Mycotoxins in Functional Beverages: A Review
Abstract
:1. Introduction
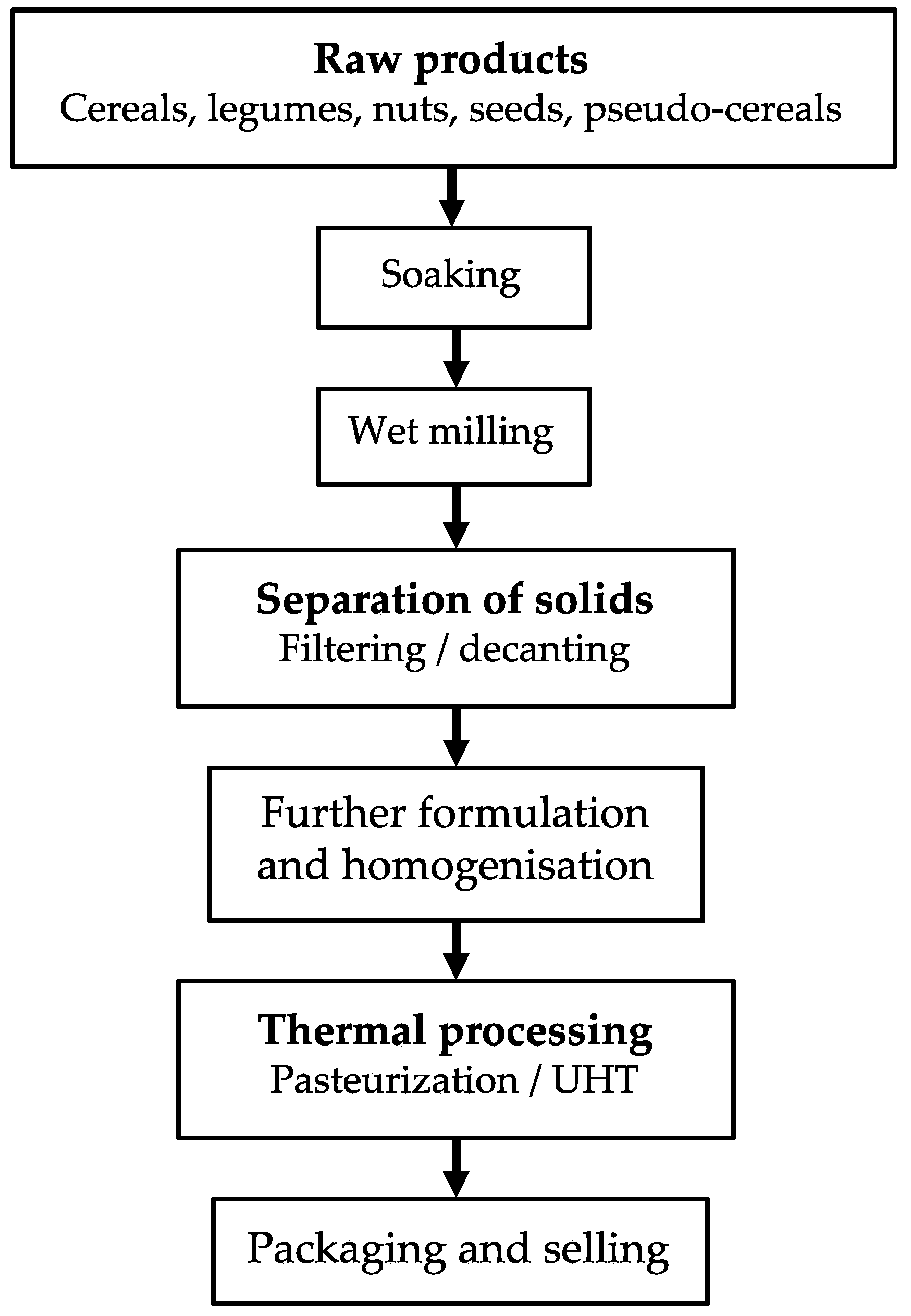
2. Plant-Based Milks
3. Fruit Juices
4. Herbal Teas
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods. Commercial trends, research, and health implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D. Functional beverages. In Beverages: Processing and Technology; Mudgil, D., Barak, S., Eds.; Scientific Publishers: Rajasthan, India, 2018; pp. 292–302. [Google Scholar]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Mycotoxins–Toxicology. In Reference Module in Food Science; Academic Press: Cambridge, MA, USA, 2019; pp. 1–7. [Google Scholar]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity and analysis of major mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Chang, A.S.; Sreedharab, A.; Schneider, K.R. Peanut and peanut products: A food safety perspective. Food Control 2013, 32, 296–303. [Google Scholar] [CrossRef]
- Egbuta, M.A.; Mwanza, M.; Phoku, J.Z.; Chilaka, C.A.; Dutton, M.F. Comparative analysis of mycotoxigenic fungi and mycotoxins contaminating soya bean seeds and processed soya bean from Nigerian Markets. Adv. Microbiol. 2016, 6, 1130–1139. [Google Scholar] [CrossRef] [Green Version]
- Gelderblom, C.A.; Shephard, G.S.; Rheeder, J.P.; Sathe, S.K. Edible nuts, oilseeds and legumes. In Food Safety Management. A Practical Guide for the Food Industry; Motarjemi, Y., Lelieveld, H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 301–324. [Google Scholar]
- Bhat, R.; Reddy, K.R.N. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from the last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef]
- Ogungbemile, O.A.; Etaware, P.M.; Odebode, A.C. Aflatoxin detection and quantification is stores cowpea seeds in Ibadan, Nigeria. J. Biomed. Biotechnol. 2020, 3, 10–18. [Google Scholar] [CrossRef]
- Marín, S.; Ramos, A.J. Molds and mycotoxins in nuts. In Food Hygiene and Toxicology in Ready-to Eat Foods; Kotzekidou, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 295–312. [Google Scholar]
- Fernández-Cruz, M.L.; Mansilla, M.L.; Tadeo, J.L. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J. Adv. Res. 2010, 1, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, J.; Jiang, Y.; Duan, X.; Qu, H.; Yang, B.; Chen, F.; Sivakumar, D. Natural occurrence, analysis, and prevention of mycotoxins in fruits and their processed products. Crit. Rev. Food Sci. Nutr. 2014, 54, 64–83. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Coppa, C.F.; de Neeff, D.V.; Corassin, C.H.; Fernandes-Oliveira, C.A. Mycotoxins in fruits and fruit-based products: Occurrence and methods for decontamination. Toxin Rev. 2019, 38, 263–272. [Google Scholar] [CrossRef]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Mycotoxins in medicinal/aromatic herbs—A review. Bol. Latinoam. Caribe Plantas Med. Aromát. 2013, 12, 119–142. [Google Scholar]
