The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage
Abstract
1. Introduction
2. Materials and Methods
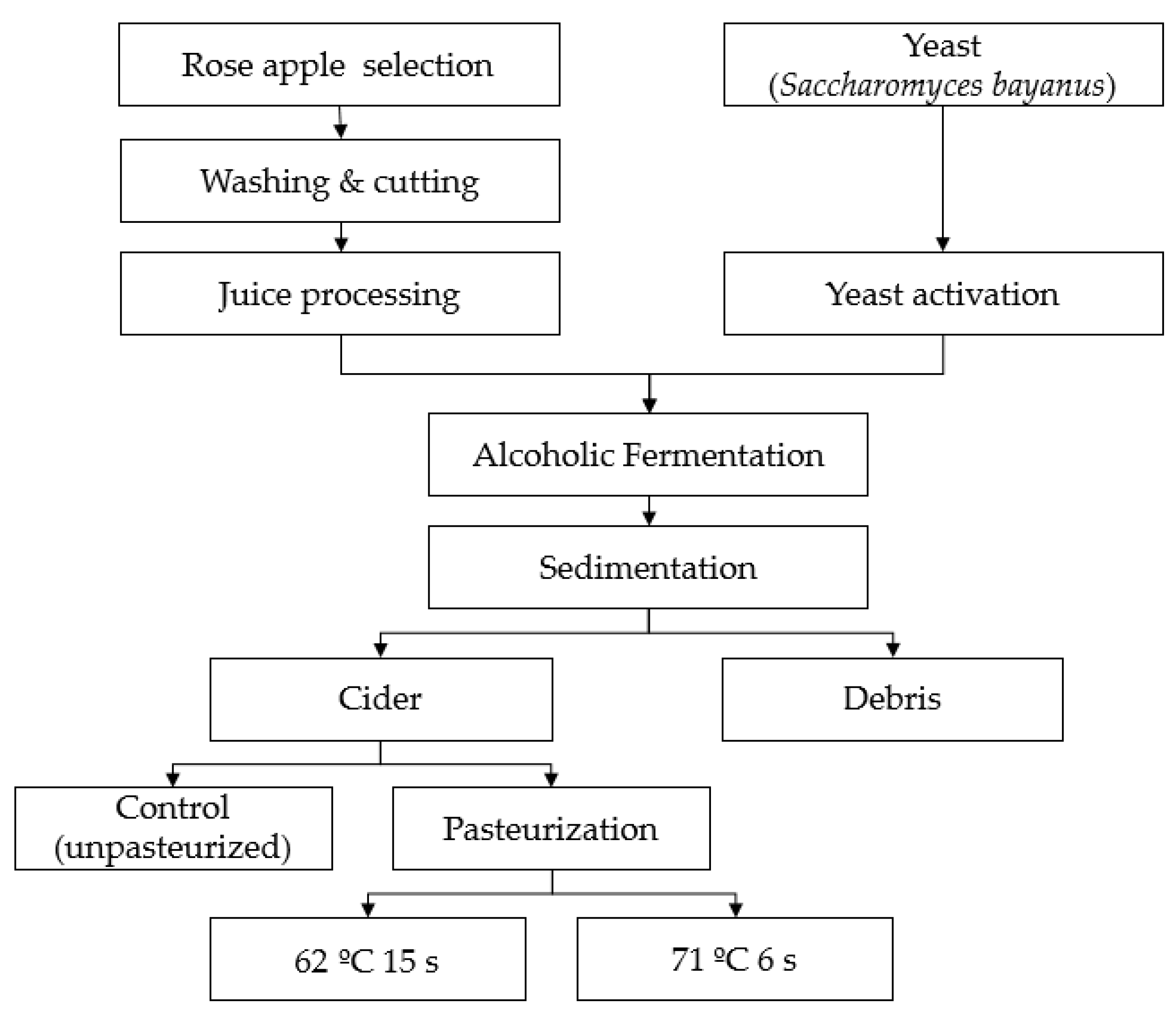
2.1. Cider Preparation and Pasteurization Process
2.2. Physicochemical Analysis
2.3. Microbiological Analysis
2.4. Vitamin C Content
2.5. Total Phenols
2.6. Antioxidant Capacity
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS•+ Radical Scavenging Activity
2.6.3. Hydroxyl Radical Scavenging Ability
2.6.4. Ferric Reducing Antioxidant Power (FRAP)
2.7. Modified Spectrum Descriptive Analysis (SDA)
2.8. Statistical Analysis
3. Results and Discussion
