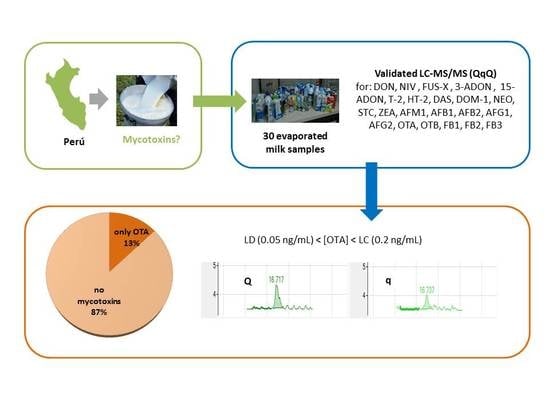
Analysis of Mycotoxins in Peruvian Evaporated Cow Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mycotoxin Stock Solutions
2.3. Sample Collection
2.4. Instrumentation and Analytical Conditions
2.5. Sample Preparation
2.6. Calibration and Control Samples Preparation
2.7. Method Validation for Mycotoxin Group B
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Binder, E.M. Managing the risk of mycotoxins in modern feed production. Anim. Feed Sci. Technol. 2007, 133, 149–166. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 39, 1–27. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Laganà, A. Multiclass mycotoxin analysis in food, environmental and biological matrices with chromatography/mass spectrometry. Mass Spectrom. Rev. 2012, 31, 466–503. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. A 2008, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; Lizarraga, E.; López de Cerain, A.; González-Peñas, E. Presence of mycotoxins in animal milk: A review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- FDA Compliance Policy Guide, CPG Sec. 527.400 Whole Milk, Lowfat Milk, Skim Milk—Aflatoxin M1, U.S. Food and Drug Administration, 2015. Available online: http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm074482.htm (accessed on 22 February 2018).
- European Commission. Commission Regulation (EU) N° 165/2010 of 26 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. OJL 2010, 50, 8–12. Available online: https://www.fsai.ie/uploadedFiles/Reg165_2010.pdf (accessed on 26 February 2018).
- Instituto Nacional de Innovación Agraria (INIA). Perú: National Report on the Status of Plant Genetic Resources for Agriculture and Food [Informe Nacional Sobre el Estado de los Recursos Fitogenéticos Para la Agricultura y la Alimentación]. Instituto Nacional de Innovación Agraria—INIA. Subdirección de Recursos Genéticos y Biotecnología (SUDIRGEB) 2009. Available online: http://www.fao.org/docrep/013/i1500e/Peru.pdf (accessed on 26 February 2018).
- Resnik, S.; Costarrica, M.L.; Pacin, A. Mycotoxins in Latin America and the Caribbean. Food Control 1995, 6, 19–28. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- MINAGRI. Livestock and Industrial Poultry Production 2013 [Producción Pecuaria e Industrial Avícola 2013]. Oficina de Estudios Económicos y Estadísticos. Ministerio de Agricultura y Riego (MINAGRI) 2014. Available online: http://siea.minag.gob.pe/siea/?q=produccion-pecuaria-e-industria-avicola (accessed on 26 February 2018).
- Ortiz, C. Analysis of Aflatoxin M1 in Fresh Milk from Dairy Farms in Arequipa. Rev. Investig. Vet. Perú 2009, 20, 139–141. Available online: http://dev.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1609-91172009000100021&lng=en (accessed on 22 February 2018).
- MINAGRI. Livestock and Industrial Poultry Production 2014 [Producción Pecuaria y Avícola 2014]. Dirección General de Seguimiento y Evaluación de Políticas. Ministerio de Agricultura y Riego (MINAGRI), 2015. Available online: http://siea.Minag.gob.pe/siea/?q=produccion-pecuaria-e-industria-avicola (accessed on 26 February 2018).
