Short Wave Ultraviolet Light (UV-C) Effectiveness in the Inactivation of Bacterial Spores Inoculated in Turbid Suspensions and in Cloudy Apple Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Spore Suspensions
2.2. Matrices Preparation
2.3. UV-C Treatments
Reynolds Number Calculation in UV-C Treatments
2.4. Microbiological Analysis
2.5. Physicochemical Analysis
2.5.1. Optical Properties
2.5.2. Browning Index
2.6. Statistical Analysis
2.7. Inactivation Kinetics
3. Results
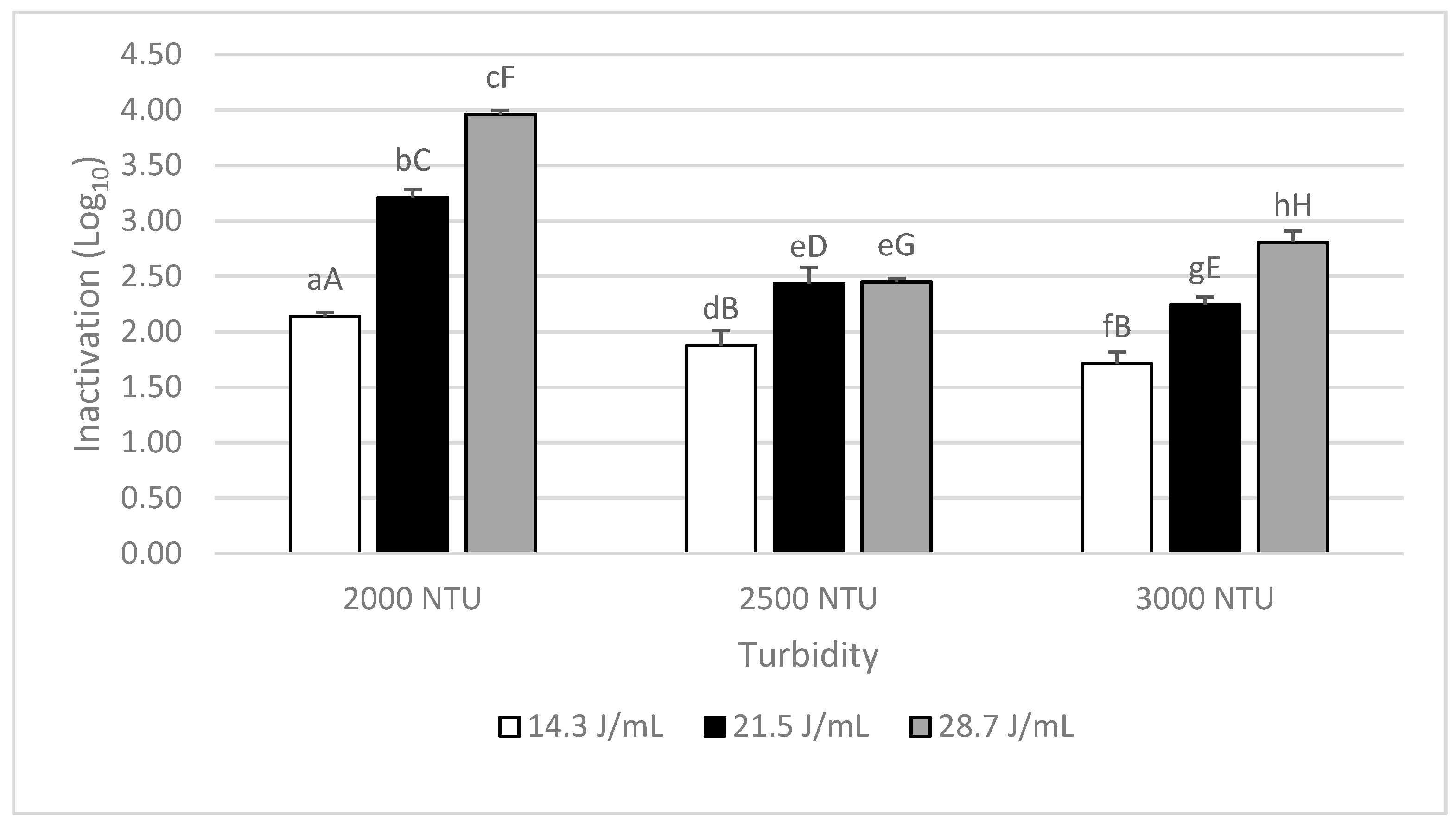
3.1. UV-C Effectiveness in the Different Turbidity Suspensions
3.2. A. acidoterrestris Inactivation in Cloudy Apple Juice with Ascorbic Acid
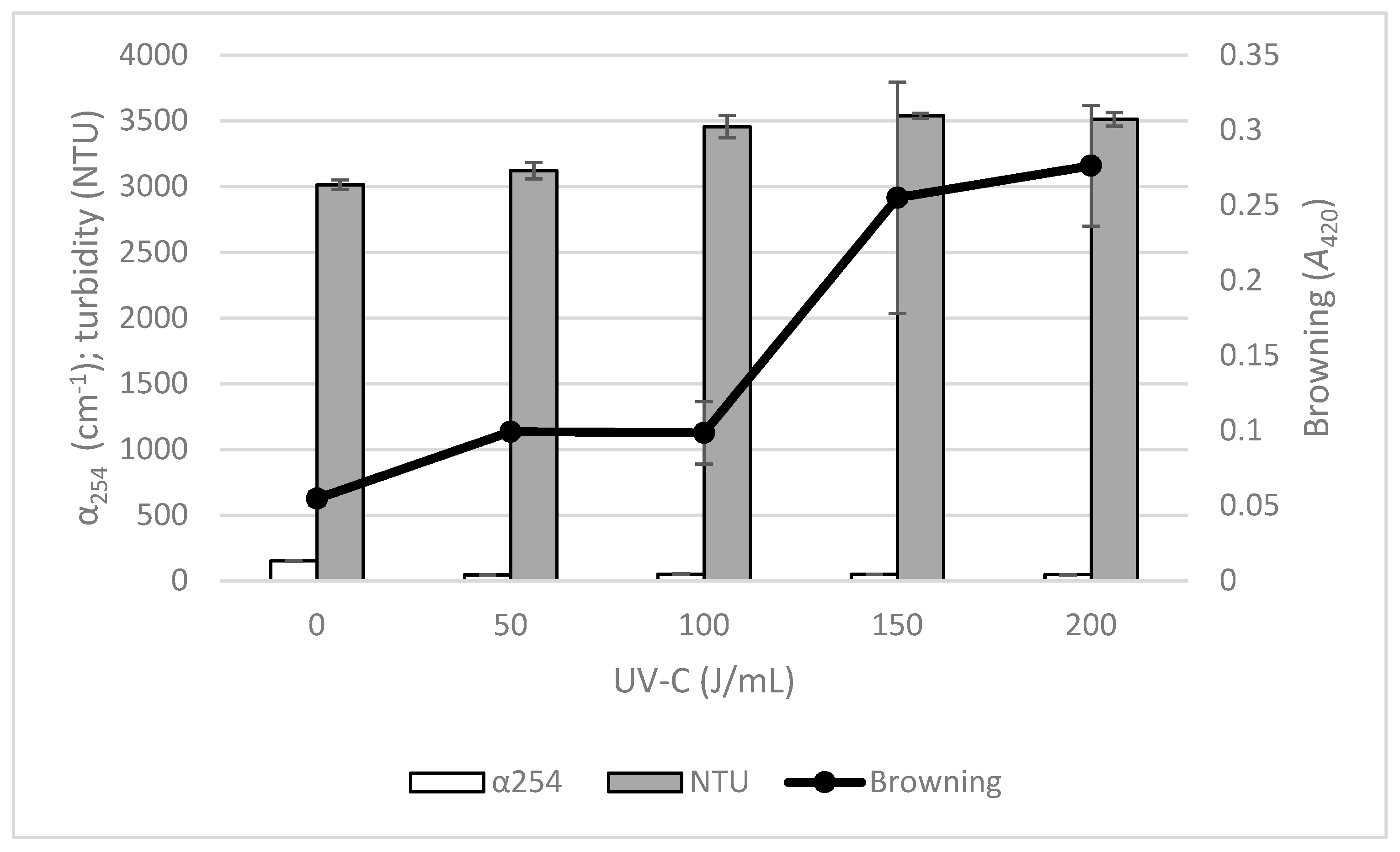
3.3. Physicochemical Changes from the Application of UV-C Treatments with Recirculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Souza, P.M.; Fernández, A. Effects of UV-C on physicochemical quality attributes and Salmonella enteritidis inactivation in liquid egg products. Food Control 2011, 22, 1385–1392. [Google Scholar] [CrossRef]
- Martín, M.F.S.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food Processing by High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef]
- Reverter-Carrión, L.; Sauceda-Gálvez, J.; Codina-Torrella, I.; Hernández-Herrero, M.; Gervilla, R.; Roig-Sagués, A. Inactivation study of Bacillus subtilis, Geobacillus stearothermophilus, Alicyclobacillus acidoterrestris and Aspergillus niger spores under Ultra-High Pressure Homogenization, UV-C light and their combination. Innov. Food Sci. Emerg. Technol. 2018, 48, 258–264. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.; Tió-Coma, M.; Martinez-Garcia, M.; Hernández-Herrero, M.; Gervilla, R.; Roig-Sagués, A. Effect of single and combined UV-C and ultra-high pressure homogenisation treatments on inactivation of Alicyclobacillus acidoterrestris spores in apple juice. Innov. Food Sci. Emerg. Technol. 2020, 60, 102299. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Cibelli, F.; Corbo, M.R.; Sinigaglia, M. Effects of high-pressure homogenization on the survival of Alicyclobacillus acidoterrestris in a laboratory medium. Lett. Appl. Microbiol. 2007, 45, 382–386. