Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Polyphenols
2.2. Determination of Total Polyphenol Content
2.3. Determination of Antioxidant Activity
2.4. Statistical Analysis
3. Results
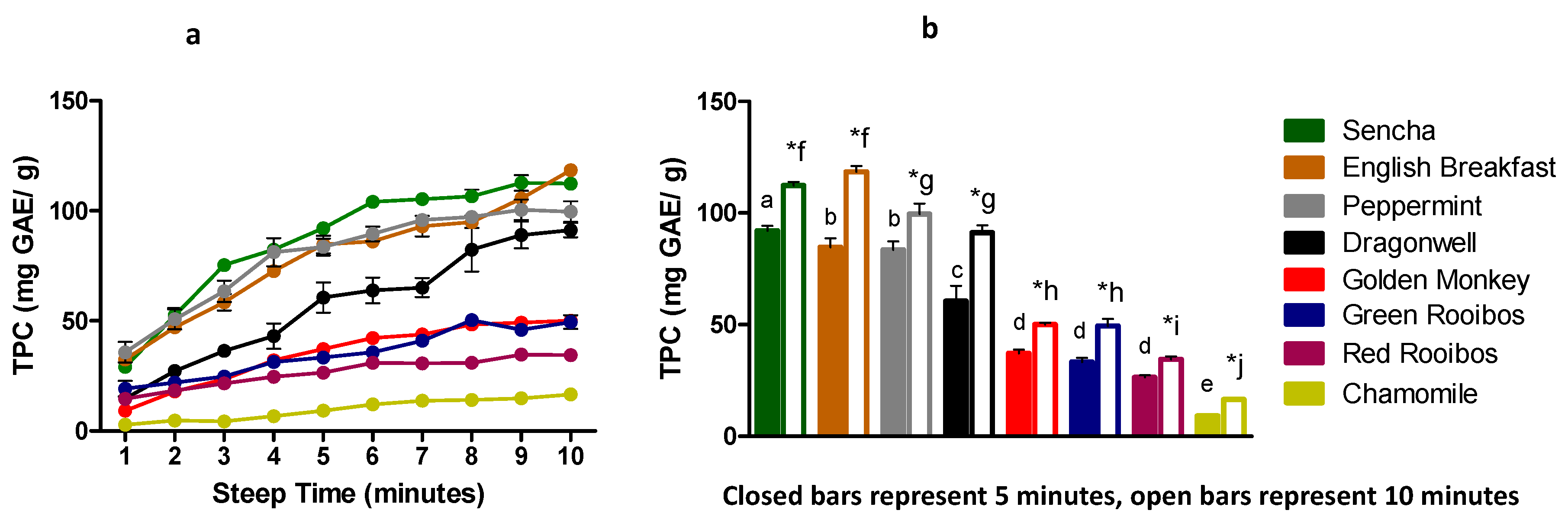
3.1. Total Polyphenol Content
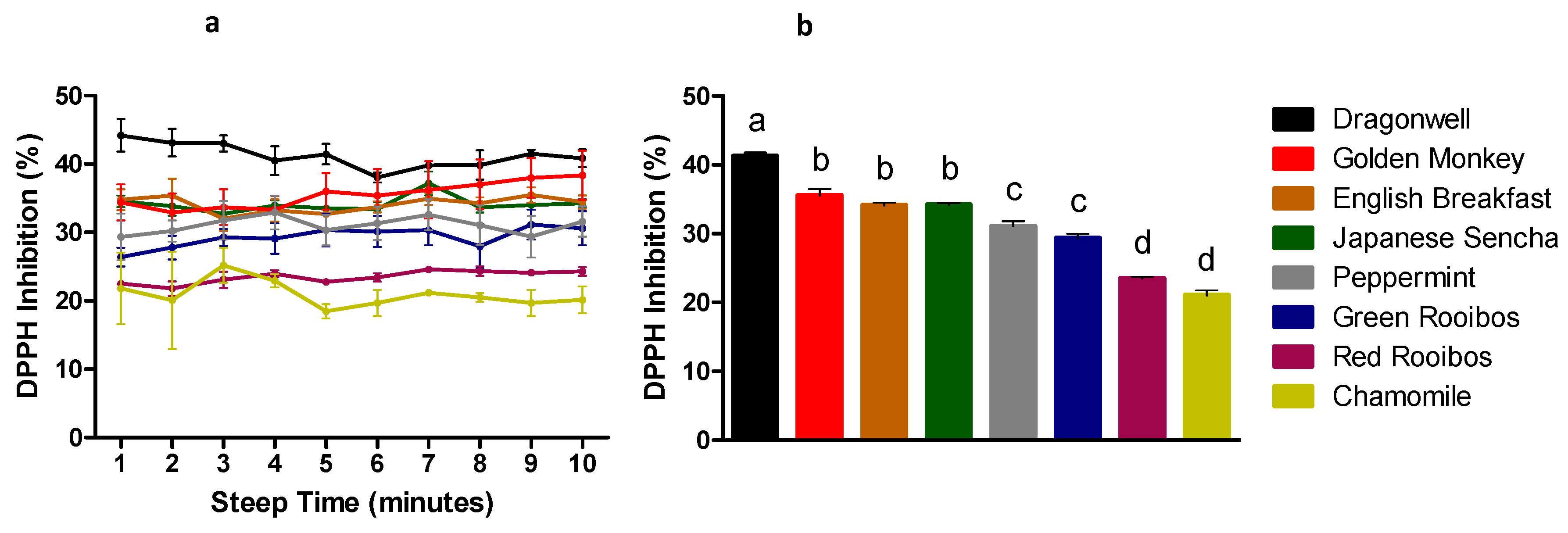
3.2. Antioxidant Capacity
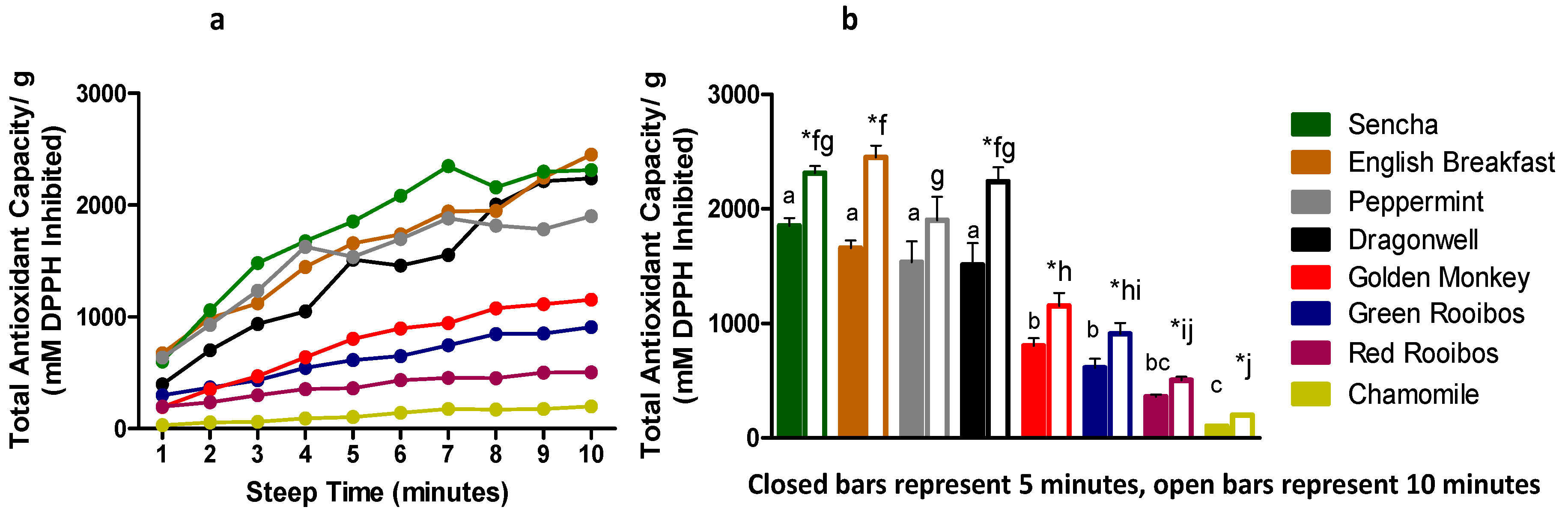
3.3. Predicted Total Antioxidant Capacity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TPC | Total Polyphenol Content |
| DW | Dragonwell |
| S | Sencha |
| EB | English Breakfast |
| GM | Golden Monkey |
| GR | Green Rooibos |
| RR | Red Rooibos |
| C | Chamomile |
| P | Peppermint |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric-Reducing Antioxidant Power |
| TRAP | Total Radical Antioxidant Trapping Parameter |
| ORAC | Oxygen Radical Absorbing Capacity |
| OVX | Ovariectomized |
| BMD | Bone Mineral Density |
| OD | Optical Density |
| GAE | Gallic Acid Equivalents |
| EGCG | Epigallocatechin Gallate |
| EGC | Epigallocatechin |
| dH2O | Distilled water |
Appendix
| Steep Time (Minutes) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||||||||
| Sencha | 29.06±4.39 | a | 52.42±6.90 | b | 75.31±4.88 | c | 82.33±1.81 | c,d | 92.11±4.37 | d,e | 104.06± 5.82 | e,f | 105.26±4.09 | f | 106.60±5.92 | f | 112.65±7.10 | f,g | 112.37±3.03 | f |
| I,II | I | I | I | I | I | I | I | I | I | |||||||||||
| English Breakfast | 32.47±3.26 | a | 47.10±5.78 | b | 58.44±7.46 | b,c | 72.55±3.85 | c,d | 84.65±7.99 | d,e | 86.08±5.68 | d,e,f | 92.99±9.40 | e,f | 94.85±5.94 | e,f | 105.59±2.35 | f,g | 118.44±5.29 | g |
| I,II | I | II | I | I | II | I | I | I | I | |||||||||||
| Peppermint | 35.71±9.38 | a | 50.68±9.07 | a,b | 63.45±9.66 | b,c | 81.21±12.77 | c,d | 83.53±7.65 | c,d | 89.52±6.59 | d | 95.85±4.70 | d | 97.16±5.00 | d | 100.48±9.25 | d | 99.59±9.43 | d |
| I | I | I,II | I | I | II | I | I | I,II | II | |||||||||||
| Dragonwell | 14.75±3.42 | a | 27.19±4.34 | a,b | 36.28±4.27 | a,b,c | 43.01±11.50 | b,c,d | 60.55±13.83 | c,d,e | 63.80±11.79 | d,e,f | 65.09±8.67 | d,e,f | 82.32±19.75 | e,f,g | 88.96±12.14 | f,g | 91.19±6.44 | g |
| III | II | III | II | II | III | II | II | II | II | |||||||||||