- Ashiq, S.; Hussain, M.; Ahmad, B. Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet. Biol. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sedova, I.; Kiseleva, M.; Tutelvan, V. Mycotoxins in tea: Occurrence, methods of determination and risk evaluation. Toxins 2018, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Humana Press: Totowa, NJ, USA, 2017; pp. 51–106. [Google Scholar]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Baker, R.C.; Ford, R.M.; Helander, M.E.; Marecki, J.; Natarajan, R.; Ray, B. Framework for managing mycotoxin risks in the food industry. J. Food Prot. 2014, 177, 2181–2188. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Ezequiel, C.N.; Ayeni, K.I.; Misihairabgwi, J.M.; Somorin, Y.M.; Chibuzor-Onyema, I.E.; Oyedele, O.A.; Abia, W.A.; Sulyok, M.; Shepard, G.S.; Krska, R. Traditionally processed beverages in Africa: A review of the mycotoxin occurrence patterns and exposure assessment. Compr. Rev. Food Sci. Food Saf. 2018, 17, 334–351. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef] [PubMed]
- McClemens, D.J.; Newman, E.; McClemens, I.F. Plant-based milks: A review of the science underpinning their design, fabrication, and performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Kabak, B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Gbashi, S.; Madal, N.E.; De Saeger, S.; De Boevre, M.; Njobeh, P.B. Numerical optimization of temperature-time degradation of multiple mycotoxins. Food Chem. Toxicol. 2019, 125, 289–304. [Google Scholar] [CrossRef]
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERs and liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 229, 336–372. [Google Scholar] [CrossRef]
- Hamed, A.M.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Determination of Fusarium toxins in functional vegetable milks applying salting-out-assisted liquid–liquid extraction combined with ultra-high-performance liquid chromatography tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 2033–2041. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Hamed, A.M.; García-Campaña, A.M.; Gámiz-Gracia, L. Plant-based milks: Unexplored source of emerging mycotoxins. A proposal for the control of enniatins and beauvericin using UHPLC-MS/MS. Food Addit. Contam. Part B 2019, 12, 296–302. [Google Scholar] [CrossRef]
