Discrimination of Juice Press Fractions for Sparkling Base Wines by a UV-Vis Spectral Phenolic Fingerprint and Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Scale Trial
2.1.1. Hydroxycinnamate Standards
2.1.2. Grape Juice Press Fractioning
2.2. Commercial Scale Trial
2.3. UV-Vis Spectcroscopy
2.4. Chemometrics
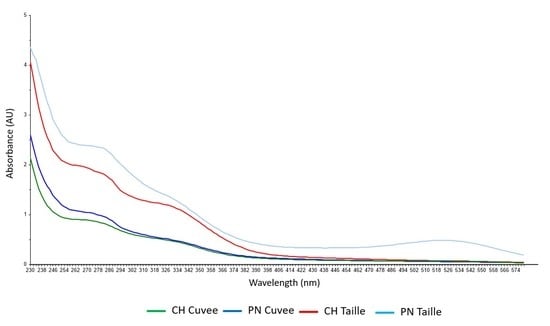
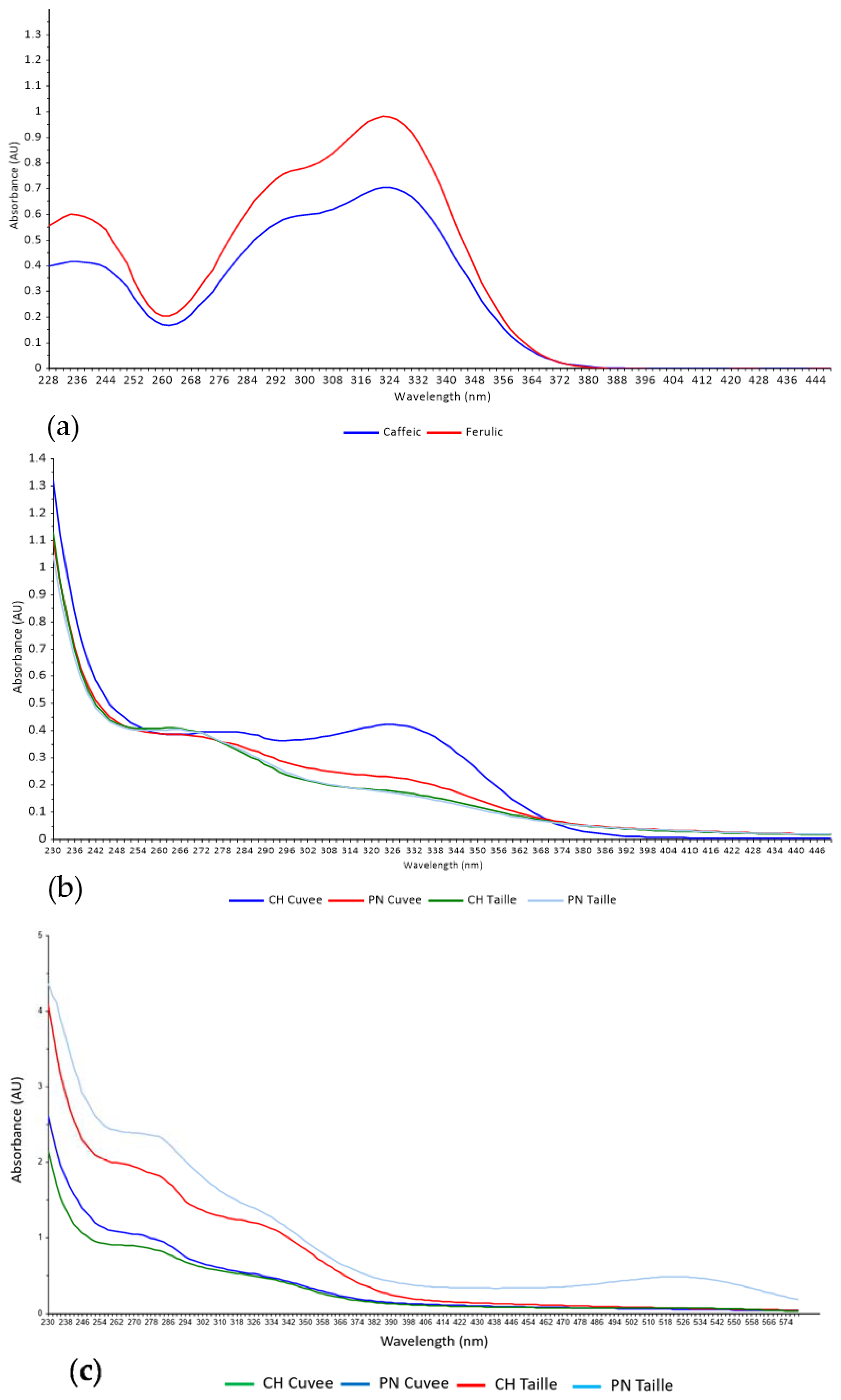
3. Results and Discussion
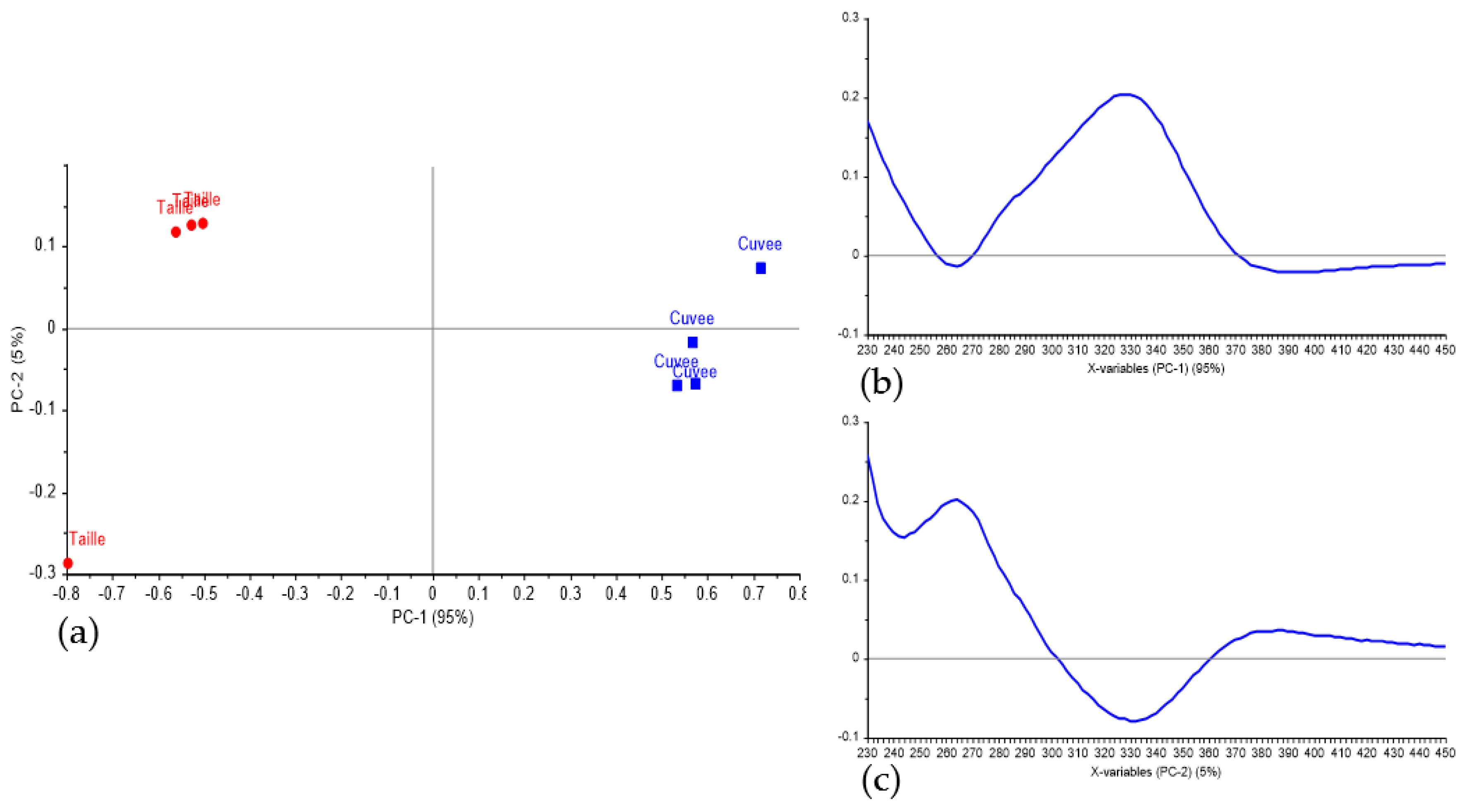
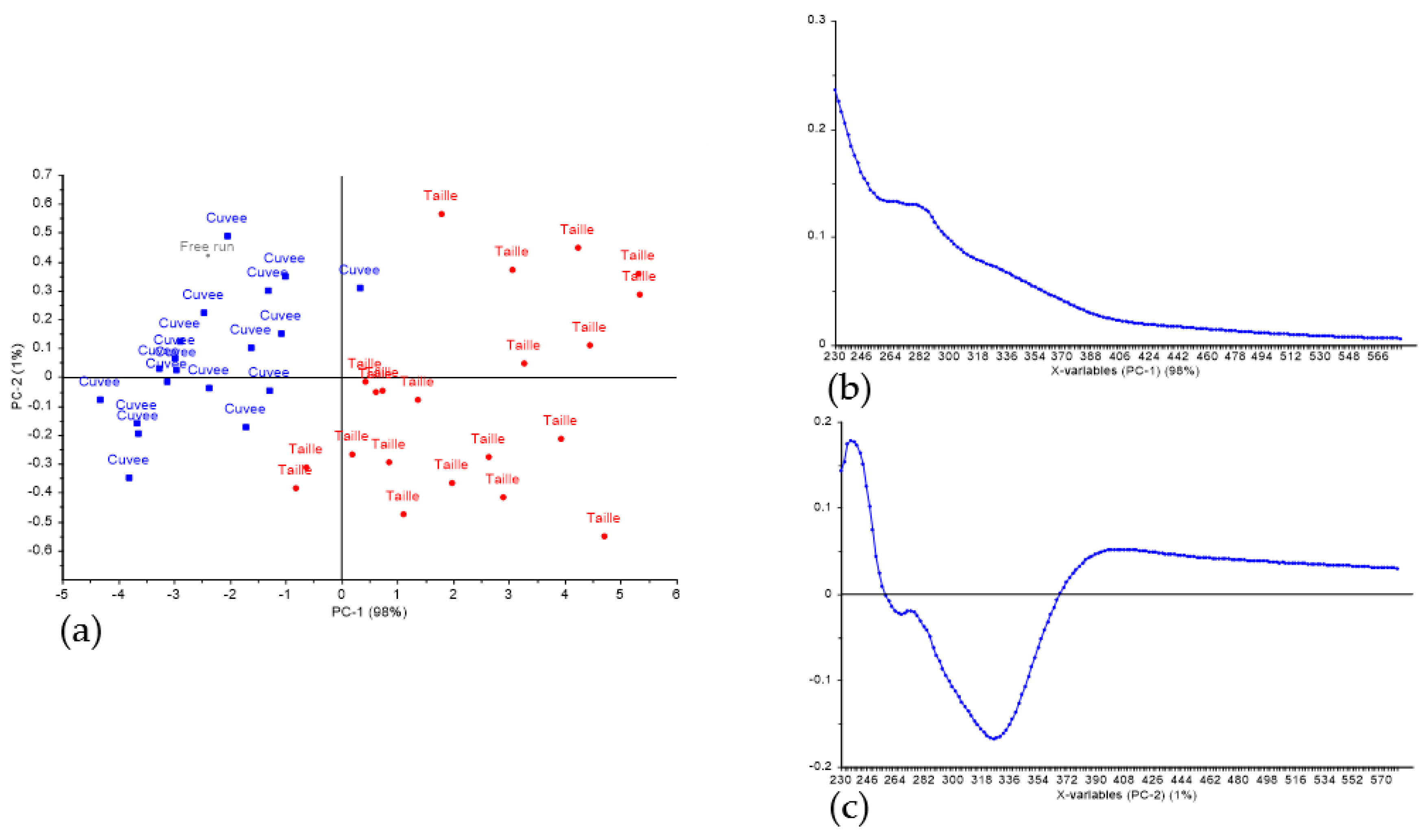
3.1. Discrimination of Press Fractions in the Laboratory Scale Trial
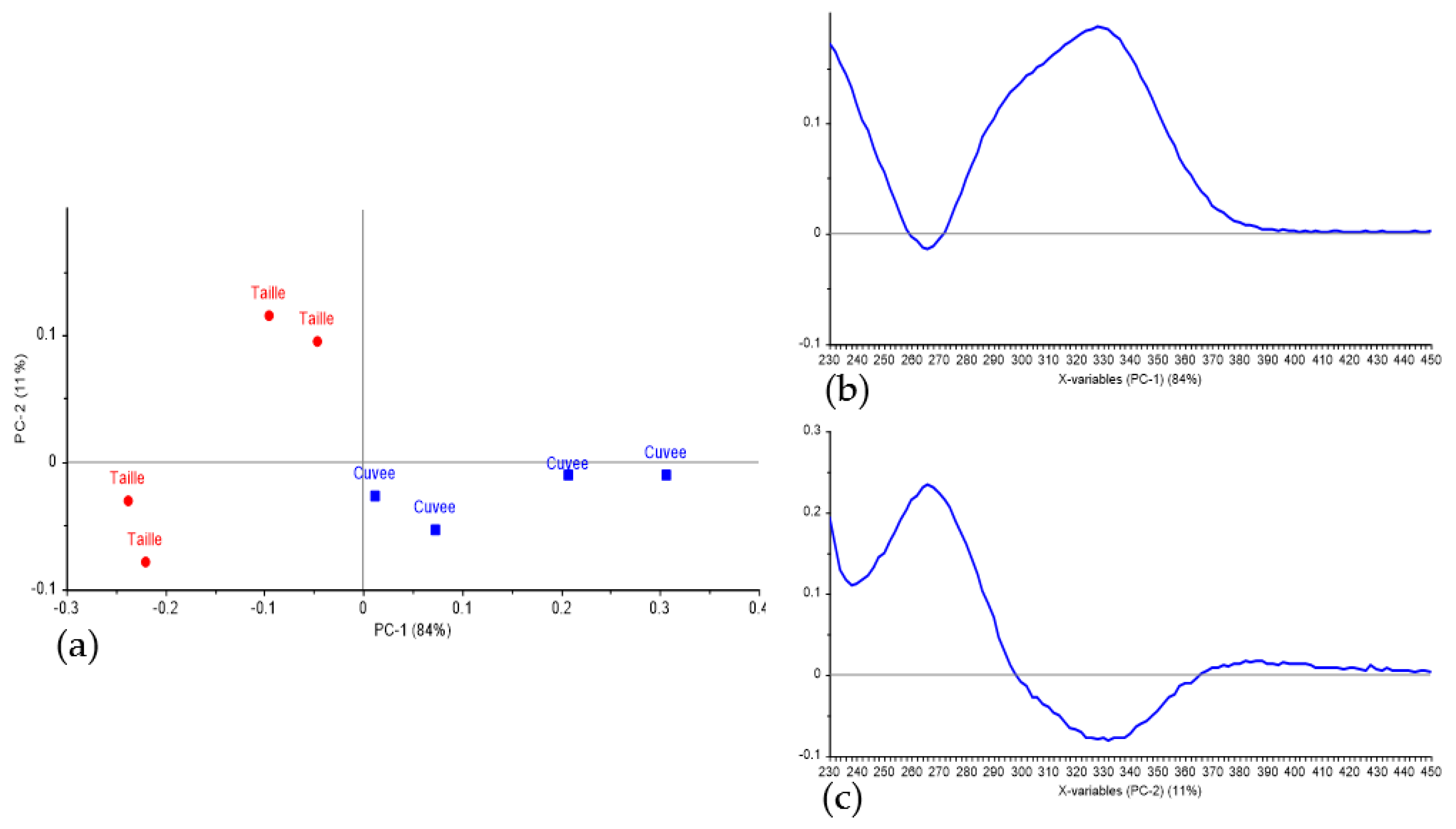
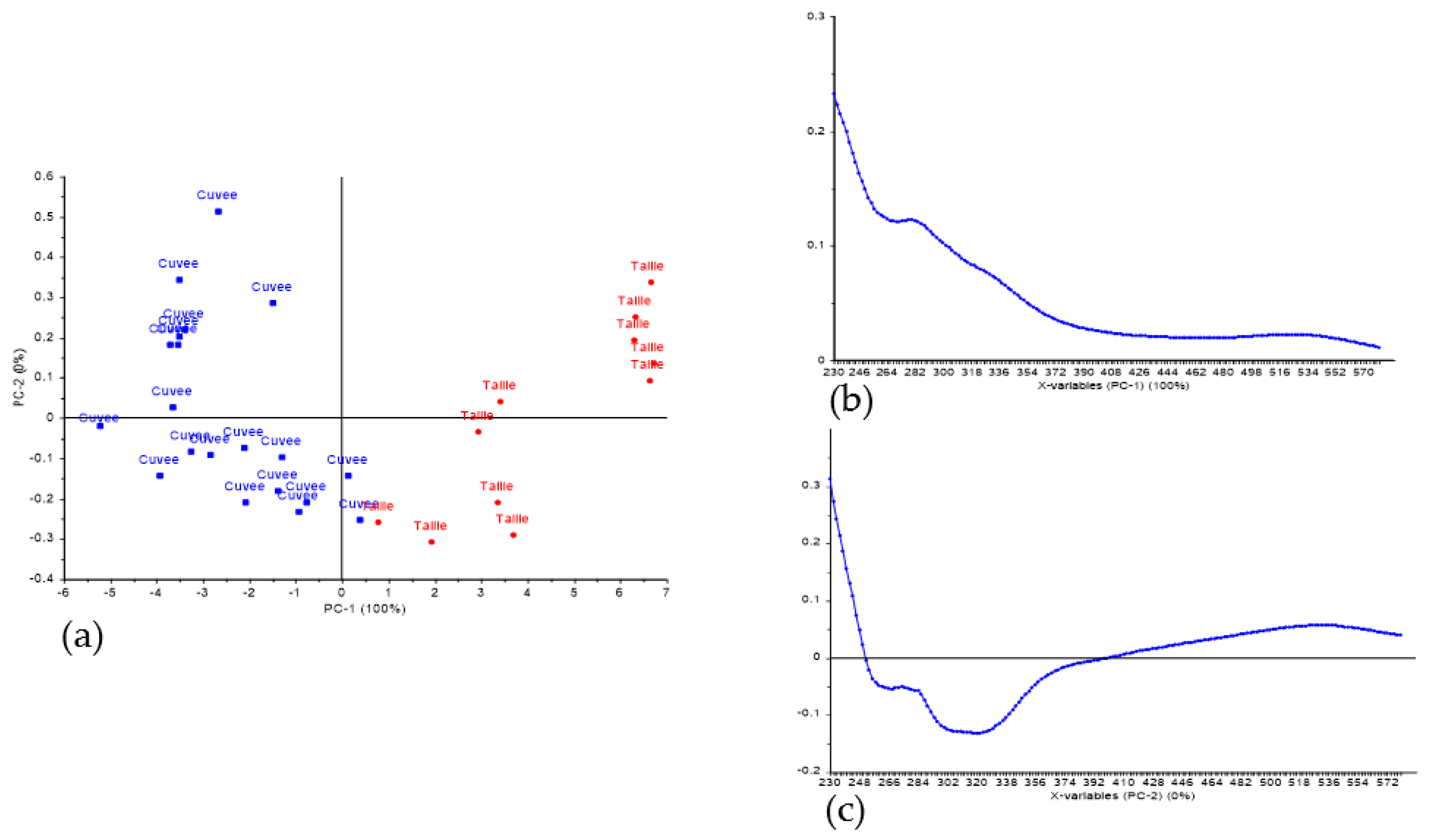
3.2. Classification of Press Fractions in the Commercial Scale Trial
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemp, B.; Kerslake, F.; Nesbitt, A. Global wine regions with a new sparkle. Australian & New Zealand Grapegrower and Winemaker, 23 December 2014. [Google Scholar]
- Culbert, J.A.; Ristic, R.; Ovington, L.A.; Saliba, A.J.; Wilkinson, K.L. Sensory profiles and consumer acceptance of different styles of Australian Moscato. Aust. J. Grape Wine Res. 2018, 24, 96–104. [Google Scholar] [CrossRef]