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Dougherty, R.H.; Kang, D.-H. Inhibitory Effects of High Pressure and Heat on Alicyclobacillus acidoterrestris Spores in Apple Juice. Appl. Environ. Microbiol. 2002, 68, 4158–4161. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-S.; Kang, D.-H. Alicyclobacillusspp. in the Fruit Juice Industry: History, Characteristics, and Current Isolation/Detection Procedures. Crit. Rev. Microbiol. 2004, 30, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Phillips, C. Alicyclobacillus acidoterrestris: An increasing threat to the fruit juice industry? Int. J. Food Sci. Technol. 2007, 43, 250–260. [Google Scholar] [CrossRef]
- Koutchma, T.; Popović, V.; Ros-Polski, V.; Popielarz, A. Effects of Ultraviolet Light and High-Pressure Processing on Quality and Health-Related Constituents of Fresh Juice Products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 844–867. [Google Scholar] [CrossRef]
- Choudhary, R.; Bandla, S. Ultraviolet Pasteurization for Food Industry. Int. J. Food Sci. Nutr. Eng. 2012, 2, 12–15. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.; Roca-Couso, R.; Martinez-Garcia, M.; Hernández-Herrero, M.; Gervilla, R.; Roig-Sagués, A. Inactivation of ascospores of Talaromyces macrosporus and Neosartorya spinosa by UV-C, UHPH and their combination in clarified apple juice. Food Control. 2019, 98, 120–125. [Google Scholar] [CrossRef]
- Adzahan, N.M.; Lau, P.L.; Hashim, N.; Shamsudin, R.; Sew, C.C.; Sobhi, B. Pineapple juice production using ultraviolet pasteurisation: Potential cost implications. J. Agribus. Mark. 2011, 4, 38–50. [Google Scholar]
- Koutchma, T.; Keller, S.; Chirtel, S.; Parisi, B. Ultraviolet disinfection of juice products in laminar and turbulent flow reactors. Innov. Food Sci. Emerg. Technol. 2004, 5, 179–189. [Google Scholar] [CrossRef]