- Flores-Flores, M.E.; González-Peñas, E. An LC–MS/MS method for multi-mycotoxin quantification in cow milk. Food Chem. 2017, 218, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; González-Peñas, E. Development and validation of a high performance liquid chromatographic–mass spectrometry method for the simultaneous quantification of 10 trichothecenes in ultra-high temperature processed cow milk. J. Chromatogr. A 2015, 1419, 37–44. [Google Scholar] [CrossRef] [PubMed]
- European Commision. Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Amended by Commission Regulations N° 178/2010 and 519/2014, 2006. Available online: https://www.fsvps.ru/fsvps-docs/ru/usefulinf/files/es401-2006.pdf (accessed on 26 February 2018).
- Food and Agriculture Organization of the United Nations (FAO). Worldwide Regulations for Mycotoxins in Food and Feed in 2003. FAO Food and Nutrition Paper 81. 2004. Available online: http://www.fao.org/docrep/007/y5499e/y5499e00.htm (accessed on 26 February 2018).
- Herzallah, S.M. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009, 114, 1141–1146. [Google Scholar] [CrossRef]
- El-Hoshy, S.M. Occurrence of zearalenone in milk, meat and their products with emphasis on influence of heat treatments on its level. Arch. Lebensmittelhyg. 1999, 50, 140–143. [Google Scholar]
- Huang, L.C.; Zheng, N.; Zheng, B.Q.; Wen, F.; Chen, J.B.; Han, R.W.; Xu, X.M.; Li, S.L.; Wang, J.Q. Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and α-zearalenol in milk by UHPLC–MS/MS. Food Chem. 2014, 146, 242–249. [Google Scholar] [CrossRef]
- Scientific Co-operation on Questions relating to Food (SCOOP). Task 3.2.10 Collection of Occurrence Data of Fusarium Toxins in Food and Assessment of Dietary Intake by the Population of EU Member States. Subtask II: Zearalenone, 2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_fusarium_task3210.pdf (accessed on 26 February 2018).
- Sørensen, L.K.; Elbæk, T.H. Determination of mycotoxins in bovine milk by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2005, 820, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, T.; Lugoboni, B.; Zironi, E.; Barbarossa, A.; Serraino, A.; Pagliuca, G. Determination of fumonisin B1 in bovine milk by LC–MS/MS. Food Control 2009, 20, 1171–1174. [Google Scholar] [CrossRef]
- Maragos, C.M.; Richard, J.L. Quantitation and stability of fumonisins B1 and B2 in milk. J. AOAC Int. 1994, 77, 1162–1167. [Google Scholar] [PubMed]
- Boudra, H.; Barnouin, J.; Dragacci, S.; Morgavi, D.P. Aflatoxin M1 and ochratoxin A in raw bulk milk from French dairy herds. J. Dairy Sci. 2007, 90, 3197–3201. [Google Scholar] [CrossRef] [PubMed]
- Elzupir, A.O.; Makawi, S.Z.A.; Elhussein, A.M. Determination of aflatoxins and ochratoxin A in dairy cattle feed and milk in Wad Medani, Sudan. J. Anim. Vet. Adv. 2009, 8, 2508–2511. [Google Scholar]
- Pattono, D.; Gallo, P.F.; Civera, T. Detection and quantification of Ochratoxin A in milk produced in organic farms. Food Chem. 2011, 127, 374–377. [Google Scholar] [CrossRef]
 ) [18] and semi-skimmed evaporated milk (
) [18] and semi-skimmed evaporated milk (  ).
).
 ) [18] and semi-skimmed evaporated milk (
) [18] and semi-skimmed evaporated milk (  ).
).
