Nuclear Magnetic Resonance and Headspace Solid-Phase Microextraction Gas Chromatography as Complementary Methods for the Analysis of Beer Samples
Abstract
:1. Introduction
2. Experimental
2.1. NMR Analysis
2.2. SPME GC Analysis
2.3. FTIR Analysis
3. Results
3.1. NMR Results
3.2. SPME GC Results
3.3. Ethanol Content
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gallup New Service. Gallup Poll Social Series: Consumption Habits. Available online: http://www.gallup.com/file/poll/174083/Favorite_Alcoholic_Drink_140723%20.pdf (accessed on 28 July 2016).
- Meilgaard, M.C.D. Tech Dissertation, Beer Flavor; Technical University of Denmark: Lyngby, Denmark, 1981. [Google Scholar]
- Nord, L.I.; Vaag, P.; Duus, J.Ø. Quantification of Organic and Amino Acids in Beer by 1H NMR Spectroscopy. Anal. Chem. 2004, 76, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.F.; Barros, A.; Almeida, C.; Spraul, M.; Gil, A.M. Multivariate Analysis of NMR and FTIR Data as a Potential Tool for the Quality Control of Beer. J. Agric. Food Chem. 2004, 52, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Duarte, I.F.; Barros, A.; Rodrigues, J.; Spraul, M.; Gil, A.M. Composition of Beer by 1H NMR Spectroscopy: Effects of Brewing Site and Date of Production. J. Agric. Food Chem. 2006, 54, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Frank, W.; Humpfer, E.; Schäfer, H.; Keller, S.; Mörtter, M.; Spraul, M. Quality Control of Beer using High-Resolution Nuclear Magnetic Resonance Spectroscopy and Multivariate Analysis. Eur. Food Res. Technol. 2005, 220, 215–221. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Wlazly, K.; Wąsowica, E.; Kamiński, E. Solid-Phase Microextraction for the Analysis of Some Alcohols and Esters in Beer: Comparison with Static Headspace Method. J. Agric. Food Chem. 1998, 46, 1469–1473. [Google Scholar] [CrossRef]
- Horák, T.; Ćulík, J.; Kellner, V.; Jurková, M.; Ćejka, P.; Hašková, D.; Dvořák, J. Analysis of Selected Esters in Beer: Comparison of Solid-Phase Microextraction and Stir Bar Sorptive Extraction. J. Inst. Brew. 2010, 116, 81–85. [Google Scholar] [CrossRef]
- Kleinová, J.; Klejdus, B. Determination of Volatiles in Beer using Solid-Phase Microextraction in Combination with Gas Chromatography/Mass Spectrometry. Czech J. Food Sci. 2014, 32, 241–248. [Google Scholar]
- Leça, J.M.; Pereira, A.C.; Vieira, A.C.; Reis, M.S.; Marques, J.C. Optimal Design of Experiments applied to Headspace Solid Phase Microextraction for the Quantification of Vicinal Diketones in Beer through Gas Chromatography-Mass Spectrometric Detection. Anal. Chim. Acta 2015, 887, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Pinho, O.; Ferreira, I.M.P.L.V.O.; Santos, L.H.M.L.M. Method Optimization by Solid-Phase Microextraction in Combination with Gas Chromatography with Mass Spectrometry for Analysis of Beer Volatile Fraction. J. Chromatogr. A 2006, 1121, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Das, A.J.; Khawas, P.; Miyaji, T.; Deka, S.C. HPLC and GC-MS Analyses of Organic Acids, Carbohydrates, Amino Acids and Volatile Aromatic Compounds in Some Varieties of Rice Beer from Northeast India. J. Inst. Brew. 2014, 120, 244–252. [Google Scholar] [CrossRef]
- Klampfl, C. Analysis of Organic Acids and Inorganic Anions in Different Types of Beer Using Capillary Zone Electrophoresis. J. Agric. Food Chem. 1999, 47, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Easy, Precise and Accurate Quantitative NMR. Application Note. Available online: https://www.agilent.com/cs/library/applications/qNMR%205990–7601.pdf (accessed on 13 March 2017).
