Significance of Grape Phenolic Compounds for Wine Characteristics: Dynamics and Extractability During Fruit Maturation
Abstract
1. Introduction
2. Phenolic Compounds in Grapevines: Structure, Reactivity, and Role in Plant Development
3. Evolution of Phenolic Compounds During Grape Ripening
3.1. Non-Flavonoid Phenolics
3.2. Flavonoid Phenolics
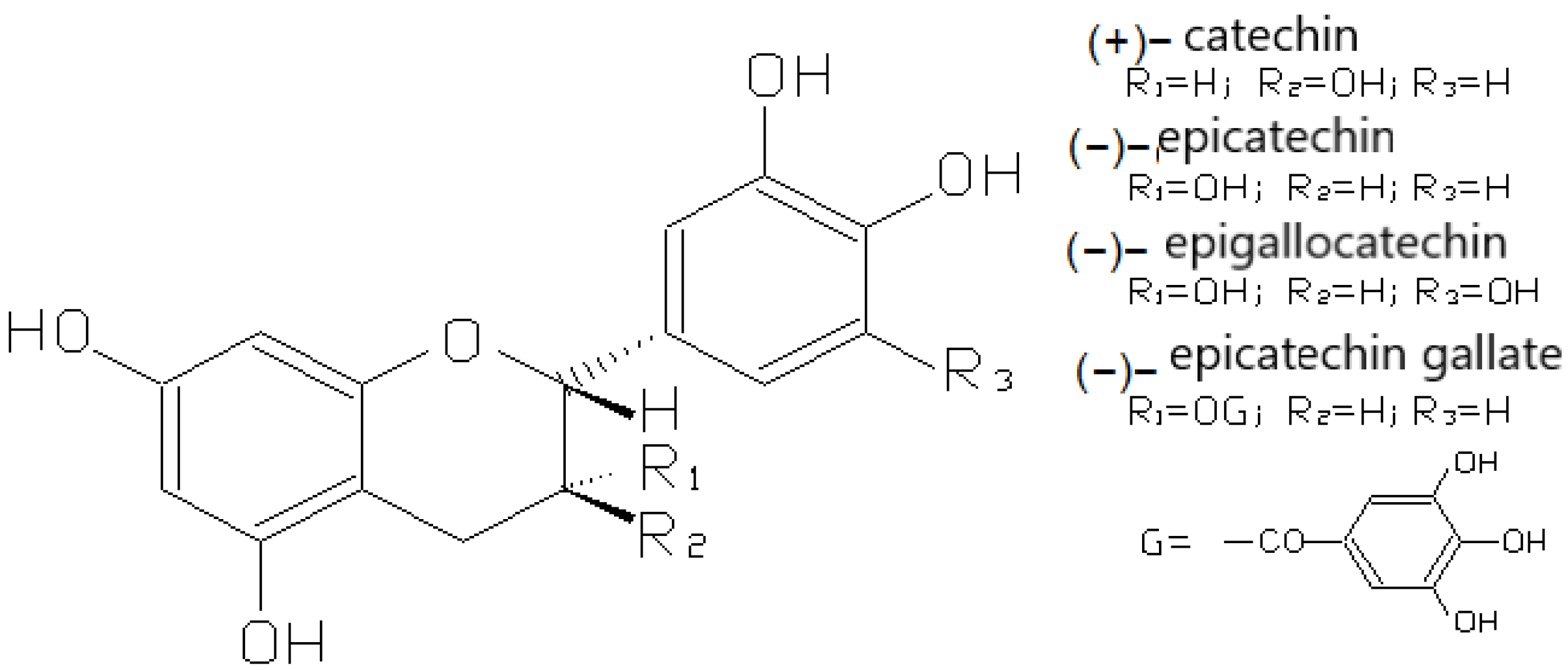
3.2.1. Flavan-3-ols
3.2.2. Proanthocyanidins
3.2.3. Flavonols
3.2.4. Anthocyanins
4. Phenolic Compounds and Wine Quality
4.1. Pigmentation and Co-Pigmentation
4.2. Influence of the Phenolic Compounds on the Wine Taste
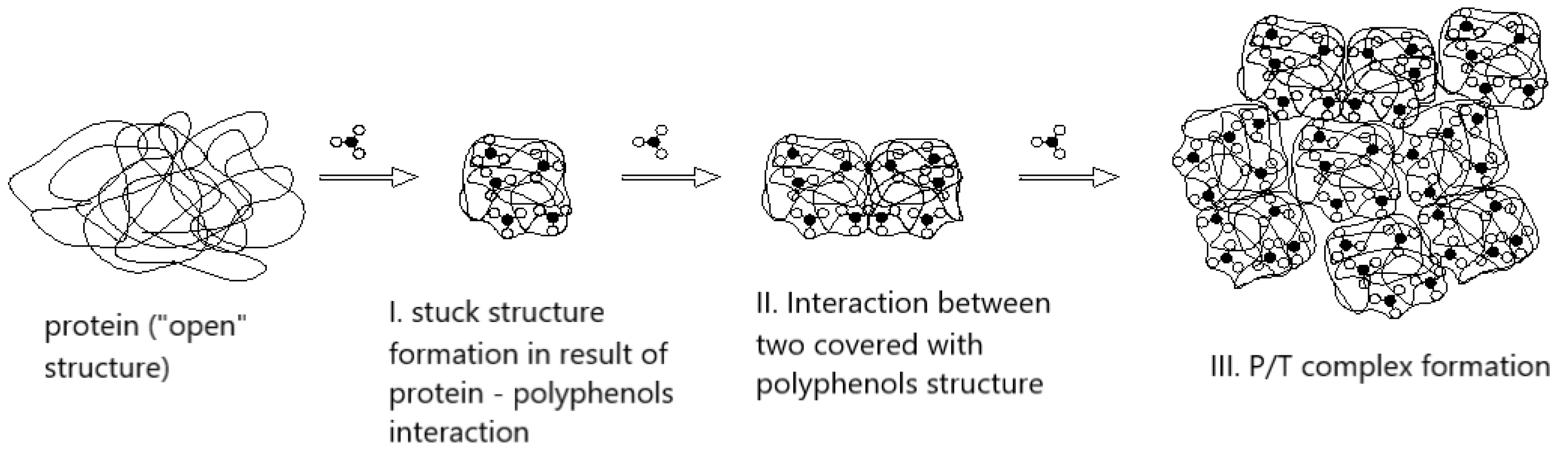
- 1.
- The initial interaction between phenolics and open, flexible protein structures, leading to the formation of compact protein–tannin complexes.
- 2.
- The further aggregation of these complexes through mutual binding.
- 3.
- The formation of larger cross-linked networks, ultimately leading to precipitation, which underlies the astringent mouthfeel.
5. Extractability of Grape Phenolic Compounds
- Sulfur dioxide additions [131],
- Grape berries texture and variety specific (discussed later).
6. Phenolic Compound Determination—Winemaking Aspects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.; Geros, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed]
- Buhman, K.; Aravena-Calvo, J.; Zaulich, C.R.; Hinz, K.; Laursen, T. Anthocyanic vacuolar inclusions: From biosynthesis to storage and possible applications. Front. Chem. 2022, 10, 913324. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.; Gattuso, G.; Nadavi, S. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Matsui, T. Polyphenol—Absorption and occurrence in the body system. Food Sci. Technol. Res. 2022, 28, 13–33. [Google Scholar] [CrossRef]
- Harbrone, J.B.; Williams, C.A. Advances in flavonoids research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Zagoskina, N.; Zubova, M.; Nechaeva, T.; Kazantseva, V.; Goncharuk, E.; Katanskaya, V.; Baranova, E.; Aksenova, M. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation, and practical applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Stress Physiology. In Plant Phisiology, 3th ed.; Sinauer Associates Publisher: Sunderland, MA, USA, 2003; p. 591. [Google Scholar]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Negi, A. Natural dyes and pigments: Sustainable applications and future scope. Sustain. Chem. 2025, 6, 23. [Google Scholar] [CrossRef]