 ) [18] and semi-skimmed evaporated milk (
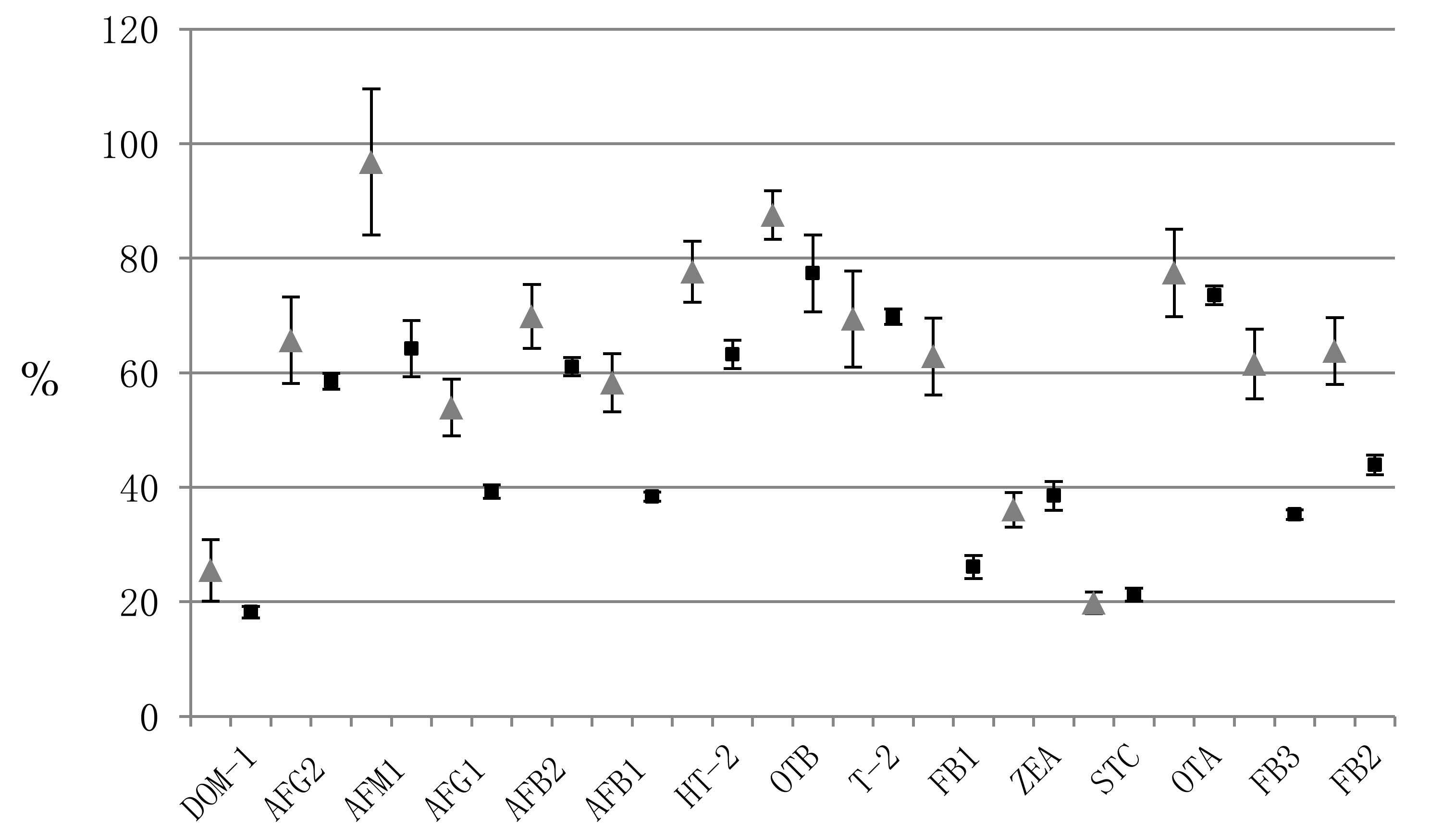
) [18] and semi-skimmed evaporated milk (  ).
).
 ) [18] and semi-skimmed evaporated milk (
) [18] and semi-skimmed evaporated milk (  ).