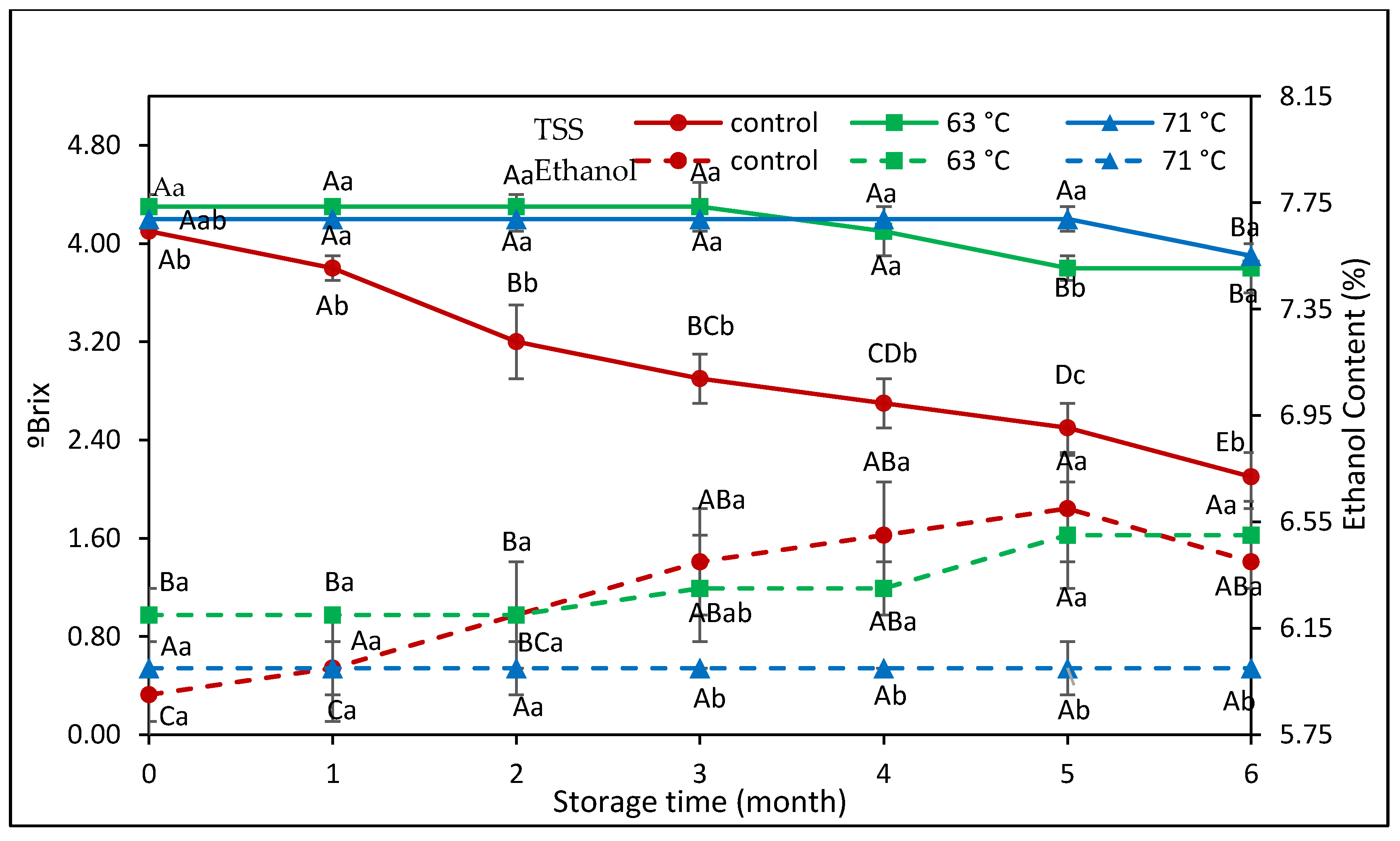
3.1. Physicochemical Properties of Rose Apple Cider
3.2. Microbial Evaluation
3.3. Vitamin C Contents of Cider Samples
3.4. Phenolic Content and Antioxidant Activity
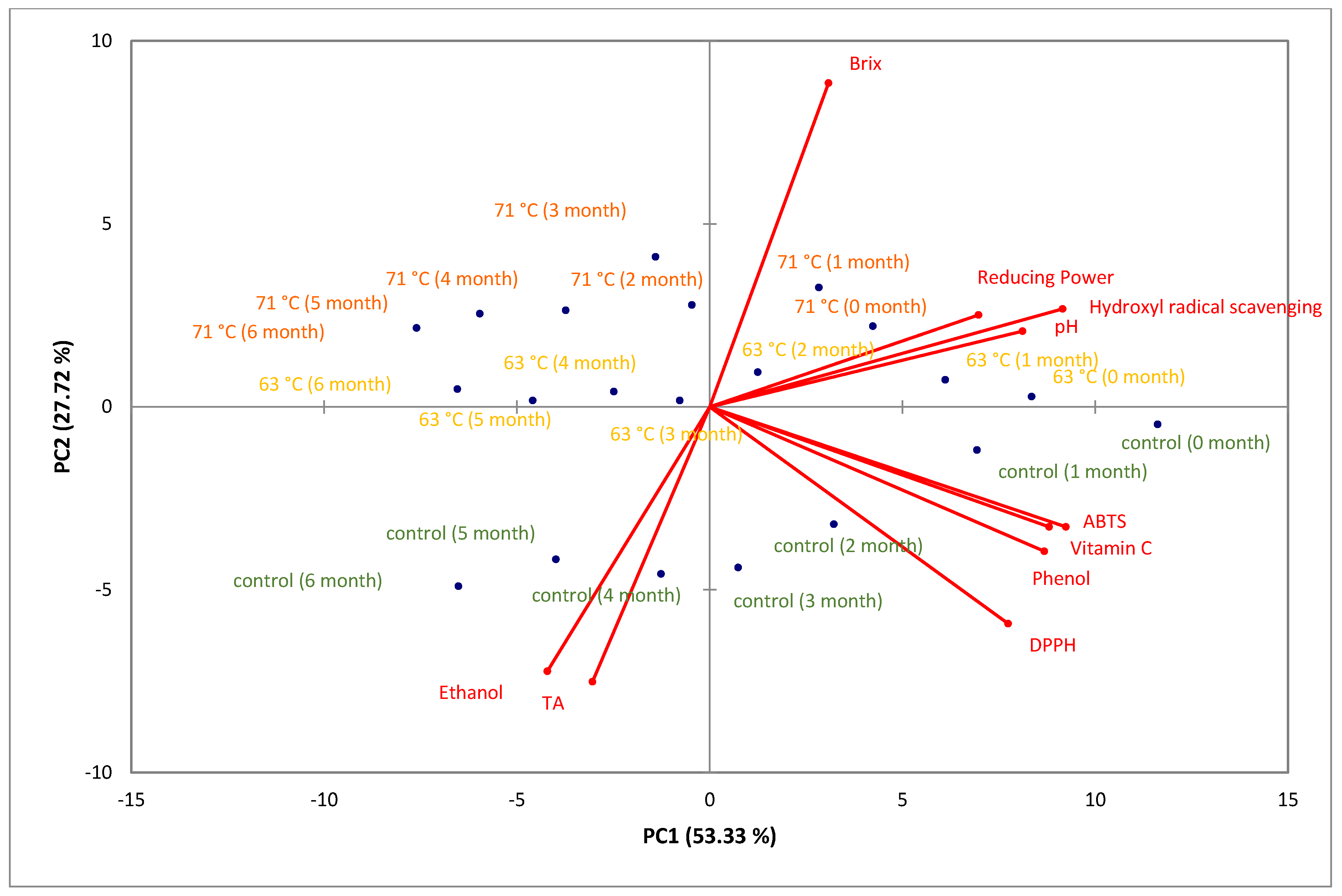
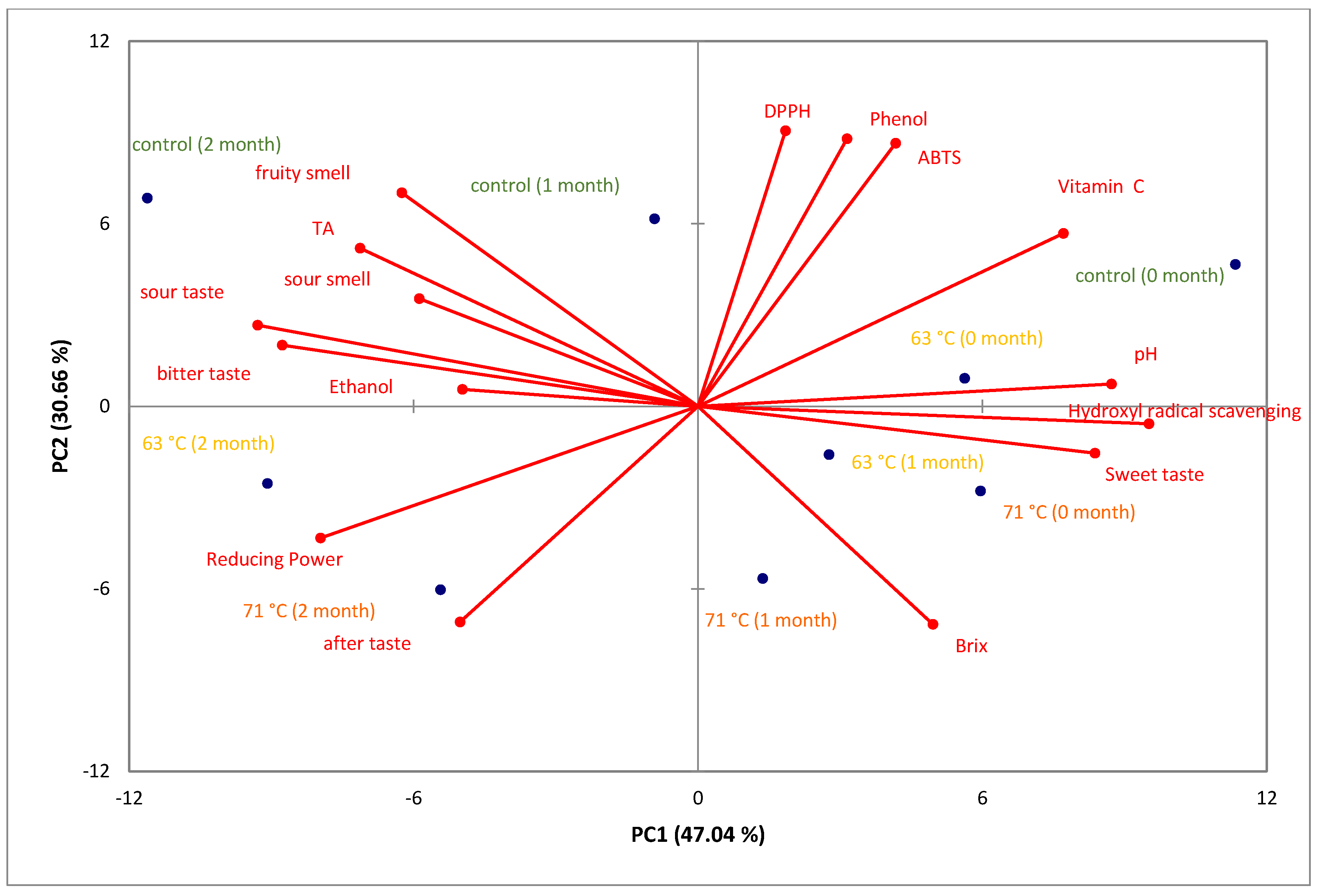
3.5. Principal Component Analysis (PCA)
3.5.1. PCA of Physicochemical Properties and Antioxidant Activity of Cider Samples during Storage
3.5.2. PCA of Physicochemical Properties, Antioxidant Activity, and Sensory Profiles of Cider Samples
3.6. Sensory Profile of Rose Apple Cider during Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shü, Z.H.; Tirtawinata, R.; Meon, Z.; Thanarut, C. Wax apple production in selected tropical Asian countries. Acta Hortic. 2008, 773, 161–164. [Google Scholar]
- Supapvanich, S.; Mitrsang, P.; Srinorkham, P.; Boonyaritthongchai, P.; Wongs-Aree, C. Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. J. Food Sci. Technol. 2016, 53, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Department of Agriculture Extension-Report Product Statistic. Available online: https://production.doae.go.th/service/report-product-statistic/index (accessed on 13 December 2019).