- Culbert, J.; Verdonk, N.; Ristic, R.; Olarte Mantilla, S.; Lane, M.; Pearce, K.; Cozzolino, D.; Wilkinson, K. Understanding consumer preferences for Australian sparkling wine vs. French Champagne. Beverages 2016, 2, 19. [Google Scholar] [CrossRef]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.Á.; Martínez-Rodríguez, A.; Pueyo, E.; Moreno-Arribas, M.V. Chemical and biochemical features involved in sparkling wine production: From a traditional to an improved winemaking technology. Trends Food Sci. Technol. 2009, 20, 289–299. [Google Scholar] [CrossRef]
- Jégou, S.; Hoang, D.A.; Salmon, T.; Williams, P.; Oluwa, S.; Vrigneau, C.; Doco, T.; Marchal, R. Effect of grape juice press fractioning on polysaccharide and oligosaccharide compositions of Pinot Meunier and Chardonnay Champagne base wines. Food Chem. 2017, 232, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Culbert, J.A.; McRae, J.M.; Condé, B.C.; Schmidtke, L.M.; Nicholson, E.L.; Smith, P.A.; Howell, K.S.; Boss, P.K.; Wilkinson, K.L. Influence of production method on the chemical composition, foaming properties, and quality of Australian carbonated and sparkling white wines. J. Agric. Food Chem. 2017, 65, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Gawel, R.; Day, M.; Van