- Songs, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Matti, G.B. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stress. Plants 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.C.; Ibanes Jara, M.A.; Rosillo, L.; Salinas, M.R. Effect of temperature and water availability on grape phenolic compounds and their extractability in Merlot grown in a worm area. Sci. Hortic. 2024, 337, 113475. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—A review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef]
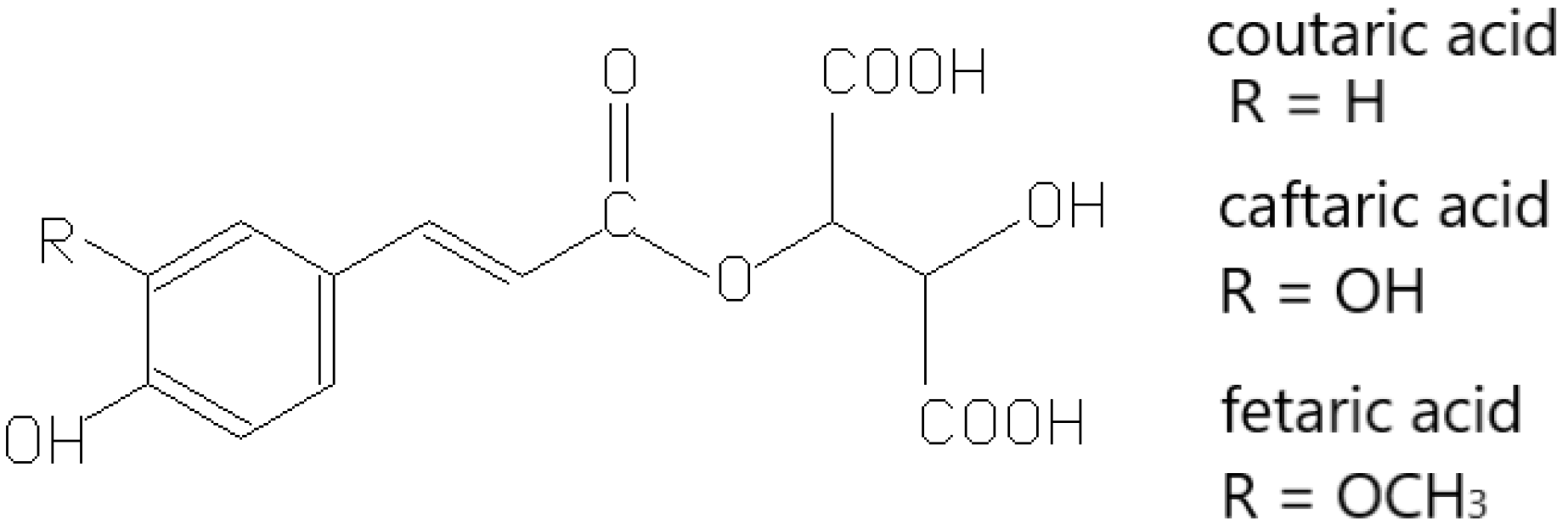
- Singleton, V.L.; Zaya, J.; Trousdale, E. Compositional changes in ripening grapes: Cafftaric and coutaric acids. Vitis 1986, 25, 107–117. [Google Scholar] [CrossRef]
- Goulao, L.; Fernandes, J.; Lopes, P.; Amancio, S. Tacking the cell wall of the grape berry. In The Biochemistry of the Grape Berry; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 172–193. [Google Scholar]
- Clifford, M. Chlorogenic acids and other cinamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Andabaka, Ž.; Tomaz, I.; Markovic, Z.; Stupic, D.; Maletic, E.; Kontic, J.; Preiner, D. Grapevine as a rich source of polyphenolic compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef]
- Buiarelli, F.; Coccioli, F.; Merolle, M.; Jasionawska, R.; Terracciano, A. Identification of hydroxycinnamic acid-tartaric acid esters in wine by HPLC—Tandem mass spectrometry. Food Chem. 2010, 123, 827–833. [Google Scholar] [CrossRef]
- Mitic, M.; Obradovic, M.; Grabovac, Z.; Pavlovic, A. Antioxidant Capacities and phenolic levels of different varieties of Serbian white wines. Molecules 2010, 15, 2016–2027. [Google Scholar] [CrossRef]
- Darias-Martin, J.; Martin-Luis, B.; Carrilo-Lopez, M.; Lamuela-Raventos, R.; Diaz-Romero, C.; Boulton, R. Effect of caffeic acid on the color of red wine. J. Agric. Food Chem. 2002, 50, 2062–2067. [Google Scholar] [CrossRef]