- Huang, C.C.; Wang, D.N.; Liou, D.T. Reduction of prevention of chilling injury by pruning and covering treatments on wax apple. Crop Environ. Bioinform. 2005, 2, 73–80. [Google Scholar]
- Shü, Z.H.; Shiesh, C.C.; Lin, H.L. Wax apple (Syzygium samarangense (Blume) Merr. and L.M. Perry) and related species. In Postharvest Biology and Technology of Tropical and Subtropical Fruits, 1st ed.; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 4, pp. 458–473. [Google Scholar]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Jarvis, B. Cider: Cyder and Hard Cider. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; Volume 1, pp. 437–443. [Google Scholar]
- Johnston, C.S. Medicinal Uses of Vinegar. In Complementary and Alternative Therapies and the Aging Population, 1st ed.; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 433–440. [Google Scholar]
- Venkatachalam, K.; Techakanon, C.; Thitithanakul, S. Impact of the ripening stage of wax apples on chemical profiles of juice and cider. ACS Omega 2018, 3, 6710–6718. [Google Scholar] [CrossRef]
- Lea, A.G.H. Bitterness and astringency: The procyanidins of fermented apple ciders. In Bitterness in Foods and Beverages; Russell, L.R., Ed.; Elsevier Science Publishers: New York, NY, USA, 1990; pp. 123–143. [Google Scholar]
- Besser, R.E.; Lett, S.M.; Weber, J.T.; Doyle, M.P.; Barrett, T.J.; Wells, J.G.; Griffin, P.M. An outbreak of diarrhea and hemolytic uremic Syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 1993, 269, 2217–2220. [Google Scholar] [CrossRef]
- Miller, L.G.; Kaspar, C.W. Escherichia coli O157: H7 acid tolerance and survival in apple cider. J. Food Prot. 1994, 57, 460–464. [Google Scholar] [CrossRef]
- Senkel, I.A., Jr.; Henderson, R.A.; Jolbitado, B.; Meng, J. Use of hazard analysis critical control point and alternative treatments in the production of apple cider. J. Food Prot. 1999, 62, 778–785. [Google Scholar] [CrossRef]
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Herbert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 398, 681–685. [Google Scholar] [CrossRef]
- US.FDA. Hazard analysis and critical control point (HACCP), Procedures for the safe and sanitary processing and importing of juice. Fed. Regist. 2001, 66, 6137–6202. [Google Scholar]
- Linton, M.; Mcclements, M.J.; Patterson, M.F. Inactivation of Escherichia coli O157:H7 in orange juice using a combination of high pressure and mild heat. J. Food Prot. 1999, 62, 277–279. [Google Scholar] [CrossRef]
- Koutchma, T.; Popović, V.; Ros-Polski, V.; Popielarz, A. Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Compr. Rev. Food Sci. Food 2016, 15, 844–867. [Google Scholar] [CrossRef]
- Tandon, K.; Worobo, R.W.; Churey, J.J.; Padilla-Zakour, O.I. Storage quality of pasteurized and UV treated apple cider. J. Food Process. Preserv. 2003, 27, 21–35. [Google Scholar] [CrossRef]
- Donahue, D.W.; Canitez, N.; Bushway, A.A. UV inactivation of E. coli O157:H7 in apple cider: Quality, sensory and shelf life analysis. J. Food Process. Preserv. 2004, 28, 368–387. [Google Scholar] [CrossRef]
- Baumann, A.R.; Martin, S.E.; Feng, H. Ultrasound treatment of Listeria monocytogenes in apple cider. J. Food Prot. 2005, 68, 2333–2340. [Google Scholar] [CrossRef]
- Ugarte-Romero, E.; Feng, H.; Martin, S.E.; Cadwallader, K.; Robinson, S.J. Inactivation of Escherichia coli with power ultrasound in apple cider. J. Food Sci. 2016, 71, 102–108. [Google Scholar] [CrossRef]
- D’amico, D.J.; Silk, T.M.; Wu, J.; Guo, M. Inactivation of microorganisms in milk and apple cider treated with Ultrasound. J. Food Prot. 2006, 69, 556–563. [Google Scholar] [CrossRef]
