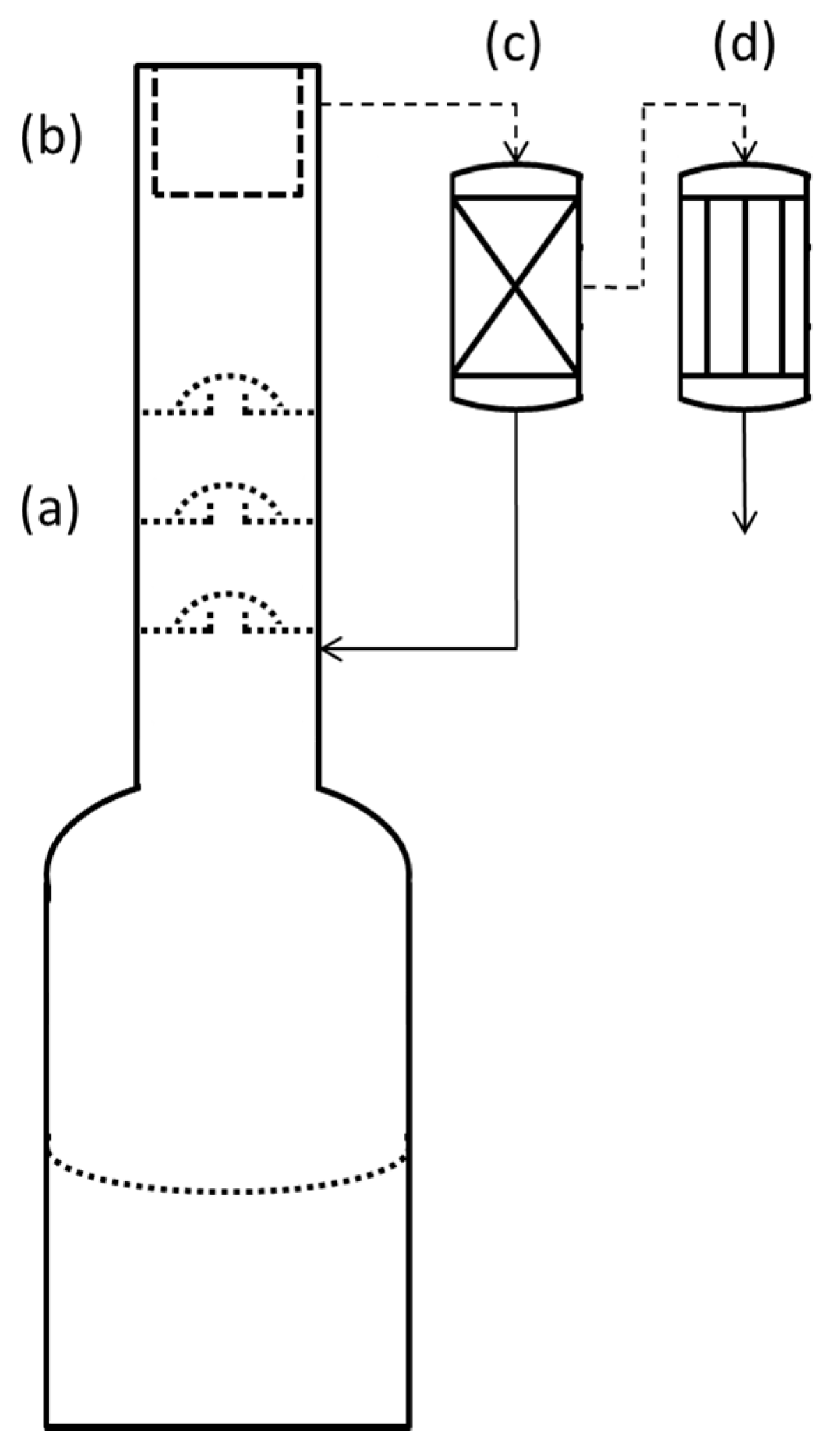
2.2. Distillation
2.3. Volatiles Analysis
2.4. Absorbance Spectroscopy of Distillate Fractions
3. Results and Discussion
3.1. Ethanol Yields
3.2. Volatiles Composition
3.3. Absorbance Spectroscopy
3.4. Subfraction Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Einfalt, D.; Meissner, K.; Kurz, L.; Intani, K.; Müller, J. Fruit Spirit Production from Coffee Cherries—Process Analysis and Sensory Evaluation. Beverages 2020, 6, 57. [Google Scholar] [CrossRef]
- García-Llobodanin, L.; Roca, J.; López, J.R.; Pérez-Correa, J.R.; López, F. The lack of reproducibility of different distillation techniques and its impact on pear spirit composition. Int. J. Food Sci. Technol. 2011, 46, 1956–1963. [Google Scholar] [CrossRef]
- Spaho, N.; Dürr, P.; Grba, S.; Velagić-Habul, E.; Blesić, M. Effects of distillation cut on the distribution of higher alcohols and esters in brandy produced from three plum varieties. J. Inst. Brew. 2013, 119, 48–56. [Google Scholar] [CrossRef]
- Jacques, K.A.; Lyons, T.P.; Kelsall, D.R. The Alcohol Textbook, 4th ed.; Nottingham University Press: Nottingham, UK, 2003. [Google Scholar]
- Spaho, N. Distillation Techniques in the Fruit Spirits Production. In Distillation-Innovative Applications and Modeling; Mendes, M., Ed.; IntechOpen: London, UK, 2017; pp. 129–152. [Google Scholar]
- Doets, E.L.; Kremer, S. The silver sensory experience—A review of senior consumers’ food perception, liking and intake. Food Qual. Prefer. 2016, 48, 316–332. [Google Scholar] [CrossRef]
- Sádecká, J.; Tóthová, J.; Májek, P. Classification of brandies and wine distillates using front face fluorescence spectroscopy. Food Chem. 2009, 117, 491–498. [Google Scholar] [CrossRef]
- Tóthová, J.; Sadecka, J.; Májek, P. Total luminescence spectroscopy for differentiating between brandies and wine distillates. Czech J. Food Sci. 2009, 27, 425–432. [Google Scholar] [CrossRef]