- Holzgrabe, U.; Diehib, B.W.K. NMR Spectroscopy in Pharmacy. J. Pharm. Biomed. Anal. 1998, 17, 557–616. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Prediction of Flavor Differences between Beers from their Chemical Compositions. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Common Brewing Faults. Available online: http://wine.appstate.edu/sites/wine.appstate.edu/files/Common%20Attributes_Taubman.pdf (accessed on 29 July 2016).
- Vanderhaegen, B.; Delvaux, F.; Daenen, L.; Verachtert, H.; Delvaux, F.R. Aging Characteristics of Different Beer Types. Food Chem. 2007, 103, 404–412. [Google Scholar] [CrossRef]
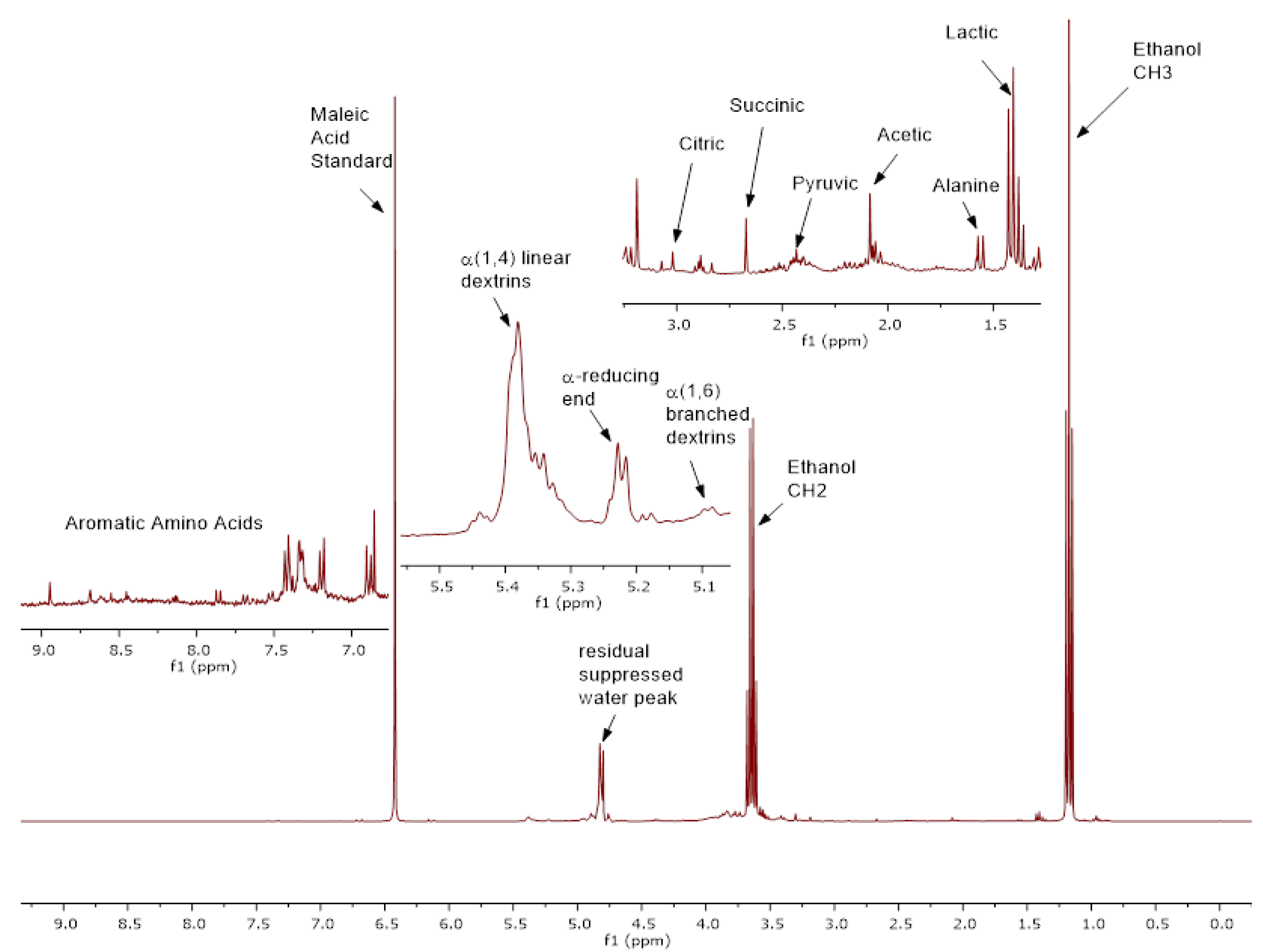

| Sample Number | Beer Style |
|---|---|
| 1 | Belgian Ale |
| 2 | IPA |
| 3 | Scotch Ale |
| 4 | Hefeweizen |
| 5 | English Brown Ale |
| 6 | Stout |
| 7 | Double IPA |
| 8 | Amber Ale |
| 9 | Patersbier |
| Component | Shift (ppm) | Multiplicity | J (Hz) | Assignment | N |
|---|---|---|---|---|---|
| Organic Acids | |||||
| Lactic Acid | 1.33 | d | 7.0 | CH3 | 3 |
| Succinic Acid | 2.67 | s | - | CH2 | 4 |
| Acetic Acid | 2.08 | s | - | CH3 | 3 |
| Malic Acid | 2.90/2.85 | dd/dd | 16.5,4.6/6.7, 16.5 | CH2 | 2 |
| Pyruvic Acid | s | - | CH3 | 3 | |
| Pyruvic Acid hydrate | 1.57 | s | - | CH3 | 3 |
| Citric Acid | 3.02, 2.86 | d, d | 15.8 | CH2 | 4 |
| Formic Acid | 8.45 | s | - | CH | 1 |
| Alcohols | |||||
| Ethanol | 1.17 | t | 7.1 | CH3 | 3 |
| iso-Butanol | 0.87 | d | 6.6 | CH3 | 6 |
| iso-Pentanol | 0.88 | d | 6.6 | CH3 | 6 |
| 1-Propanol | 0.87 | t | 7.1 | CH3 | 3 |
| 2,3-Butandiol | 1.13 | m | - | CH3 | 6 |
| Carbohydrates | |||||