- Iu, J.; Mittal, G.S.; Griffiths, M.W. Reduction in levels of Escherichia coli O157:H7 in apple cider by pulsed electric fields. J. Food Prot. 2001, 64, 964–969. [Google Scholar] [CrossRef]
- Turk, M.F.; Vorobiev, E.; Baron, E. Improving apple juice expression and quality by pulsed electric field on an industrial scale. LWT Food Sci. Technol. 2012, 49, 245–250. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Noci, F.; Muñoz, A.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 2011, 124, 1387–1392. [Google Scholar] [CrossRef]
- Splittstoesser, D.F.; Mclellan, M.R. Destruction of Escherichia coli 0157:H7 in cider Topic. In Proceedings of the Kansas Apple Cider Seminar, Topeka, KS, USA, 10 January 1997. [Google Scholar]
- Splittstoesser, D.F.; Mclellan, M.R.; Churey, J.J. Heat resistance of Escherichia coli 0157:H7 in apple juice. J. Food Prot. 1995, 59, 226–229. [Google Scholar] [CrossRef] [PubMed]
- AFDO. Apple Cider Processing Operations Requirements and Guidelines; Association of Food and Drug Officials: York, UK, 2003. [Google Scholar]
- Alberti, A.; Machadodos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT-Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Babashahi-Kouhanestani, M.; Salehi, M.; Mazloomi, S.M.; Almasi-Hashyani, A. Quantitative evaluation of vitamin C in industrial lemon juice by titration method. J. Biol. Today’s World 2014, 3, 139–141. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Delgado, T.; Malheiro, R.; Pereira, J.A.; Ramalhosa, E. Hazelnut (Corylus avellana L.) kernels as a source of antioxidants and their potential in relation to other nuts. Ind. Crop. Prod. 2010, 32, 621–626. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Wanapu, C.; Boonkerd, N.; Dithavibool, L. Winemaker 1; Sombun Printing House: Bangkok, Thailand, 2004. [Google Scholar]
- Chanthai, S.; Danvirutai, P. Chemical analysis of locally produced wines. Part I. Acidity and organic compounds. J. Acad. Serv. Cent. Khon Kaen Univ. 2004, 12, 23–29. [Google Scholar]
- Dennis, C. Breakdown of cellulose by yeast species. Microbiology 1972, 71, 409–411. [Google Scholar] [CrossRef]
- Akin, H.; Brandam, C.; Meyer, X.M.; Strehaiano, P.A. model for pH determination during alcoholic fermentation of a grape must by Saccharomyces cerevisiae. Chem. Eng. Process Process Intensif. 2008, 47, 1986–1993. [Google Scholar] [CrossRef]
- Won, J.I.; Yang, Y.L.; Kim, B.G.; Choi, C.Y. Adaptive control of specific growth rate based on proton production in anaerobic fed-batch culture. Biotechnol. Lett. 1993, 15, 511–516. [Google Scholar] [CrossRef]
- Sigler, K.; Knotková, A.; Kotyk, A. Factors governing substrate-induced generation and extrusion of protons in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA)-Biomembr. 1981, 643, 572–582. [Google Scholar] [CrossRef]
- Castrillo, J.I.; De Miguel, I.; Ugalde, U.O. Proton production and consumption pathways in yeast metabolism. A chemostat culture analysis. Yeast 1995, 11, 1353–1365. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Jindal, V.K. Variation in electrical conductivity of selected fruit juices during continuous Ohmic heating. KMUTNB Int. J. Appl. Sci. Technol. 2014, 7, 47. [Google Scholar] [CrossRef][Green Version]
- Carstensen, E.L.; Cox, H.A.; Mercer, W.B.; Natale, L.A. Passive electrical properties of microorganisms. Biophys. J. 1965, 5, 289–300. [Google Scholar] [CrossRef]
- Sosa-Hernández, O.; Popat, S.C.; Parameswaran, P.; Alemán-Nava, G.S.; Torres, C.I.; Buitrón, G.; Parra-Saldívar, R. Application of microbial electrolysis cells to treat spent yeast from an alcoholic fermentation. Bioresour. Technol. 2016, 200, 342–349. [Google Scholar] [CrossRef]