Sluyter, S.C.; Holt, H.; Waters, E.J.; Smith, P.A. White wine taste and mouthfeel as affected by juice extraction and processing. J. Agric. Food Chem. 2014, 62, 10008–10014. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lima, N.E.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Impact of pressing conditions on the phenolic composition, radical scavenging activity and glutathione content of Brazilian Vitis vinifera white wines and evolution during bottle ageing. Food Bioprocess Technol. 2016, 9, 944–957. [Google Scholar] [CrossRef]
- Hardy, G. Le pressurage, élément primordial de la qualité des vins de base en méthode champenoise. Rev. OEnologues 1990, 55, 17–25. [Google Scholar]
- Blanck, G.; Valade, M. Le fractionnement des moûts. Le Vign. Champen. 1989, 5, 266–277. [Google Scholar]
- Bosch-Fusté, J.; Sartini, E.; Flores-Rubio, C.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Viability of total phenol index value as quality marker of sparkling wines, “cavas”. Food Chem. 2009, 114, 782–790. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hayes, J.; Nolden, A. Biologically driven differences in sensation: Implications for the wine industry. In Proceedings of the 16th Australian Wine Industry Technical Conference, Adelaide, Australia, 24–28 July 2016; Beames, K., Robinson, E., Dry, P., Johnson, D., Eds.; Glen Osmond, South Australia, The Australian Wine Industry Technical Conference Inc.: Adelaide, Australia, 2017; pp. 120–127. [Google Scholar]
- Saurina, J. Characterization of wines using compositional profiles and chemometrics. Trends Anal. Chem. 2010, 29, 234–245. [Google Scholar] [CrossRef]
- Azcarate, S.M.; Cantarelli, M.Á.; Pellerano, R.G.; Marchevsky, E.J.; Camiña, J.M. Classification of Argentinean Sauvignon Blanc wines by uv spectroscopy and chemometric methods. J. Food Sci. 2013, 78, C432–C436. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Oliveri, P.; Armanino, C.; Lanteri, S.; Forina, M. Nir and UV-Vis spectroscopy, artificial nose and tongue: Comparison of four fingerprinting techniques for the characterisation of Italian red wines. Anal. Chim. Acta 2010, 668, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Girschik, L.; Jones, J.E.; Kerslake, F.L.; Robertson, M.; Dambergs, R.G.; Swarts, N.D. Apple variety and maturity profiling of base ciders using UV spectroscopy. Food Chem. 2017, 228, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Olivieri, A.; Aleixandre, J.L.; du