| Maltooligosaccharides | 4.3–4.8/4.9–5.5 | d | - | CH | 1 |
| Lactose | 4.45 | d | 7.7 | CH | 1 |
| Glycerol | 3.55 | m | 5.4 | CH2 | 2 |
| Amino Acids | |||||
| Histidine | 8.7 | s | - | CH | 1 |
| Uridine | 7.86 | d | 8.1 | CH | 1 |
| Tryptophan | 7.58 | s | - | CH | 1 |
| Phenylalanine | 7.37 | m | - | CH | 5 |
| Tyrosine | 7.17 | d | 8.6 | CH | 1 |
| Proline | 2.12/2.39 | m | - | CH2 | 2 |
| Alanine | 1.55 | d | 7.3 | CH3 | 3 |
| Valine | 1.06/1.02 | d/d | 7.1 | CH3 | 3 |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 | Sample 8 | Sample 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Organic Acids | |||||||||
| Lactic Acid (mg/L) | 738 | 1042 | 626 | 966 | 47 | 761 | 1118 | 661 | 421 |
| Succinic Acid (mg/L) | 299 | 262 | 161 | 201 | 98 | 247 | 201 | 144 | 448 |
| Acetic Acid (mg/L) | 135 | 133 | 205 | 139 | 119 | 178 | 111 | 107 | 88 |
| Pyruvic Acid (mg/L) | 57 | 97 | 103 | 94 | 103 | 83 | 89 | 74 | 57 |
| Pyruvic Acid hydrate (mg/L) | 0 | 109 | 103 | 74 | 126 | 37 | 123 | 77 | 54 |
| Citric Acid (mg/L) | 140 | 89 | 94 | 94 | 260 | 94 | 70 | 70 | 61 |
| Formic Acid (mg/L) | 31 | 0 | 22 | 0 | 9 | 40 | 0 | 0 | 0 |
| Alcohols | |||||||||
| Ethanol (% v/v) | 6.6 | 8.0 | 8.6 | 6.1 | 6.2 | 8.0 | 8.9 | 4.9 | 5.5 |
| iso-Butanol (mg/L) | 34 | 29 | 12 | 24 | 17 | 17 | 22 | 19 | 12 |
| iso-Pentanol (mg/L) | 123 | 126 | 72 | 77 | 60 | 77 | 86 | 49 | 60 |
| 1-Propanol (mg/L) | 47 | 156 | 78 | 62 | 62 | 70 | 78 | 47 | 55 |
| 2,3-Butandiol (mg/L) | 65 | 74 | 79 | 57 | 51 | 90 | 75 | 59 | 38 |
| Glycerol (mg/L) | 1699 | 2276 | 1667 | 2372 | 1506 | 2660 | 2724 | 1346 | 1699 |