- Adrean, M.; Jeandet, P.; Breuil, A.; Levite, D.; Debord, S.; Bessis, R. Assay of resveratrol and derivative stilbenes in wines by direct injection high performance liquid chromatography. Am. J. Enol. Vitic. 2000, 51, 37–41. [Google Scholar] [CrossRef]
- Haygarov, V.; Yoncheva, T.; Dimitrov, D. Study of resveratrol content in grapes and wine of the varieties Storgozia, Kaylashki Rubin, Trapezitsa, Rubin, Bouquet and Pinot noir. J. Mt. Agric. Balk. 2017, 20, 300–311. [Google Scholar]
- Tintunen, S.; Lehtonen, P. Distinguishing organic wines from normal wines on the basis of concentration of phenolic compounds and spectral data. Eur. Food Res. Technol. 2001, 212, 90–394. [Google Scholar] [CrossRef]
- Jordão, A.; Ricardo-da-Silva, J.; Laureano, O. Evolution of catechins and oligomeric procyanidins during grape maturation of Castelão francês and Touriga Francesa. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar] [CrossRef]
- Mateus, N.; Marques, S.; Gonçalves, A.; Machado, J.; De Freitas, V. Proanthocyanidin composition of red Vitis Vinifera varieties from the Douro valley during ripening: Influence of cultivation altitude. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar] [CrossRef]
- Monagas, M.; Gomez-Cordoves, C.; Bartolome, B.; Laureano, O.; Ricardo da Silva, J. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. cv. Graciano, Tempranillo and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- Sun, B.; Pinto, T.; Leandro, M.; Ricardo-da-Silva, J.; Spranger, M. Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. Am. J. Enol. Vitic. 1999, 50, 179–183. [Google Scholar] [CrossRef]
- Kennedy, J.; Troup, G.; Pilbrow, J.; Hutton, D.; Hewitt, D.; Humter, C.; Ristic, R.; Iland, P.; Jones, G. Development of seed polyphenols in berries from Vitis vinifera cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Kennedy, J.; Hayasaka, Y.; Vidal, S.; Waters, E.; Jones, E. Composition of grape skin proanthocyanidins at different stage of berry development. J. Agric. Food Chem. 2001, 49, 5348–5355. [Google Scholar] [CrossRef]
- Blancquaert, E.; Oberholster, A.; Ricardo-da-Silva, J.; Deloire, A. Grape flavonoid evolution and composition under altered light and temperature conditions in Cabernet Sauvignone (Vitis vinifere L.). Front. Plant Sci. 2019, 8, 1062. [Google Scholar]