| Golden Monkey | 9.20±2.49 | a | 17.94±2.51 | b | 23.49±2.74 | b | 32.11±3.06 | c | 37.19±3.13 | c,d | 42.14±2.19 | d,e | 43.82±4.94 | d,e,f | 48.31±2.25 | e,f | 49.21±4.68 | e,f | 50.11±1.41 | f |
| III | II | IV | II,III | III | IV | III | III | III | III | |||||||||||
| Green Rooibos | 19.20±7.06 | a | 21.91±3.28 | a,b | 24.64±0.81 | a,b,c | 31.26±2.68 | b,c,d | 33.32±3.67 | c,d | 35.72±3.11 | d | 40.97±0.21 | d,e | 50.31±2.88 | e | 46.00±5.15 | e | 49.43±6.29 | e |
| II,III | II | III,IV | II,III | III | IV | III | III | III | III | |||||||||||
| Red Rooibos | 14.60±2.14 | a | 18.34±2.14 | a,b | 21.56±2.50 | b,c | 24.61±3.44 | b,c,d | 26.48±1.84 | c,d,e,f | 30.98±3.36 | e,f | 30.72±1.90 | d,e,f | 30.92±2.83 | d,e,f | 34.75±1.52 | f | 34.41±2.70 | f |
| III | II | IV | III | III | IV | III | IV | III | IV | |||||||||||
| Chamomile | 2.83±1.71 | a | 2.62±1.71 | a | 4.31±1.58 | a,b | 6.70±0.70 | a,b,c | 9.23±1.73 | b,c,d | 12.04±2.83 | c,d,e | 13.73±5.03 | d,e | 14.01±0.79 | d,e | 14.81±2.10 | d,e | 16.60±1.68 | e |
| IV | III | V | IV | IV | V | IV | IV | IV | V | |||||||||||
| Steep Time (Minutes) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||||||||
| Sencha | 601.50±85.06 | a | 1057.94±93.54 | b | 1480.33±127.67 | c | 1675.10±78.64 | c,d | 1854.90±132.22 | d,e | 2084.07±64.57 | e,f | 2349.76±243.34 | f | 2157.71±194.38 | e,f | 2299.70±136.56 | f | 2312.67±125.51 | f |
| II | I | I | I | I | I | I | I | I | II | |||||||||||
| English Breakfast | 674.48±42.98 | a | 990.00±95.26 | b | 1120.43±177.86 | b | 1445.30±150.38 | c | 1658.33±133.32 | c,d | 1739.75±74.55 | c,d | 1943.00±141.46 | d,e | 1948.27±93.75 | d,e | 2245.94±105.08 | e,f | 2451.43±203.14 | f |
| I | I | I,II | I,II | I | I,II | I,II | I | I,II | I,II | |||||||||||
| Peppermint | 635.72±220.29 | a | 928.68±251.66 | a,b | 1232.83±406.34 | a,b,c | 1625.12±455.93 | b,c | 1535.25±361.30 | a,b,c | 1697.26±387.90 | b,c | 1881.27±335.91 | c | 1816.39±395.73 | b,c | 1782.59±489.83 | b,c | 1900.51±413.89 | c |
| I,II | I,II | I,II | I | I | I,II | II | I | II | II | |||||||||||
| Dragonwell | 396.31±127.86 | a | 700.94±103.27 | a | 936.51±118.99 | a,b | 1048.78±296.22 | a,b | 1511.51±380.75 | b,c | 1459.79±301.23 | b,c | 1554.44±208.09 | b,c,d | 2002.76±695.15 | c,d | 2214.93±294.72 | c,d | 2239.11±248.71 | d |