Toit, W. Phenolic profiling of grapes, fermenting samples and wines using UV-Visible spectroscopy with chemometrics. Food Control 2018, 85, 11–22. [Google Scholar] [CrossRef]
- Pearce, K.; Culbert, J.; Cass, D.; Cozzolino, D.; Wilkinson, K. Influence of sample storage on the composition of carbonated beverages by MIR spectroscopy. Beverages 2016, 2, 26. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Smith, J.P.; Müller, M.C.; Holzapfel, B.P. Rapid monitoring of grapevine reserves using ATR–FT-IR and chemometrics. Anal. Chim. Acta 2012, 732, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Culbert, J.; Cozzolino, D.; Ristic, R.; Wilkinson, K. Classification of sparkling wine style and quality by MIR spectroscopy. Molecules 2015, 20, 8341–8356. [Google Scholar] [CrossRef] [PubMed]
- Schueuermann, C.; Khakimov, B.; Engelsen, S.B.; Bremer, P.; Silcock, P. GC-MS metabolite profiling of extreme southern Pinot Noir wines: Effects of vintage, barrel maturation, and fermentation dominate over vineyard site and clone selection. J. Agric. Food Chem. 2016, 64, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L.; Mukhopadhyay, S.; Robbins, R.J.; Finley, J.W.; Banuelos, G.S.; Harnly, J.M. UV