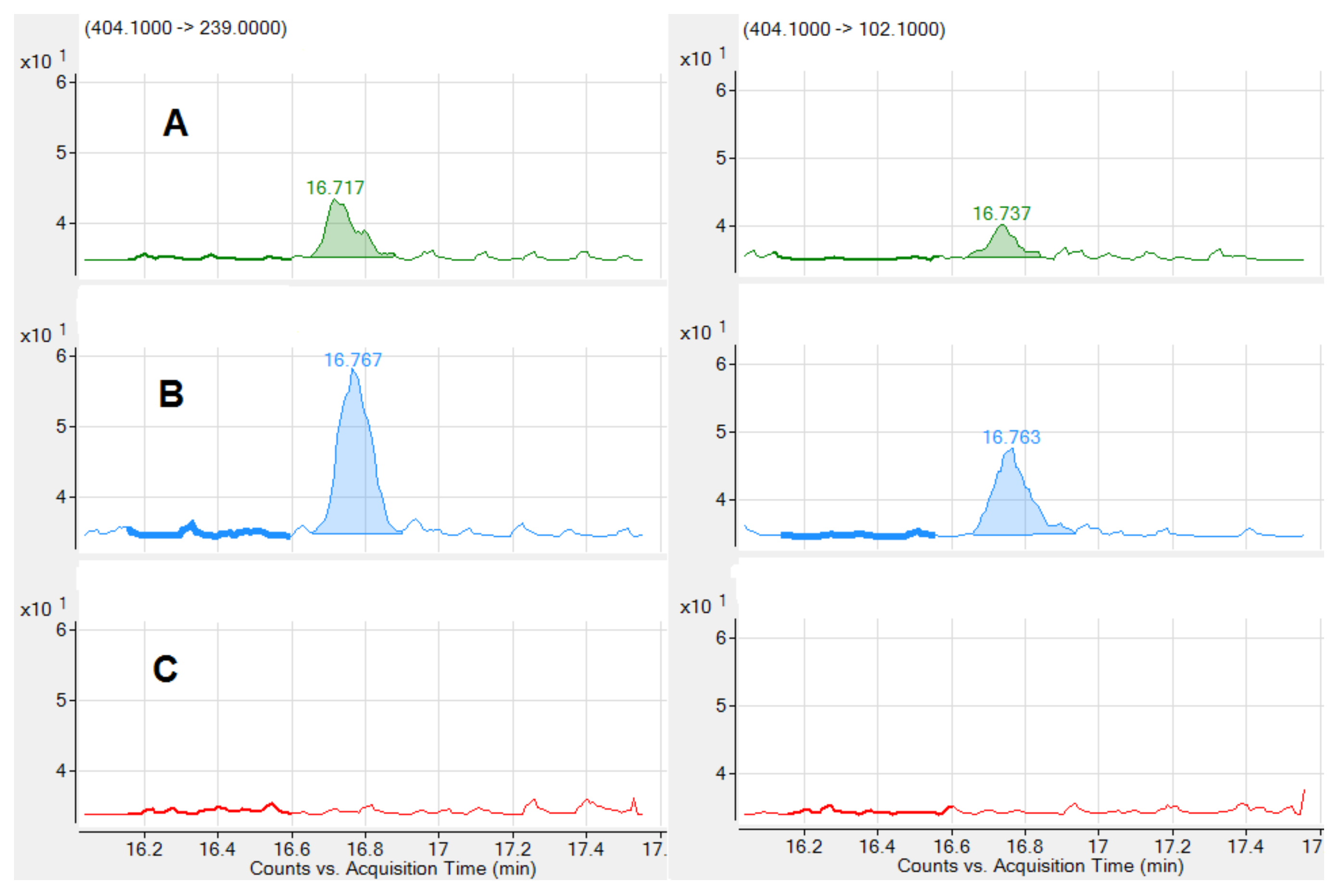
).