| II,III | II | II | II,III | I | II | II | I | I,II | I,II | |||||||||||
| Golden Monkey | 193.78±74.61 | a | 350.82±55.46 | a,b | 469.78±43.73 | a,b,c | 639.90±77.18 | b,c,d | 803.98±133.63 | c,d,e | 896.20±214.78 | d,e | 943.57±193.25 | d,e | 1076.97±236.50 | e | 1114.22±141.97 | e | 1153.90±225.87 | e |
| III | III | III | III,IV | I | III | III | II | III | III | |||||||||||
| Green Rooibos | 298.69±89.03 | a | 368.80±95.92 | a,b | 433.49±43.51 | a,b | 543.08±67.68 | a,b,c | 612.68±156.65 | b,c,d | 649.27±125.34 | b,c,d | 746.30±106.98 | c,d | 847.11±218.20 | c,d | 851.84±77.60 | c,d | 907.93±193.01 | d |
| III | III | III,IV | IV,V | II | III | III,IV | II,III | III,IV | III,IV | |||||||||||
| Red Rooibos | 197.41±30.63 | a | 236.94±23.50 | a,b | 299.75±54.93 | b,c | 353.33±42.34 | c,d | 362.28±31.89 | c,d,e | 434.92±43.08 | d,e,f | 453.60±32.26 | e,f | 452.07±52.12 | d,e,f | 502.31±10.95 | f | 503.31±62.68 | f |
| III,IV | III | III,IV | IV,V | II,III | III,IV | IV,V | II,III | IV,V | IV,V | |||||||||||
| Chamomile | 30.61±9.85 | a | 56.14±28.53 | a,b | 61.89±14.57 | a,b | 92.08±12.31 | a,b,c | 102.67±25.85 | a,b,c | 140.73±40.14 | b,c,d | 175.99±71.36 | c,d | 172.44±13.21 | c,d | 176.62±54.48 | c,d | 198.98±33.66 | d |
| IV | III | IV | V | III | IV | V | III | V | V | |||||||||||
References
- Butt, M.S.; Sultan, M.T. Green tea: Nature’s defense against malignancies. Crit. Rev. Food Sci. Nutr. 2009, 49, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Dreger, H.; Stangl, K.; Lorenz, M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc. Res. 2007, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (mentha piperita l.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity of south african herbal teas: Rooibos (aspalathus linearis) and honeybush (cyclopia intermedia). Phytother. Res. 2007, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Nash, L.A.; Ward, W.E. Tea and bone health: Findings from human studies, potential mechanisms, and identification of knowledge gaps. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 2008, 3, S181–S184. [Google Scholar] [CrossRef] [PubMed]
- Christen, Y. Oxidative stress and alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [PubMed]