spectral fingerprinting and analysis of variance-principal component analysis: A useful tool for characterizing sources of variance in plant materials. J. Agric. Food Chem. 2008, 56, 5457–5462. [Google Scholar] [CrossRef] [PubMed]
- Dambergs, R.G.; Mercurio, M.D.; Kassara, S.; Cozzolino, D.; Smith, P.A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 2012, 66, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chamkha, M.; Cathala, B.; Cheynier, V.; Douillard, R. Phenolic composition of Champagnes from Chardonnay and Pinot Noir vintages. J. Agric. Food Chem. 2003, 51, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Vérette, E.; Noble, A.C.; Somers, T.C. Hydroxycinnamates of Vitis vinifera: Sensory assessment in relation to bitterness in white wines. J. Sci. Food Agric. 1988, 45, 267–272. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics adaptation and validation of methyl cellulose precipitable tannin assay and modified somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Cynkar, W.U.; Shah, N.; Dambergs, R.G.; Smith, P.A. A brief introduction to multivariate methods in grape and wine analysis. Int. J. Wine Res. 2009, 2009, 123–130. [Google Scholar] [CrossRef]
- Yokotsuka, K. Effect of press design and pressing pressures on grape juice components. J. Ferment. Bioeng. 1990, 70, 15–21. [Google Scholar] [CrossRef]
- Cheynier, V.; Masson, G.; Rigaud, J.; Moutounet, M. Estimation of must oxidation during pressing in champagne. Am. J. Enol. Vitic. 1993, 44, 393–399. [Google Scholar]
- Gómez-Míguez, M.J.; González-Miret, M.L.; Hernanz, D.; Fernández, M.Á.; Vicario, I.M.; Heredia, F.J. Effects of prefermentative skin contact conditions on colour and phenolic content of white wines. J. Food Eng. 2007, 78, 238–245. [Google Scholar] [CrossRef]
| Variety | Press Fraction | Number of Samples | Press Cycle | Pressure (Bar) |
|---|---|---|---|---|
| Chardonnay | Cuvée (481 L ton−1) | 21 | Free run | Free run |
| 1 | 0.2, 0.4, 0.6, 0.8, 1.1 | |||
| 2 | 0.2, 0.4, 0.6, 0.8, 1.1 | |||
| 3 | 0.2, 0.4, 0.6, 0.8, 1.1, 1.4 | |||
| 4 | 0.4, 0.6, 0.8, 1.1 | |||
| Taille (228 L ton−1) | 21 | 4 | 1.4 | |
| 5 | 0.6, 0.8, 1.1, 1.4, 1.7 | |||
| 6 | 0.6, 0.8, 1.1, 1.4, 1.7 | |||
| 7 | 0.6, 0.8, 1.1, 1.4, 1.7 | |||
| 8 | 0.6, 0.8, 1.1, 1.4, 1.7 | |||
| Pinot noir | Cuvée (502 L ton−1) | 22 | Free run | Free run |
| 1 | 0.2, 0.4, 0.6, 0.8, 1.1 | |||