- Tomková, M.; Sádecká, J.; Hroboňová, K. Synchronous Fluorescence Spectroscopy for Rapid Classification of Fruit Spirits. Food Anal. Methods 2014, 8, 1258–1267. [Google Scholar] [CrossRef]
- Sádecká, J.; Uríčková, V.; Hroboňová, K.; Májek, P. Classification of Juniper-Flavoured Spirit Drinks by Multivariate Analysis of Spectroscopic and Chromatographic Data. Food Anal. Methods 2014, 8, 58–69. [Google Scholar] [CrossRef]
- Sádecká, J.; Jakubíková, M.; Májek, P.; Kleinová, A. Classification of plum spirit drinks by synchronous fluorescence spectroscopy. Food Chem. 2016, 196, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Dambergs, R.G.; Kambouris, A.; Francis, I.L.; Gishen, M. Rapid Analysis of Methanol in Grape-Derived Distillation Products Using Near-Infrared Transmission Spectroscopy. J. Agric. Food Chem. 2002, 50, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Anjos, O.; Santos, A.J.; Estevinho, L.M.; Caldeira, I. FTIR–ATR spectroscopy applied to quality control of grape-derived spirits. Food Chem. 2016, 205, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Casolino, M.C.; Hysenaj, V.; Oliveri, P.; Zunin, P. A screening method based on UV–Visible spectroscopy and multivariate analysis to assess addition of filler juices and water to pomegranate juices. Food Chem. 2013, 140, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.R.; Talhavini, M.; Vieira, M.L.; Zacca, J.J.; Braga, J.W.B. Discrimination of whisky brands and counterfeit identification by UV–Vis spectroscopy and multivariate data analysis. Food Chem. 2017, 229, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsuta, M.; Tanaka, F.; Tsukahara, M.; Tsukahara, K. Assessment of Japanese Awamori Spirits Using UV–VIS Spectroscopy. Food Anal. Methods 2020, 13, 726–734. [Google Scholar] [CrossRef]
- German Customs, Abfindungs- und Stoffbesitzerbrennen—Zugelassene Rohstoffe (§§ 9 und 11 Alkoholsteuergesetz) und Festgelegte Amtliche Ausbeutesätze; Generalzolldirektion: Bonn, Germany, 2018.