- Bayly, F.C.; Berg, H.W. Grape and wine proteins of white wine varietals. Am. J. Enol. Vitic. 1967, 18, 18–32. [Google Scholar]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Fermentativa, I.D.A.N.A.; Vinho, D. The impact of nitrogen on yeast fermentation and wine quality. Ciência Técnica Vitiv 2011, 26, 17–32. [Google Scholar]
- Walton, E.F.; Pringle, J.R. Effect of growth temperature upon heat sensitivity in Saccharomyces cerevisiae. Arch. Microbiol. 1980, 124, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Laslo, V.; Socaci, S.; Teusdea, A.; Timar, A.; Tofana, M.; Ioana-Vicas, S. The effect of pasteurization time on phytochemical composition and antioxidant capacity of two apple cultivars juices. Bull. UASVM Food Sci. Technol. 2018, 75, 67–77. [Google Scholar] [CrossRef]
- Sadecka, J.; Polovka, M.; Kolek, E.; Belajova, E.; Tobolkova, B.; Daško, L.; Durec, J. Orange juice with pulp: Impact of pasteurization and storage on flavour, polyphenols, ascorbic acid and antioxidant activity. J. Food Nutr. Res. 2014, 53, 371–388. [Google Scholar]
- Narwojsz, A.; Borowska, E.J. Cranberry and strawberry juices-influence of method production on antioxidants content and antioxidative capacity. Pol. J. Nat. Sci. 2010, 25, 209–214. [Google Scholar] [CrossRef][Green Version]
- Pérez-Conesa, D.; García-Alonso, J.; García-Valverde, V.; Iniesta, M.D.; Jacob, K.; Sánchez-Siles, L.M.; Periago, M.J. Changes in bioactive compounds and antioxidant activity during homogenization and thermal processing of tomato puree. Innov. Food Sci. Emerg. Technol. 2009, 10, 179–188. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. J. Agric. Food Chem. 2000, 48, 4597–4605. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín Belloso, O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, J.J., I; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Jirumarn, J.; Srihanam, P. Oxidants and Antioxidants: Sources and Mechanism. Acadamic J. Karasin Rajabhat Univ. 2011, 1, 59–70. [Google Scholar]
- Rabie, M.A.; Soliman, A.Z.; Diaconeasa, Z.S.; Constantin, B. Effect of pasteurization and shelf life on the physicochemical properties of physalis (Physalis peruviana L.) Juice. J. Food Process. Preserv. 2015, 39, 1051–1060. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci. Hum. Wellness 2014, 3, 16–25. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Wu, J.C.; Pan, M.H. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef]
- Lu, A.T.; Whitaker, J.R. Some factors affecting rates of heat inactivation and reactivation of horseradish peroxidase. J. Food Sci. 1974, 39, 1173–1178. [Google Scholar] [CrossRef]
- Clemente, E.; Robinson, D.S. The thermostability of purified oranges isoperoxidases. Arq. Biol. Tecnol. 1995, 38, 109–118. [Google Scholar]

| Condition | Storage Time (Month) | ||||||
|---|---|---|---|---|---|---|---|
| Conductivity (µS/cm) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| control | 2921 ± 16 E,a | 2952 ± 1 D,a | 2977 ± 2 C,a | 2986 ± 3 BC,b | 2987 ± 1 BC,b | 2991 ± 1 B,b | 3104 ± 1 A,a |
| 63 °C | 2757 ± 2 E,c | 2893 ± 0 D,c | 2911 ± 1 C,a | 2911 ± 3 C,c | 2931 ± 4 B,c | 2933 ± 0 B,c | 2976 ± 1 A,c |
| 71 °C | 2799 ± 27 C,b | 2949 ± 1 B,b | 2989 ± 18 AB,a | 3000 ± 1 A,a | 3000 ± 1 A,a | 3001 ± 1 A,a | 3008 ± 0 A,b |
| Total nitrogen (%) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| control | 0.063 ± 0.001 A,a | 0.063 ± 0.001 A,a | 0.064 ± 0.001 A,a | 0.064 ± 0.001 A,a | 0.056 ± 0.002 B,a | 0.050 ± 0.007 C,a | 0.044 ± 0.001 D,a |
| 63 °C | 0.063 ± 0.001 A,a | 0.062 ± 0.001 A,a | 0.063 ± 0.001 A,a | 0.063 ± 0.001 A,a | 0.056 ± 0.002 B,a | 0.049 ± 0.001 C,a | 0.038 ± 0.003 D,b |