| 2 | 0.2, 0.4, 0.6, 0.8, 1.1 | |||
| 3 | 0.4, 0.6, 0.8, 1.1, 1.4 | |||
| 4 | 0.4, 0.6, 0.8, 1.1 | |||
| 4 | 1.4, 1.7 | |||
| Taille (200 L ton−1) | 9 | 5 | 0.6, 0.8, 1.1, 1.4 | |
| 6 | 0.6, 0.8, 1.1, 1.4, 1.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerslake, F.; Longo, R.; Dambergs, R. Discrimination of Juice Press Fractions for Sparkling Base Wines by a UV-Vis Spectral Phenolic Fingerprint and Chemometrics. Beverages 2018, 4, 45. https://doi.org/10.3390/beverages4020045
Kerslake F, Longo R, Dambergs R. Discrimination of Juice Press Fractions for Sparkling Base Wines by a UV-Vis Spectral Phenolic Fingerprint and Chemometrics. Beverages. 2018; 4(2):45. https://doi.org/10.3390/beverages4020045
Chicago/Turabian StyleKerslake, Fiona, Rocco Longo, and Robert Dambergs. 2018. "Discrimination of Juice Press Fractions for Sparkling Base Wines by a UV-Vis Spectral Phenolic Fingerprint and Chemometrics" Beverages 4, no. 2: 45. https://doi.org/10.3390/beverages4020045
APA StyleKerslake, F., Longo, R., & Dambergs, R. (2018). Discrimination of Juice Press Fractions for Sparkling Base Wines by a UV-Vis Spectral Phenolic Fingerprint and Chemometrics. Beverages, 4(2), 45. https://doi.org/10.3390/beverages4020045