- Mukarev, M.I.; Walsh, K.B. Prediction of Brix Values of Intact Peaches with Least Squares-Support Vector Machine Regression Models. J. Near Infrared Spectrosc. 2012, 20, 647–655. [Google Scholar] [CrossRef]
- Sahamishirazi, S.; Moehring, J.; Claupein, W.; Graeff-Hoenninger, S. Quality assessment of 178 cultivars of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 2017, 214, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Tewari, J.; Joshi, M.; Gupta, A.; Mehrotra, R.; Chandra, S. Determination of Sugars and Organic Acid Concentration in Apple Juices Using Infrared Spectroscopy. J. Sci. Ind. Res. 1999, 58, 19–24. [Google Scholar]
- Claus, M.J.; Berglund, K.A. Fruit brandy production by batch dolumn distillation with reflux. J. Food Process Eng. 2005, 28, 53–67. [Google Scholar] [CrossRef]
- García-Llobodanin, L.; Senn, T.; Ferrando, M.; Güell, C.; López, F. Influence of the fermentation pH on the final quality of Blanquilla pear spirits. Int. J. Food Sci. Technol. 2010, 45, 839–848. [Google Scholar] [CrossRef]
- Faletar, J.; Blesic, M.; Smajic, M.; Begic-Akagic, A.; Alihodzic, A.; Spaho, N. Dynamics of Evaporation of Certain Volatiles during Plum Brandy Distillation. In Proceedings of the 24th International Scientific-Expert-Conference of Agriculture and Food Industry, Sarajevo, Bosnia and Herzegovina, 25–28 September 2013. [Google Scholar]
- Boscolo, M.; Andrade-Sobrinho, L.G.; Lima-Neto, B.S.; Franco, D.W.; Ferreira, M.M.C. Spectrophotometric determination of caramel content in spirits aged in oak casks. J. Assoc. Off. Anal. Chem. 2002, 85, 744–750. [Google Scholar] [CrossRef]



| Mash No. | Substrate | Extract (°Brix) | Mash Volume (L) |
|---|---|---|---|
| 1 | apple juice | 13.4 | 20 |
| 2 | apple juice | 13.4 | 20 |
| 3 | apple juice | 13.4 | 20 |
| 4 | grape juice | 16.9 | 20 |
| 5 | grape juice | 16.9 | 20 |
| 6 | grape juice | 16.9 | 20 |
| 7 | zwetschge plum | 14.3 | 88 |
| 8 | peach | 11.4 | 90 |
| 9 | mirabelle plum | 19.2 | 92 |
| 10 | pear | 11.9 | 120 |
| 11 | pear | 11.7 | 100 |
| 12 | apple ‘Jona Gold’ | 13.6 | 98 |
| 13 | apple ‘Jona Gold’ | 13.6 | 93 |
| Mash No. | Heads | Hearts | Tails | Total Ethanol Yield Based on 100 L Mash | |||
|---|---|---|---|---|---|---|---|
| Volume | Ethanol | Volume | Ethanol | Volume | Ethanol | ||
| L | % (v/v) aa | L | % (v/v) aa | L | % (v/v) aa | % (v/v) | |