- Hamed, A.M.; Abdel-Hamid, M.; Gámiz-Gracia, L.; García-Campaña, A.M.; Arroyo-Manzanares, N. Determination of aflatoxins in plant-based milk and dairy products by dispersive liquid–liquid microextraction and high- performance liquid chromatography with fluorescence detection. Anal. Lett. 2019, 52, 363–372. [Google Scholar] [CrossRef]
- El-Badry, S. Determination of ochratoxin A residues in some animal and plant milk products. Zagazig Vet. J. 2016, 44, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Roselló-Soto, E.; García, C.; Fessard, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F. Nutritional and microbiological quality of tiger nut tubers (Cyperus esculentus) derived plant-based and lactic fermented beverages. Fermentation 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Sebastia, N.; Soler, C.; Soriano, J.M.; Mañes, J. Occurrence of aflatoxins in tigernuts and their beverages commercialized in Spain. J. Agric. Food Chem. 2010, 58, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Sebastia, N.; Soriano, J.M.; Soler, C.; Mañes, J. One-year monitoring of aflatoxins and ochratoxin A in tiger-nuts and their beverages. Food Chem. 2011, 127, 822–826. [Google Scholar] [CrossRef]
- Yang, Y.; Guoliang, L.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Mandappa, I.M.; Basavaraj, K.; Manonmani, H.K. Analysis of mycotoxins in fruit juices. In Fruit Juices. Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 763–777. [Google Scholar]
- Pallarés, N.; Carballo, D.; Ferrer, E.; Fernández-Franzón, M.; Berrada, H. Mycotoxin dietary exposure assessment through fruit juices consumption in children and adult population. Toxins 2019, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Mehmood, S.; Yuan, Y.; Yue, T. Mycotoxin patulin in food matrices: Occurrence and its biological degradation strategies. Drug Metab. Rev. 2019, 51, 105–120. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- European Commission. Regulation N° 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 50, 8–12. [Google Scholar]
- Ministerio da Saúde do Brasil. RDC N° 7 de 18 de fevereiro de 2011–Dispoe sobre limites máximos tolerados (LMT) para micotoxinas en alimentos. Diário Oficial da União 2011, 37, 66–67. [Google Scholar]
- China Food and Drug Administration (CFDA). National Food Safety Standard for Maximum Levels of Mycotoxins in Foods (GB 2761-2011). Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Maximum%20Levels%20of%20Mycotoxins%20in%20Foods_Beijing_China%20-%20Peoples%20Republic%20of_12-29-2014.pdf (accessed on 9 June 2020).