| Carbohydrates | |||||||||
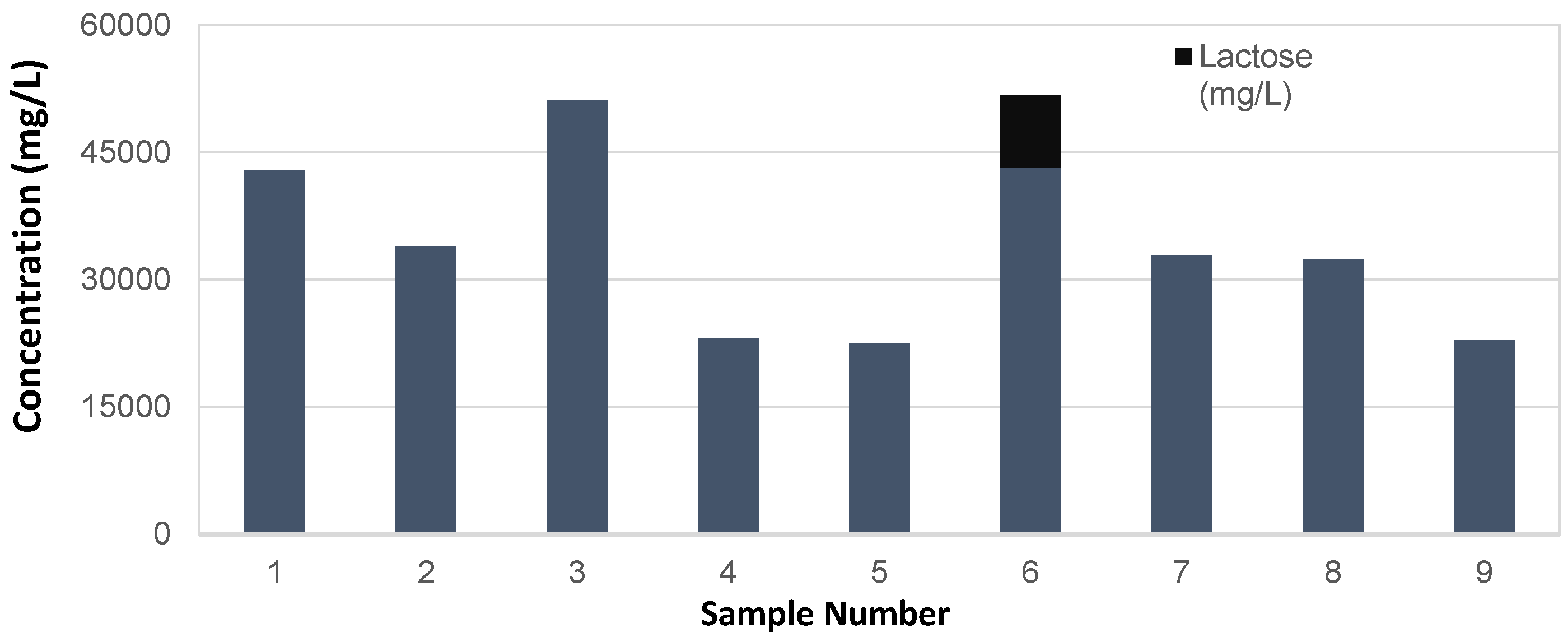
| Residual Dextrins (mg/L) | 42,814 | 33,910 | 51,173 | 23,093 | 22,478 | 43,165 | 32,822 | 32,347 | 22,829 |
| Lactose (mg/L) | 0 | 0 | 0 | 0 | 0 | 8606 | 0 | 0 | 0 |
| Glycerol (mg/L) | 1699 | 2276 | 1667 | 2372 | 1506 | 2660 | 2724 | 1346 | 1699 |
| Amino Acids | |||||||||
| Histidine (mg/L) | 60 | 60 | 91 | 106 | 30 | 106 | 0 | 0 | 0 |
| Uridine (mg/L) | 24 | 24 | 95 | 119 | 24 | 167 | 214 | 24 | 143 |
| Tryptophan (mg/L) | 60 | 40 | 119 | 179 | 60 | 219 | 239 | 0 | 219 |
| Phenylalanine (mg/L) | 277 | 306 | 377 | 406 | 203 | 425 | 509 | 167 | 261 |
| Tyrosine (mg/L) | 194 | 247 | 300 | 380 | 159 | 362 | 415 | 203 | 177 |
| Gallic Acid (mg/L) | 66 | 50 | 50 | 0 | 25 | 83 | 91 | 0 | 33 |
| GABA (mg/L) | 487 | 643 | 713 | 668 | 266 | 558 | 477 | 362 | 352 |
| Proline (mg/L) | 1122 | 1149 | 1463 | 1274 | 754 | 1274 | 1301 | 628 | 870 |
| Alanine (mg/L) | 336 | 315 | 333 | 315 | 136 | 246 | 420 | 98 | 200 |
| Valine (mg/L) | 0 | 384 | 221 | 145 | 129 | 303 | 411 | 120 | 204 |
| Calculated Calories | |||||||||
| Cal/L | 561 | 607 | 709 | 462 | 452 | 682 | 654 | 417 | 418 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Average | Std. Dev. | %RSD | |
|---|---|---|---|---|---|---|---|---|---|---|
| Organic Acids | ||||||||||
| Lactic Acid (mg/L) | 3934 | 3969 | 3945 | 3966 | 3966 | 3983 | 3972 | 3962 | 17 | 0.4 |
| Succinic Acid (mg/L) | 201 | 216 | 204 | 216 | 210 | 201 | 201 | 207 | 7 | 3.2 |
| Acetic Acid (mg/L) | 256 | 275 | 258 | 287 | 252 | 256 | 254 | 262 | 13 | 5.1 |
| Alcohols | ||||||||||