- Harwood, C.R. Bacillus Subtilis as a Model for Bacterial Systems Biology. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Martinez-Garcia, M.; Sauceda-Gálvez, J.N.; Codina-Torrella, I.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Evaluation of Continuous UVC Treatments and its Combination with UHPH on Spores of Bacillus subtilis in Whole and Skim Milk. Foods 2019, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Gervilla, R.; Salas, F.; Salas, J.; Guamis, A.; Guamis, D.; Moreta, L. System and Method for Sterilizing A Fluid. European Patent Application No. EP-2965766-A1, 11 November 2014. [Google Scholar]
- Rahn, R.O. Potassium Iodide as a Chemical Actinometer for 254 nm Radiation: Use of Iodate as an Electron Scavenger. Photochem. Photobiol. 1997, 66, 885. [Google Scholar] [CrossRef]
- Ozbayoglu, M.E.; Omurlu, C. Analysis of the effect of eccentricity on the flow characteristics of annular flow of non-Newtonian fluids using finite-element method. In Proceedings of the SPE/ICoTA Coiled Tubing and Well Intervention Conference and Exhibition, The Woodlands, TX, USA, 4–5 April 2006; Volume 2006, pp. 293–298. [Google Scholar]
- Chang, S.; Kang, D.-H. Development of novel Alicyclobacillus spp. isolation medium. J. Appl. Microbiol. 2005, 99, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Meydav, S.; Saguy, I.; Kopelman, I.J. Browning determination in citrus products. J. Agric. Food Chem. 1977, 25, 602–604. [Google Scholar] [CrossRef]
- Geeraerd, A.; Herremans, C.; Van Impe, J. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Berg, R.W. Investigation of L(+)—Ascorbic acid with Raman spectroscopy in visible and UV light. Appl. Spectrosc. Rev. 2014, 50, 193–239. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef]
- Falguera, V.; Garvín, A.; Garza, S.; Pagán, J.; Ibarz, A. Effect of UV-Vis Photochemical Processing on Pear Juices from Six Different Varieties. Food Bioprocess Technol. 2014, 7, 84–92. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Anantheswaran, R.C.; Elias, R.J.; Laborde, L.F. Ultraviolet-Induced Oxidation of Ascorbic Acid in a Model Juice System: Identification of Degradation Products. J. Agric. Food Chem. 2011, 59, 8244–8248. [Google Scholar] [CrossRef] [PubMed]