- Caramori, G.; Papi, A. Oxidants and asthma. Thorax 2004, 59, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Leenen, R.; Roodenburg, A.J.; Tijburg, L.B.; Wiseman, S.A. A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur. J. Clin. Nutr. 2000, 54, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Neshchadin, D.; Batchelor, S.N.; Bilkis, I.; Gescheidt, G. Short-lived phenoxyl radicals formed from green-tea polyphenols and highly reactive oxygen species: An investigation by time-resolved epr spectroscopy. Angew. Chem. Int. Ed. Engl. 2014, 53, 13288–13292. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Puddey, I.B.; Croft, K.D.; Burke, V.; Mori, T.A.; Caccetta, R.A.; Beilin, L.J. Acute effects of ingestion of black and green tea on lipoprotein oxidation. Am. J. Clin. Nutr. 2000, 71, 1103–1107. [Google Scholar] [PubMed]
- Cherubini, A.; Beal, M.F.; Frei, B. Black tea increases the resistance of human plasma to lipid peroxidation in vitro, but not ex vivo. Free Radic. Biol. Med. 1999, 27, 381–387. [Google Scholar] [CrossRef]
- Mehrabian, S. The study of antioxidant and anticarcinogenic green tea and black tea. Pak. J. Biol. Sci. 2007, 10, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Bunkova, R.; Marova, I.; Nemec, M. Antimutagenic properties of green tea. Plant Foods Hum. Nutr. 2005, 60, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chaudhuri, T.; Seth, P.; Ganguly, D.K.; Giri, A.K. Antimutagenic effects of black tea (world blend) and its two active polyphenols theaflavins and thearubigins in salmonella assays. Phytother Res 2002, 16, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Mukherjee, M.; Das, D.; Mitra, C. Protective action of aqueous black tea (camellia sinensis) extract (bte) against ovariectomy-induced oxidative stress of mononuclear cells and its associated progression of bone loss. Phytother. Res. 2009, 23, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int. 2008, 19, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Luximon-Ramma, A.; Gunness, T.K.; Sookar, D.; Bhoyroo, S.; Jugessur, R.; Reebye, D.; Googoolye, K.; Crozier, A.; Aruoma, O.I. Black tea reduces uric acid and c-reactive protein levels in humans susceptible to cardiovascular diseases. Toxicology 2010, 278, 68–74. [Google Scholar] [CrossRef]