| Ethanol (mg/L) | 55,693 | 55,916 | 55,889 | 55,891 | 56,006 | 56,118 | 56,118 | 55,947 | 150 | 0.3 |
| Ethanol (v/v) | 7.06 | 7.09 | 7.08 | 7.08 | 7.10 | 7.11 | 7.11 | 7.09 | 0 | 0.3 |
| Carbohydrates | ||||||||||
| Residual Dextrin (mg/L) | 20,880 | 21,143 | 20,950 | 21,056 | 21,424 | 20,301 | 19,949 | 20,815 | 512 | 2.5 |
| Amino Acids | ||||||||||
| Alanine (mg/L) | 174 | 179 | 168 | 165 | 171 | 159 | 179 | 171 | 7 | 4.4 |
| Sample | Isoamyl Acetate (mg/L) | Ethyl Caprylate (mg/L) | Ethyl Caprate (mg/L) | Other Compounds Identified |
|---|---|---|---|---|
| 1 | 2.1 | 1.6 | 0.8 | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate |
| 2 | 0.2 | 0.2 | BDL | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate, glyceraldehyde, butyl butyrate, β-pinene |
| 3 | ND | BDL | 0.9 | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate |
| 4 | BDL | BDL | BDL | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate, linalool |
| 5 | ND | BDL | BDL | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate |
| 6 | BDL | BDL | BDL | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate |
| 7 | 1.0 | BDL | 0.8 | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate, butyl butyrate, isoamyl propionate, β-pinene, amyl butyrate |
| 8 | 0.6 | 0.4 | 0.2 | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate, amyl butyrate |
| 9 | 3.4 | BDL | 0.6 | Ethanol, ethyl acetate, isoamyl alcohol, ethyl butyrate, ethyl valerate |
| Sample | Stated %ABV | FTIR %ABV | NMR %ABV |
|---|---|---|---|
| 1 | 6.5 | 6.5 | 6.6 |
| 2 | 7.1 | 8.6 | 8.0 |
| 3 | 8.2 | 8.8 | 8.6 |
| 4 | 5.2 | 6.3 | 6.1 |
| 5 | 5.4 | 6.1 | 6.2 |
| 6 | 7.5 | 7.1 | 8.0 |