| Mixed Stock Solution 1 | Mixed Stock Solution 2 | Mixed Stock Solution 3 | |||
|---|---|---|---|---|---|
| Mycotoxin | ng/mL | Mycotoxin | ng/mL | Mycotoxin | ng/mL |
| NIV | 1011.4 | DOM-1 | 151.5 | FB1 | 507.0 |
| DON | 251.3 | AFG2 | 7.5 | FB3 | 125.0 |
| FUS-X | 185.0 | AFM1 | 2.5 | FB2 | 125.0 |
| NEO | 10.0 | AFG1 | 5.1 | ||
| 3-ADON | 50.2 | AFB2 | 2.0 | ||
| 15-ADON | 101.1 | AFB1 | 2.0 | ||
| DAS | 8.0 | HT-2 | 20.1 | ||
| OTB | 2.5 | ||||
| T-2 | 2.5 | ||||
| ZEA | 25.5 | ||||
| STC | 25.1 | ||||
| OTA | 10.0 | ||||
| Number | Amount (g) | Presentation | Year | Comments |
|---|---|---|---|---|
| 1 | 400 | tetrabrik | 2015 | evaporated semi-skimmed milk 0% Lactose |
| 2 | 500 | tetrabrik | 2015 | evaporated semi-skimmed milk with DHA * for children |
| 3 | 520 | tetrabrik | 2015 | evaporated semi-skimmed milk |
| 4 | 500 | tetrabrik | 2015 | evaporated semi-skimmed milk |
| 5 | 400 | tetrabrik | 2015 | evaporated whole cream milk |
| 6 | 400 | bag | 2015 | evaporated whole cream milk |
| 7 | 170 | can | 2015 | evaporated semi-skimmed milk with DHA for children |
| 8 | 170 | can | 2015 | evaporated semi-skimmed milk 0% Lactose |
| 9 | 170 | can | 2015 | evaporated whole cream milk |
| 10 | 410 | can | 2015 | evaporated skimmed milk |
| 11 | 410 | can | 2015 | evaporated whole cream milk with Ca and Fe |
| 12 | 400 | can | 2014 | evaporated whole cream milk |
| 13 | 410 | can | 2015 | evaporated semi-skimmed milk |
| 14 | 410 | can | 2015 | evaporated whole cream milk |
| 15 | 400 | can | 2015 | evaporated semi-skimmed milk with DHA |
| 16 | 410 | can | 2015 | evaporated semi-skimmed milk for children |
| 17 | 410 | can | 2015 | evaporated semi-skimmed milk for babies |
| 18 | 165 | can | 2013 | evaporated whole cream milk |
| 19 | 170 | can | 2013 | evaporated semi-skimmed milk |