- Hughes, L.A.; Arts, I.C.; Ambergen, T.; Brants, H.A.; Dagnelie, P.C.; Goldbohm, R.A.; van den Brandt, P.A.; Weijenberg, M.P.; Netherlands Cohort, S. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in bmi over time in women: A longitudinal analysis from the netherlands cohort study. Am. J. Clin. Nutr. 2008, 88, 1341–1352. [Google Scholar] [PubMed]
- Lorenz, M.; Urban, J.; Engelhardt, U.; Baumann, G.; Stangl, K.; Stangl, V. Green and black tea are equally potent stimuli of no production and vasodilation: New insights into tea ingredients involved. Basic Res. Cardiol. 2009, 104, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chang, S.C.; Goldstein, B.Y.; Scheider, W.L.; Cai, L.; You, N.C.; Tarleton, H.P.; Ding, B.; Zhao, J.; Wu, M.; et al. Green tea consumption, inflammation and the risk of primary hepatocellular carcinoma in a chinese population. Cancer Epidemiol. 2011, 35, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Damiani, E.; Astolfi, P.; Carloni, P. Influence of steeping conditions (time, temperature, and particle size) on antioxidant properties and sensory attributes of some white and green teas. Int. J. Food Sci. Nutr. 2015, 66, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nash, L.A.; Ward, W.E. Comparison of black, green and rooibos tea on osteoblast activity. Food Funct. 2016, 7, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Fernando, C.D.; Soysa, P. Extraction kinetics of phytochemicals and antioxidant activity during black tea (camellia sinensis l.) brewing. Nutr. J. 2015, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of dpph method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; van De Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in the netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food Chem. 2000, 48, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Lakenbrink, C.; Lapczynski, S.; Maiwald, B.; Engelhardt, U.H. Flavonoids and other polyphenols in consumer brews of tea and other caffeinated beverages. J. Agric. Food Chem. 2000, 48, 2848–2852. [Google Scholar] [CrossRef] [PubMed]
- Price, W.; Spitzer, J. The kinetics of extraction of individual flavanols and caffeine from a japanese green tea (sen cha uji tsuyu) as a function of temperature. Food Chem. 1994, 50, 19–23. [Google Scholar] [CrossRef]