| 1 | 0.30 | 82.0 | 0.95 | 84.8 | 0.55 | 53.3 | 6.7 |
| 2 | 0.35 | 82.7 | 1.05 | 83.3 | 0.38 | 42.3 | 6.6 |
| 3 | 0.28 | 84.1 | 0.93 | 87.3 | 0.70 | 37.8 | 6.5 |
| 4 | 0.25 | 83.1 | 1.40 | 88.8 | 0.50 | 55.4 | 8.6 |
| 5 | 0.25 | 80.9 | 1.40 | 88.4 | 0.40 | 63.2 | 8.5 |
| 6 | 0.20 | 81.9 | 1.40 | 87.2 | 0.55 | 61.7 | 8.6 |
| 7 | 0.39 | 86.0 | 4.00 | 84.6 | 2.50 | 35.2 | 5.2 |
| 8 | 0.65 | 88.7 | 3.00 | 84.4 | 1.30 | 44.6 | 4.1 |
| 9 | 0.55 | 80.9 | 5.10 | 85.6 | 1.80 | 37.1 | 6.0 |
| 10 | 0.99 | 87.3 | 6.00 | 82.9 | 2.50 | 45.0 | 5.8 |
| 11 | 0.50 | 87.1 | 4.50 | 85.3 | 3.20 | 39.0 | 5.5 |
| 12 | 0.40 | 85.9 | 5.10 | 84.0 | 2.60 | 55.0 | 6.2 |
| 13 | 0.40 | 92.6 | 5.70 | 85.4 | 2.50 | 44.3 | 6.9 |
| Substance | Heads | Hearts | Tails |
|---|---|---|---|
| 1-propanol | 0.5 ± 0.1 a | 1.5 ± 0.6 b | 0.7 ± 0.3 ab |
| 2-propanol | <0.1 | <0.1 | <0.1 |
| acetaldehyde | 1.2 ± 0.5 a | 0.1 ± 0.1 b | 0.1 ± 0 b |
| acetone | <0.1 | <0.1 | <0.1 |
| ethyl acetate | 4.7 ± 1.9 a | 0.3 ± 0.1 b | 0.1 ± 0.0 b |
| isobutanol | 0.3 ± 0.2 a | 0.6 ± 0.2 a | 0.5 ± 0.1 a |
| methanol | 8.8 ± 5.7 a | 5.3 ± 3.2 a | 7.3 ± 4.5 a |
| Distillate Fraction | Fraction Label | Area | Zero Point |
|---|---|---|---|
| heads | f − 300 | 20.7 ± 4.9 a | 70.2 ± 32.8 a |
| f − 200 | 13.0 ± 4.1 b | 24.5 ± 18.1 b | |
| f − 100 | 9.9 ± 1.4 b | 11.2 ± 5.5 b | |
| hearts | f0 | 7.9 ± 0.5 b | 5.9 ± 1.3 b |
| f + 100 | 7.3 ± 0.8 b | 5.5 ± 1.4 b | |
| f + 200 | 8.0 ± 0.5 b | 4.6 ± 0.6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohn, J.; Roj, S.; Hoppert, L.; Heller, D.; Einfalt, D. Absorbance Spectroscopy of Heads, Hearts and Tails Fractions in Fruit Spirits. Beverages 2021, 7, 21. https://doi.org/10.3390/beverages7020021
Bohn J, Roj S, Hoppert L, Heller D, Einfalt D. Absorbance Spectroscopy of Heads, Hearts and Tails Fractions in Fruit Spirits. Beverages. 2021; 7(2):21. https://doi.org/10.3390/beverages7020021
Chicago/Turabian StyleBohn, Jens, Simon Roj, Luis Hoppert, Daniel Heller, and Daniel Einfalt. 2021. "Absorbance Spectroscopy of Heads, Hearts and Tails Fractions in Fruit Spirits" Beverages 7, no. 2: 21. https://doi.org/10.3390/beverages7020021
APA StyleBohn, J., Roj, S., Hoppert, L., Heller, D., & Einfalt, D. (2021). Absorbance Spectroscopy of Heads, Hearts and Tails Fractions in Fruit Spirits. Beverages, 7(2), 21. https://doi.org/10.3390/beverages7020021