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An overview. J. Food Qual. 2017, 2017, 347526. [Google Scholar] [CrossRef] [Green Version]
- Arcella, D.; Eskola, M.; Gómez-Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control. 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Hussain, S.; Asi, M.R.; Iqbal, M.; Khalid, N.; Wajih-ul-Hassan, S.; Ariño, A. Patulin mycotoxin in mango and orange fruits, juices, pulps, and jams marketed in Pakistan. Toxins 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Palumbo, R.; Guimaraes, A.; Gkrillas, A.; Dall’Asta, C.; Dorne, J.L.; Battilani, P.; Venancio, A. The route of mycotoxins in the grape food chain. Am. J. Enol. Vitic. 2020, 71, 89–104. [Google Scholar] [CrossRef]
- Logrieco, A.; Ferracane, R.; Cozzi, G.; Haidukowsky, M.; Susca, A.; Mulè, G.; Ritieni, A. Fumonisin B2 by Aspergillus niger in the grape-wine chain: An additional potential mycotoxicological risk. Ann. Microbiol. 2011, 61, 1–3. [Google Scholar] [CrossRef]
- Juan, C.; Mañes, J.; Font, G.; Juan-García, A. Determination of mycotoxins in fruit berry by-products using QuEChERS extraction method. LWT 2017, 86, 344–351. [Google Scholar] [CrossRef]
- Carballo, D.; Pinheiro-Fernandes, P.; Tolosa, J.; Font, G.; Berrada, H.; Ferrer, E. Dietary exposure to mycotoxins through fruits juice consumption. Rev. Toxicol. 2018, 35, 2–6. [Google Scholar]
- Abdallah, M.F.; Audenaert, K.; Lust, L.; Landschooot, S.; Bekaert, B.; Haesaert, G.; De Boevre, M.; De Saeger, S. Risk characterization and quantification of mycotoxins and their producing fungi in sugarcane juice: A neglected problem in a widely-consumed traditional beverage. Food Control 2020, 108, 106811. [Google Scholar] [CrossRef]
- Diao, E.; Hou, H.; Hu, W.; Dong, H.; Li, X. Removing and detoxifying methods of patulin: A review. Trends Food Sci. Technol. 2018, 81, 139–145. [Google Scholar] [CrossRef]
- Cheng, W.; Li, C.; Zhang, B.; Zhou, Z.; Shen, Y.; Liao, X.; Yang, J.; Wang, Y.; Li, X.; Li, Y.; et al. Advances in biodetoxification of ochratoxin A—A review of the past five decades. Front. Microbiol. 2018, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marceddu, S.; Jaoua, S.; Migheli, Q. Adsorption of ochratoxin A from grape juice by yeast cells immobilised in calcium alginate beads. Int. J. Food Microbiol. 2016, 217, 29–34. [Google Scholar] [CrossRef]
- Poswal, F.S.; Rusell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal teas and their health benefits: A scoping review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shadidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Duarte, S.C.; Salvador, N.; Machado, F.; Costa, E.; Almeida, A.; Silva, L.J.G.; Pereira, A.M.P.T.; Lino, C.; Pena, A. Mycotoxins in teas and medicinal plants destined to prepare infusions in Portugal. Food Control. 2020, 115, 107290. [Google Scholar] [CrossRef]
- Mannani, N.; Tabarani, A.; Abdennegi, E.H.; Zinedine, A. Assessment of aflatoxins levels in herbal green tea available on the Moroccan market. Food Control 2020, 108, 106882. [Google Scholar] [CrossRef]
- Pouretedal, Z.; Mazaheri, M. Aflatoxins in black tea in Iran. Food Addit. Contam. Part B 2013, 6, 127–129. [Google Scholar] [CrossRef]
- Ismail, A.; Akhtar, S.; Riaz, M.; Gong, Y.Y.; Routledge, M.N.; Naeem, I. Prevalence and exposure assessment of aflatoxins through black tea consumption in the Multan City of Pakistan and the impact of tea making process on aflatoxins. Front. Microbiol. 2020, 11, 446. [Google Scholar] [CrossRef]