- Kleinwächter, M.; Selmar, D. A novel approach for reliable activity determination of ascorbic acid depending myrosinases. J. Biochem. Biophys. Methods 2004, 59, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.E.M.; Nogueira, J.N. The control of polyphenol oxidase activity in fruits and vegetables. Plant Foods Hum. Nutr. 1995, 47, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, J.A.; Barbosa-Cénovas, G.V. Inactivation of Saccharomyces cerevisiae and polyphenoloxidase in mango nectar treated with UV light. J. Food Prot. 2006, 69, 362–368. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J.; Wojdyło, A. Effect of l-ascorbic acid addition on quality, polyphenolic compounds and antioxidant capacity of cloudy apple juices. Eur. Food Res. Technol. 2013, 236, 777–798. [Google Scholar] [CrossRef]


| UV-C Dose (J/mL) | Treatment Time Per L of Juice | Number of Entrances |
|---|---|---|
| 25 | 14′52″ | 60 |
| 50 | 29′43″ | 120 |
| 75 | 44′35″ | 180 |
| 100 | 59′26″ | 240 |
| 125 | 74′18″ | 300 |
| 150 | 89′10″ | 360 |
| 200 | 118′53″ | 480 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauceda-Gálvez, J.N.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Short Wave Ultraviolet Light (UV-C) Effectiveness in the Inactivation of Bacterial Spores Inoculated in Turbid Suspensions and in Cloudy Apple Juice. Beverages 2021, 7, 11. https://doi.org/10.3390/beverages7010011
Sauceda-Gálvez JN, Martinez-Garcia M, Hernández-Herrero MM, Gervilla R, Roig-Sagués AX. Short Wave Ultraviolet Light (UV-C) Effectiveness in the Inactivation of Bacterial Spores Inoculated in Turbid Suspensions and in Cloudy Apple Juice. Beverages. 2021; 7(1):11. https://doi.org/10.3390/beverages7010011
Chicago/Turabian StyleSauceda-Gálvez, Jezer N., María Martinez-Garcia, Ma Manuela Hernández-Herrero, Ramón Gervilla, and Artur X. Roig-Sagués. 2021. "Short Wave Ultraviolet Light (UV-C) Effectiveness in the Inactivation of Bacterial Spores Inoculated in Turbid Suspensions and in Cloudy Apple Juice" Beverages 7, no. 1: 11. https://doi.org/10.3390/beverages7010011
APA StyleSauceda-Gálvez, J. N., Martinez-Garcia, M., Hernández-Herrero, M. M., Gervilla, R., & Roig-Sagués, A. X. (2021). Short Wave Ultraviolet Light (UV-C) Effectiveness in the Inactivation of Bacterial Spores Inoculated in Turbid Suspensions and in Cloudy Apple Juice. Beverages, 7(1), 11. https://doi.org/10.3390/beverages7010011