| 71 °C | 0.055 ± 0.002 A,b | 0.054 ± 0.001 AB,b | 0.048 ± 0.003 C,b | 0.046 ± 0.001 C,b | 0.049 ± 0.007 BC,a | 0.044 ± 0.001 C,a | 0.036 ± 0.001 D,b |
| Density (g/cm3) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| control | 1000 ± 0.001 A,a | 0.998 ± 0.000 B,b | 0.997 ± 0.001 BC,b | 0.997 ± 0.001 BC,b | 0.997 ± 0.001 C,b | 0.996 ± 0.001 C,b | 0.996 ± 0.001 C,b |
| 63 °C | 0.999 ± 0.001 A,a | 0.998 ± 0.001 B,b | 0.998 ± 0.001 BC,b | 0.997 ± 0.001 C,b | 0.997 ± 0.001 C,b | 0.997 ± 0.000 C,ab | 0.997 ± 0.000 C,ab |
| 71 °C | 1000 ± 0.001 AB,a | 1000 ± 0.000 A,a | 1000 ± 0.001 AB,a | 0.999 ± 0.001 B,a | 0.999 ± 0.001 B,a | 0.999 ± 0.001 B,a | 0.999 ± 0.001 B,a |
| Storage Time (Months) | Total Plate Count (log10 CFU/mL) | Yeast/Mold (log10 CFU/mL) | E. Coli (log10 CFU/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 63 °C | 71 °C | Control | 63 °C | 71 °C | Control | 63 °C | 71 °C | |
| (4 °C) | (4 °C) | (4 °C) | (4 °C) | (4 °C) | (4 °C) | (4 °C) | (4 °C) | (4 °C) | |
| 0 | 1.03 | ND | ND | 2.00 | ND | ND | ND | ND | ND |
| 1 | 3.62 | 2.26 | ND | 3.62 | 2.26 | ND | ND | ND | ND |
| 2 | 3.99 | 2.98 | 1.49 | 3.99 | 2.98 | ND | ND | ND | ND |
| 3 | 4.53 | 3.91 | 2.66 | 4.52 | 3.91 | ND | ND | ND | ND |
| 4 | 4.89 | 4.15 | 3.34 | 4.88 | 4.15 | ND | ND | ND | ND |
| 5 | 4.94 | 4.27 | 3.77 | 4.94 | 4.26 | ND | ND | ND | ND |
| 6 | 5.07 | 4.39 | 3.84 | 5.04 | 4.38 | ND | ND | ND | ND |
| Condition | Storage Time (Month) | ||||||
|---|---|---|---|---|---|---|---|
| Vitamin C (mg/100 mL) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control | 5.87 ± 0.29 A,a | 4.58 ± 0.00 B,b | 4.29 ± 0.01 C,a | 4.12 ± 0.01 CD,a | 4.00 ± 0.06 DE,a | 3.91 ± 0.05 DE,a | 3.78 ± 0.09 E,a |
| 63 °C | 5.22 ± 0.73 A,ab | 4.93 ± 0.00 A,a | 3.21 ± 0.00 B,c | 3.16 ± 0.01 B,b | 3.03 ± 0.06 B,b | 2.96 ± 0.02 B,b | 2.85 ± 0.00 B,b |
| 71 °C | 4.52 ± 0.17 A,b | 3.80 ± 0.00 B,c | 3.39 ± 0.00 C,b | 3.12 ± 0.04 D,b | 2.93 ± 0.04 E,b | 2.80 ± 0.06 EF,c | 2.66 ± 0.02 F,c |
| Total phenols (mg/L) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control | 87.15 ± 0.01 A,a | 79.11 ± 0.78 B,a | 77.89 ± 0.18 C,a | 76.27 ± 0.35 D,a | 70.57 ± 0.02 E,a | 61.56 ± 1.15 F,a | 60.03 ± 0.33 G,a |
| 63 °C | 78.33 ± 1.98 A,b | 73.31 ± 0.55 B,b | 69.57 ± 0.47 C,b | 67.91 ± 0.69 D,b | 66.57 ± 0.47 D,b | 62.19 ± 0.12 E,a | 59.32 ± 0.84 F,a |
| 71 °C | 65.15 ± 1.39 A,c | 63.32 ± 0.71 B,c | 61.46 ± 0.02 C,c | 59.52 ± 0.01 D,c | 58.23 ± 0.19 D,c | 55.58 ± 0.71 E,b | 55.48 ± 1.28 E,b |
| Reducing power (mmol Fe2+/100 mL) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control | 30.90 ± 0.26 B,b | 31.03 ± 0.03 B,c | 31.69 ± 0.03 A,ab | 31.06 ± 0.03 B,b | 30.49 ± 0.81 B,ab | 29.61 ± 0.02 C,a | 29.17± 0.18 C,a |
| 63 °C | 31.41 ± 0.05 B,a | 31.39 ± 0.03 B,b | 32.02 ± 0.29 A,a | 31.02 ± 0.29 C,b | 29.72 ± 0.12 D,b | 29.21 ± 0.15 E,b | 29.11 ± 0.26 E,a |
| 71 °C | 31.15 ± 0.06 B,ab | 31.56 ± 0.06 A,a | 31.67 ± 0.00 A,b | 31.57 ± 0.09 A,a | 30.81 ± 0.29 C,a | 29.69 ± 0.14 D,a | 29.45 ± 0.22 D,a |
| DPPH scavenging activity (%) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control | 83.29 ± 0.74 A,a | 80.49 ± 0.07 B,a | 78.95 ± 0.03 C,a | 75.28 ± 0.37 D,a | 73.53 ± 0.07 E,a | 68.82 ± 0.45 F,a | 63.96 ± 0.18 G,a |
| 63 °C | 77.31 ± 0.00 A,b | 74.30 ± 0.74 B,b | 71.06 ± 0.03 C,b | 67.13 ± 0.34 D,b | 64.46 ± 0.56 E,b | 61.70 ± 0.12 F,b | 57.31 ± 0.00 G,b |
| 71 °C | 63.82 ± 0.02 A,c | 61.51 ± 0.39 B,c | 56.95 ± 0.06 C,c | 55.16 ± 0.01 D,c | 52.75 ± 0.09 E,c | 50.66 ± 1.14 F,c | 48.82 ± 0.02 G,c |
| ABTS scavenging activity (%) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control | 69.87 ± 0.31 A,a | 67.36 ± 0.54 B,a | 65.85 ± 0.67 C,a | 64.09 ± 0.12 D,a | 63.02 ± 0.86 E,a | 61.98 ± 0.13 F,a | 60.38 ± 0.70 G,a |