| 20 | 165 | can | 2014 | evaporated whole cream milk |
| 21 | 170 | can | 2013 | evaporated whole cream milk |
| 22 | 165 | can | 2014 | evaporated whole cream milk |
| 23 | 170 | can | 2015 | evaporated whole cream milk |
| 24 | 165 | can | 2015 | evaporated whole cream milk |
| 25 | 165 | can | 2015 | evaporated whole cream milk |
| 26 | 400 | tetrabrik | 2015 | evaporated whole cream milk |
| 27 | 400 | tetrabrik | 2015 | evaporated whole cream milk for children |
| 28 | 500 | tetrabrik | 2014 | evaporated semi-skimmed milk |
| 29 | 250 | tetrabrik | 2015 | evaporated semi-skimmed milk |
| 30 | 410 | can | 2015 | evaporated semi-skimmed milk with DHA for children |
| tR (min) | Mycotoxin | Range (ng/mL) | LOD (ng/mL) | R2 | Slope (Confidence Interval 95%) | Intercept | q/Q Sample % | q/Q Standard % | |
|---|---|---|---|---|---|---|---|---|---|
| 1.52 | DOM-1 | 3.03–30.3 | 0.758 | 0.9994 | 79.6 | (77.5, 81.8) | 63.8 | 89 | 93 |
| 2.71 | AFG2 | 0.15–1.50 | 0.038 | 0.9975 | 2229.9 | (2146.6, 2313.3) | −50.7 | 75 | 74 |
| 2.84 | AFM1 | 0.05–0.50 | 0.025 | 0.9963 | 1659.8 | (1587.4, 1732.2) | 24.5 | 95 | 98 |
| 3.39 | AFG1 | 0.10–1.02 | 0.025 | 0.9979 | 4428.9 | (4261.5, 4596.4) | −93.3 | 70 | 71 |
| 4.21 | AFB2 | 0.04–0.40 | 0.010 | 0.9937 | 4852.5 | (4596.8, 5108.2) | −62.4 | 97 | 96 |
| 5.21 | AFB1 | 0.04–0.40 | 0.020 | 0.9947 | 7582.5 | (7190.8, 7974.2) | −44.7 | 62 | 61 |
| 10.19 | HT-2 | 0.40–4.02 | 0.200 | 0.9948 | 197.3 | (186.5, 208) | −20.9 | 53 | 55 |
| 12.48 | OTB | 0.05–0.50 | 0.013 | 0.9996 | 3853.2 | (3775.3, 3931) | −19.8 | 44 | 41 |
| 14.17 | T-2 | 0.05–0.50 | 0.025 | 0.9988 | 1743.3 | (1689.8, 1796.9) | −12.2 | 60 | 66 |
| 13.77 | FB1 | 10.1–101.4 | 5.07 | 0.9935 | 8.2 | (7.5, 8.8) | −24.5 | 72 | 72 |
| 15.79 | ZEA | 0.51–5.09 | 0.510 | 0.9996 | 254.2 | (249.9, 258.6) | 0.9 | 101 | 98 |