- Tosun, H.; Ergönul, P.G.; Üçok, E.F. Occurrence of aflatoxins (B1, B2, G1, G2) in herbal teas consumed in Turkey. J. Consum. Prot. Food Saf. 2016, 11, 265–269. [Google Scholar] [CrossRef]
- Viswanath, P.; Nanjegowda, D.K.; Govindegowda, H.; Dattatreya, A.M.; Siddappa, V. Aflatoxin determination in black tea (Camellia sinensis)–status and development of a protocol. J. Food Saf. 2012, 32, 13–21. [Google Scholar] [CrossRef]
- Martins, M.L.; Martins, H.M.; Bernardo, F. Fumonisin B1 and B2 in black tea and medicinal plants. J. Food Prot. 2001, 64, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Reinholds, I.; Bogdanova, E.; Pugajeva, I.; Bartkevics, V. Mycotoxins in herbal teas marketed in Latvia and dietary exposure assessment. Food Addit. Contam. Part B 2019, 12, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, N.; Font, G.; Mañes, J.; Ferrer, E. Multimycotoxin LC-MS/MS analysis in tea beverages after dispersive liquid-liquid microextraction (DLLME). J. Agric. Food Chem. 2017, 65, 10282–10289. [Google Scholar] [CrossRef]
- Pallarés, N.; Tolosa, J.; Mañes, J.; Ferrer, E. Occurrence of mycotoxins in botanical dietary supplement beverages. J. Nat. Prod. 2019, 82, 403–406. [Google Scholar] [CrossRef]
- Toman, J.; Malir, F.; Ostry, V.; Kilic, M.A.; Roubal, T.; Grosse, Y.; Pfohl-Leszkowicz, A. Transfer of ochratoxin A from raw black tea to tea infusions prepared according to the Turkish tradition. J. Sci. Food Agric. 2017, 98, 261–265. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Toman, J.; Bazin, I.; Roubal, T. Transfer of ochratoxin A into tea and coffee beverages. Toxins 2014, 6, 3438–3453. [Google Scholar] [CrossRef]
- Monbaliu, S.; Wu, A.; Zhang, D.; Van Peteghem, C.; De Saeger, S. Multimycotoxin UPLC-MS/MS for tea, herbal infusions and the derived drinkable products. J. Agric. Food Chem. 2010, 58, 12664–12671. [Google Scholar] [CrossRef]
- Pallarés, N.; Berrada, H.; Fernández-Franzón, M.; Ferrer, E. Risk Assessment and Mitigation of the Mycotoxin Content in Medicinal Plants by the Infusion Process. Plant Foods Hum. Nutr. 2020, 75. [Google Scholar] [CrossRef]
- Iha, M.H.; Trucksess, M.W. Aflatoxins and ochratoxin A in tea prepared from naturally contaminated powdered ginger. Food Addit. Contam. 2010, 27, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
| Mycotoxins | Fungal Species | Product |
|---|---|---|
| Aflatoxins (B1, B2, G1, G2) | A. flavus A. parasiticus | Cereals, nuts, legumes, herbs |
| Ochratoxin A | A. carbonarius A. niger A. welwitschiae A. westerdijkiae A. steynii | Cereals, fruits, legumes, herbs |
| Fumonisins (B1, B2) | F. verticillioides F. proliferatum | Cereal, legumes, herbs |
| Trichothecenes (DON, T-2, HT-2) | F. graminearum, F. culmorum F. sporotrichioides F. langsethiae F. equiseti | Cereals |
| Zearalenone | F. graminearum F. culmorum | Cereals, herbs |
| Patulin | P. expansum | Fruits |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Serna, J.; Vázquez, C.; Patiño, B. Mycotoxins in Functional Beverages: A Review. Beverages 2020, 6, 52. https://doi.org/10.3390/beverages6030052
Gil-Serna J, Vázquez C, Patiño B. Mycotoxins in Functional Beverages: A Review. Beverages. 2020; 6(3):52. https://doi.org/10.3390/beverages6030052
Chicago/Turabian StyleGil-Serna, Jéssica, Covadonga Vázquez, and Belén Patiño. 2020. "Mycotoxins in Functional Beverages: A Review" Beverages 6, no. 3: 52. https://doi.org/10.3390/beverages6030052
APA StyleGil-Serna, J., Vázquez, C., & Patiño, B. (2020). Mycotoxins in Functional Beverages: A Review. Beverages, 6(3), 52. https://doi.org/10.3390/beverages6030052