| 63 °C | 66.09 ± 0.13 A,b | 64.71 ± 0.35 B,b | 63.20 ± 0.35 C,b | 62.47 ± 0.40 D,b | 61.83 ± 0.46 E,a | 61.02 ± 0.51 F,b | 59.48 ± 0.11 G,a |
| 71 °C | 63.10 ± 0.99 A,c | 62.02 ± 0.27 AB,c | 61.64 ± 0.48 BC,c | 60.38 ± 0.70 CD,c | 59.58 ± 0.99 DE,b | 58.90 ± 0.27 EF,c | 57.68 ± 0.89 F,b |
| Hydroxyl radical scavenging (%) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control | 66.69 ± 0.63 A,a | 59.43 ± 1.15 B,b | 55.02 ± 0.51 C,b | 53.76 ± 0.77 C,b | 52.21 ± 0.58 Da | 49.59 ± 0.83 E,ab | 44.37 ± 0.35 F,a |
| 63 °C | 66.69 ± 1.43 A,a | 60.54 ± 0.95 B,ab | 56.13 ± 0.88 C,b | 55.04 ± 0.58 C,b | 53.24 ± 0.95 D,a | 50.18 ± 0.22 E,a | 44.35 ± 0.87 F,a |
| 71 °C | 65.51 ± 0.73 A,a | 61.70 ± 0.17 B,a | 58.94 ± 0.85 C,a | 56.76 ± 0.70 D,a | 53.57 ± 0.47 E,a | 48.93 ± 0.24 F,b | 43.51 ± 0.73 G,a |
| Attribute | Storage (Month) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||
| Sweet taste | Control | 3.80 ± 0.41 A,a | 3.20 ± 0.41 B,b | 3.07 ± 0.26 B,b | ND | ND | ND | ND |
| 63 °C | 3.87 ± 0.35 A,a | 3.60 ± 0.51 A,a | 3.20 ± 0.41 B,b | 3.00 ± 0.00 B | ND | ND | ND | |
| 71 °C | 3.80 ± 0.51 A,a | 3.80 ± 0.41 A,a | 3.67 ± 0.49 A,a | 3.67 ± 0.49 A | 3.60 ± 0.51 A | 3.47 ± 0.52 A | 3.47 ± 0.52 A | |
| Bitter taste | Control | 2.07 ± 0.46 B,b | 3.47 ± 0.52 A,a | 3.60 ± 0.51 A,a | ND | ND | ND | ND |
| 63 °C | 2.87 ± 0.35 B,a | 2.87 ± 0.35 B,b | 3.47 ± 0.52 A,a | 3.67 ± 0.49 A | ND | ND | ND | |
| 71 °C | 2.60 ± 0.26 C,a | 2.87 ± 0.35 BC,b | 2.93 ± 0.26 B,b | 3.47 ± 0.52 A | 3.67 ± 0.49 A | 3.80 ± 0.41 A | 3.80 ± 0.41 A | |
| Sour taste | Control | 2.07 ± 0.26 C,a | 2.80 ± 0.41 B,a | 3.27 ± 0.46 A,a | ND | ND | ND | ND |
| 63 °C | 2.07 ± 0.26 C,a | 2.80 ± 0.41 B,a | 2.87 ± 0.35 B,b | 3.20 ± 0.41 A | ND | ND | ND | |
| 71 °C | 1.93 ± 0.41 B,a | 2.07 ± 0.26 AB,b | 2.20 ± 0.41 AB,c | 2.27 ± 0.46 A | 2.20 ± 0.41 AB | 2.27 ± 0.46 A | 2.20 ± 0.41 AB | |
| Sour smell | Control | 2.67 ± 0.49 B,a | 3.47 ± 0.52 A,a | 3.40 ± 0.51 A,a | ND | ND | ND | ND |
| 63 °C | 1.80 ± 0.41 C,b | 2.87 ± 0.35 B,b | 3.13 ± 0.35 B,a | 3.47 ± 0.52 A | ND | ND | ND | |
| 71 °C | 1.80 ± 0.41 C,b | 2.07 ± 0.26 BC,c | 2.20 ± 0.41 AB,b | 2.40 ± 0.51 AB | 2.20 ± 0.41 AB | 2.47 ± 0.52 A | 2.33 ± 0.49 AB | |
| Fruity smell | Control | 1.93 ± 0.46 B,a | 2.73 ± 0.46 A,a | 2.80 ± 0.41 A,a | ND | ND | ND | ND |
| 63 °C | 1.60 ± 0.51 C,a | 2.07 ± 0.26 B,b | 2.27 ± 0.46 B,b | 2.60 ± 0.51 A | ND | ND | ND | |
| 71 °C | 1.80 ± 0.41 C,a | 1.93 ± 0.46 BC,b | 2.00 ± 0.38 ABC,b | 2.27 ± 0.46 A | 2.13 ± 0.35 AB | 1.80 ± 0.41 C | 1.93 ± 0.26 BC | |
| Aftertaste | Control | 1.73 ± 0.46 B,a | 1.87 ± 0.35 AB,b | 2.07 ± 0.26 A,b | ND | ND | ND | ND |
| 63 °C | 1.87 ± 0.35 B,a | 1.87 ± 0.35 B,b | 2.07 ± 0.26 AB,b | 2.27 ± 0.46 A | ND | ND | ND | |
| 71 °C | 1.80 ± 0.41 D,a | 2.60 ± 0.51 C,a | 2.87 ± 0.35 BC,a | 3.13 ± 0.35 AB | 3.27 ± 0.46 A | 3.20 ± 0.41 A | 3.27 ± 0.46 A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Techakanon, C.; Sirimuangmoon, C. The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage. Beverages 2020, 6, 43. https://doi.org/10.3390/beverages6030043
Techakanon C, Sirimuangmoon C. The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage. Beverages. 2020; 6(3):43. https://doi.org/10.3390/beverages6030043
Chicago/Turabian StyleTechakanon, Chukwan, and Chirat Sirimuangmoon. 2020. "The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage" Beverages 6, no. 3: 43. https://doi.org/10.3390/beverages6030043
APA StyleTechakanon, C., & Sirimuangmoon, C. (2020). The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage. Beverages, 6(3), 43. https://doi.org/10.3390/beverages6030043