| 16.74 | STC | 0.50–5.02 | 0.125 | 0.9939 | 361.2 | (332.9, 389.6) | 40.9 | 90 | 91 |
| 16.71 | OTA | 0.20–2.00 | 0.050 | 0.9997 | 701.3 | (686.6, 715.9) | −53.5 | 75 | 72 |
| 17.72 | FB3 | 2.50–25.0 | 0.625 | 0.9992 | 132.5 | (128.8, 136.2) | −39.6 | 66 | 66 |
| 20.08 | FB2 | 2.50–25.0 | 1.25 | 0.9989 | 100.9 | (97.6, 104.2) | 7.9 | 44 | 44 |
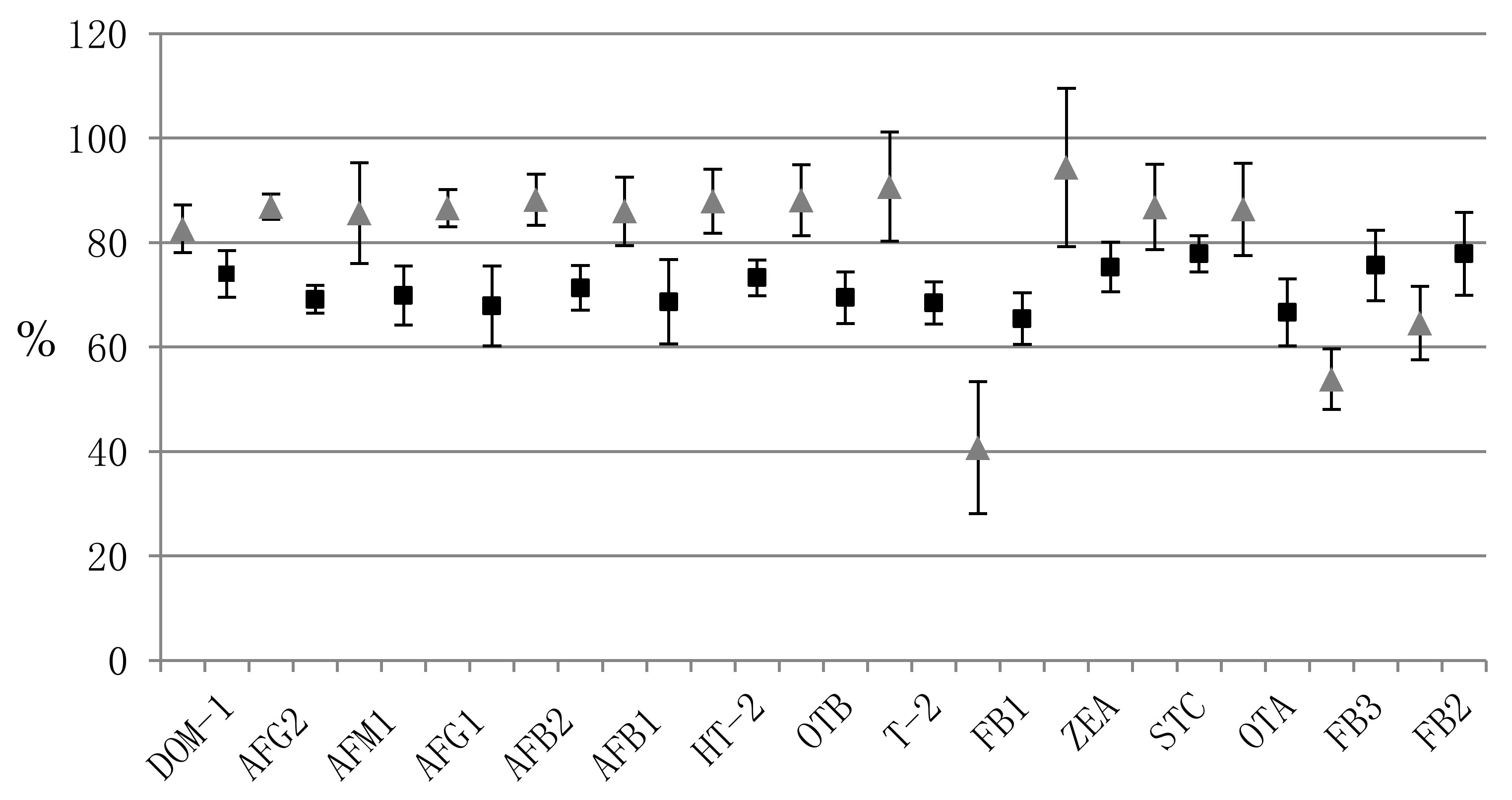
| Mycotoxin | Precision (%RSD) | Accuracy (%RE) | Matrix Effect | Recovery | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within-Run (n = 3) | Between-Run (n = 9) | Within-Run (n = 3) | Between-Run (n = 9) | Within-Run (n = 3) | Within-Run (n = 3) | |||||||||||||||
| L * | M | H | L | M | H | L | M | H | L | M | H | L | M | H | (%RSD) | L | M | H | (%RSD) | |
| DOM-1 | 10.5 | 4.1 | 10.6 | 10.5 | 4.1 | 10.6 | 4.8 | 1.1 | 1.1 | 4.8 | 1.1 | 1.1 | 19.3 | 17.9 | 17.4 | (5.3) | 79.1 | 71.8 | 71.0 | (6.1) |
| AFG2 | 6.3 | 2.3 | 2.0 | 6.3 | 2.3 | 2.0 | 0.4 | 2.8 | 1.2 | 0.4 | 2.8 | 1.2 | 59.9 | 58.5 | 57.1 | (2.3) | 67.2 | 68.0 | 72.2 | (3.8) |
| AFM1 | 5.3 | 5.0 | 5.1 | 5.3 | 5.0 | 5.1 | 10.0 | 1.5 | 0.8 | 10.0 | 1.5 | 0.8 | 69.7 | 62.7 | 60.2 | (7.6) | 63.5 | 71.7 | 74.4 | (8.1) |
| AFG1 | 8.9 | 1.2 | 11.5 | 9.5 | 1.1 | 5.1 | 0.7 | 2.0 | 2.4 | 3.6 | 1.7 | 5.7 | 39.5 | 38.0 | 40.3 | (3.0) | 61.2 | 76.2 | 66.1 | (11.3) |
| AFB2 | 2.4 | 6.6 | 1.3 | 2.4 | 6.6 | 1.3 | 12.2 | 4.3 | 2.2 | 12.2 | 4.3 | 2.2 | 59.9 | 62.9 | 60.4 | (2.6) | 70.3 | 67.6 | 76.0 | (6.0) |
| AFB1 | 9.7 | 2.1 | 2.7 | 9.7 | 2.1 | 8.0 | 6.1 | 0.9 | 1.0 | 6.1 | 0.9 | 0.3 | 38.5 | 37.5 | 39.1 | (2.1) | 60.3 | 76.5 | 69.2 | (11.8) |
| HT-2 | 2.2 | 2.1 | 9.5 | 15.4 | 2.1 | 9.5 | 15.7 | 1.1 | 0.2 | 7.9 | 1.1 | 0.2 | 61.7 | 66.1 | 61.9 | (3.9) | 76.9 | 70.1 | 72.7 | (4.7) |