| 7 | 9.0 | 9.2 | 8.9 |
| 8 | 5.0 | 5.4 | 4.9 |
| 9 | 5.4 | 5.9 | 5.5 |
| Compound | Signal Precision (%RSD) for a 5.0 mg/L Standard (n = 4) | Mean R2 Value (SD) | Detection Limit (mg/L) |
|---|---|---|---|
| Isoamyl acetate | 15.7 | 0.990 (0.015) | 0.086 |
| Ethyl caprylate | 16.8 | 0.986 (0.010) | 0.044 |
| Ethyl Caprate | 14.8 | 0.994 (0.005) | 0.023 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, S.R.; Soprano, S.E.; Wickham, L.M.; Fitzgerald, N.; Edwards, J.C. Nuclear Magnetic Resonance and Headspace Solid-Phase Microextraction Gas Chromatography as Complementary Methods for the Analysis of Beer Samples. Beverages 2017, 3, 21. https://doi.org/10.3390/beverages3020021
Johnson SR, Soprano SE, Wickham LM, Fitzgerald N, Edwards JC. Nuclear Magnetic Resonance and Headspace Solid-Phase Microextraction Gas Chromatography as Complementary Methods for the Analysis of Beer Samples. Beverages. 2017; 3(2):21. https://doi.org/10.3390/beverages3020021
Chicago/Turabian StyleJohnson, Sarah R., Samantha E. Soprano, Laura M. Wickham, Neil Fitzgerald, and John C. Edwards. 2017. "Nuclear Magnetic Resonance and Headspace Solid-Phase Microextraction Gas Chromatography as Complementary Methods for the Analysis of Beer Samples" Beverages 3, no. 2: 21. https://doi.org/10.3390/beverages3020021
APA StyleJohnson, S. R., Soprano, S. E., Wickham, L. M., Fitzgerald, N., & Edwards, J. C. (2017). Nuclear Magnetic Resonance and Headspace Solid-Phase Microextraction Gas Chromatography as Complementary Methods for the Analysis of Beer Samples. Beverages, 3(2), 21. https://doi.org/10.3390/beverages3020021