- Matthews, C.M. Steep your genes in health: Drink tea. Proc. (Bayl. Univ. Med. Cent.) 2010, 23, 142–144. [Google Scholar] [PubMed]
- Luczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Torun, M.; Dincer, C.; Topuz, A.; Sahin-Nadeem, H.; Ozdemir, F. Aqueous extraction kinetics of soluble solids, phenolics and flavonoids from sage (Salvia fruticosa miller) leaves. J. Food Sci. Technol. 2015, 52, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Chong, K.L.; Tan, J.B.L.; Wong, S.K. Antioxidant properties of temperate and tropical herbal teas. J. Food Comp. Anal. 2010, 185–189. [Google Scholar] [CrossRef]
- Stewart, A.J.; Mullen, W.; Crozier, A. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 2005, 49, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.; Alberte, R.S. Nettle extract (Urtica dioica) affects key receptors and enzymes associated with allergic rhinitis. Phytother. Res. 2009, 23, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.L.; Gelderblom, W.C.; Joubert, E. An investigation on the antimutagenic properties of south african herbal teas. Mutat. Res. 2000, 471, 157–166. [Google Scholar] [CrossRef]
| Sample Identification | Common Name | Scientific Name (Variety) | Type |
|---|---|---|---|
| EB | English Breakfast | C. sinensis | Black Tea |
| GM | Golden Monkey | C. sinensis | Black Tea |
| DW | Dragonwell | C. sinensis | Green Tea |
| S | Sencha | C. sinensis | Green Tea |
| GR | Green Rooibos | A. linearis | Herbal |
| RR | Red Rooibos | A. linearis | Herbal |
| P | Peppermint | M. piperita | Herbal |
| C | Chamomile | M. chamomilla | Herbal |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAlpine, M.D.; Ward, W.E. Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas. Beverages 2016, 2, 17. https://doi.org/10.3390/beverages2030017
McAlpine MD, Ward WE. Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas. Beverages. 2016; 2(3):17. https://doi.org/10.3390/beverages2030017
Chicago/Turabian StyleMcAlpine, Michael D., and Wendy E. Ward. 2016. "Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas" Beverages 2, no. 3: 17. https://doi.org/10.3390/beverages2030017
APA StyleMcAlpine, M. D., & Ward, W. E. (2016). Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas. Beverages, 2(3), 17. https://doi.org/10.3390/beverages2030017