| OTB | 0.2 | 7.6 | 6.7 | 0.2 | 7.6 | 6.7 | 2.0 | 8.1 | 0.2 | 2.0 | 8.1 | 0.2 | 84.7 | 71.5 | 75.9 | (8.7) | 64.4 | 69.6 | 74.3 | (7.1) |
| T-2 | 10.1 | 8.5 | 4.2 | 10.1 | 8.5 | 4.2 | 1.1 | 4.2 | 2.5 | 1.1 | 4.2 | 2.5 | 70.5 | 70.6 | 68.2 | (1.9) | 64.8 | 67.7 | 72.8 | (5.9) |
| FB1 | 6.4 | 3.2 | 11.5 | 11.2 | 3.2 | 11.5 | 12.2 | 0.8 | 0.9 | 9.8 | 0.8 | 0.9 | 27.6 | 26.9 | 23.8 | (7.8) | 70.9 | 61.3 | 64.1 | (7.6) |
| ZEA | 5.5 | 2.2 | 5.5 | 5.5 | 2.2 | 5.5 | 2.3 | 1.0 | 3.2 | 2.3 | 1.0 | 3.2 | 35.7 | 40.7 | 39.1 | (6.6) | 79.7 | 70.3 | 75.9 | (6.3) |
| STC | 12.5 | 10.1 | 10.3 | 11.8 | 9.3 | 10.3 | 1.1 | 3.7 | 1.3 | 8.7 | 4.9 | 2.1 | 20.0 | 22.0 | 21.8 | (5.3) | 81.8 | 75.8 | 75.8 | (4.4) |
| OTA | 4.0 | 6.2 | 2.6 | 14.1 | 7.6 | 5.9 | 6.5 | 8.3 | 0.7 | 6.2 | 2.4 | 3.2 | 71.8 | 73.7 | 75.1 | (2.2) | 60.3 | 66.4 | 73.1 | (9.5) |
| FB3 | 1.4 | 4.9 | 6.3 | 13.4 | 8.0 | 6.9 | 17.0 | 2.1 | 9.0 | 4.3 | 2.0 | 3.9 | 34.8 | 36.2 | 34.7 | (2.5) | 73.1 | 70.5 | 83.3 | (9.0) |
| FB2 | 7.4 | 4.7 | 9.1 | 10.6 | 7.3 | 8.0 | 19.4 | 2.5 | 7.3 | 13.1 | 0.7 | 1.3 | 43.9 | 45.6 | 42.2 | (3.9) | 80.8 | 68.9 | 83.9 | (11.2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Flores, M.E.; González-Peñas, E. Analysis of Mycotoxins in Peruvian Evaporated Cow Milk. Beverages 2018, 4, 34. https://doi.org/10.3390/beverages4020034
Flores-Flores ME, González-Peñas E. Analysis of Mycotoxins in Peruvian Evaporated Cow Milk. Beverages. 2018; 4(2):34. https://doi.org/10.3390/beverages4020034
Chicago/Turabian StyleFlores-Flores, Myra Evelyn, and Elena González-Peñas. 2018. "Analysis of Mycotoxins in Peruvian Evaporated Cow Milk" Beverages 4, no. 2: 34. https://doi.org/10.3390/beverages4020034
APA StyleFlores-Flores, M. E., & González-Peñas, E. (2018). Analysis of Mycotoxins in Peruvian Evaporated Cow Milk. Beverages, 4(2), 34. https://doi.org/10.3390/beverages4020034