- De Freitas, V.; Glories, Y.; Monique, A. Development changes of procyanidins in grape of red Vitis vinifera varieties and their composition in respective wines. Am. J. Vitic. Enol. 2000, 51, 397–403. [Google Scholar] [CrossRef]
- Kennedy, J.; Matthews, M.; Waterhouse, A. Changes in grape seed polyphenols during fruit ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Jordão, A.; Ricardo-da-Silva, J.; Laureano, O. Evolution of proanthocyanidins in bunch stems during berry development (Vitis vinifera L.). Vitis 2001, 40, 17–22. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Ricardo-da-Silva, J.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Padilla-Gonzalez, G.; Grosskorf, E.; Sadgrove, N.; Simmonds, M. Chemical diversity of flavan-3-ols in grape seeds: Modulating factors and quality requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutonet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Souquet, J.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric procyanidins from grape skin. Phytochemistry 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional component of grape pomace: Their composition, biological properties and potential applications. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- De Freitas, V.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skins of white Vitis vinifera varieties. J. Sci. Food Agric. 1998, 79, 1601–1606. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Pena-Neira, A.; Lopez-Solis, R.; Zamora-Marin, F.; Ricardo-da-Silva, J.; Laureno, O. Comparative study of the phenolic composition of seeds and skins from Carmenere and Cabernet Sauvignon grape varieties (Vitis vinifera L.) during ripening. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef]
- Harbertson, J.; Kennedy, J.; Adams, D. Tannin in skins and seeds of Cabernet sauvignon, Syrah and Pinot noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- Stoyanov, N.; Mitev, P.; Galabova, M.; Tagareva, S. Phenolic compounds extractability from Melnik 55 grape solid parts during grape maturity. BIO Web Conf. 2023, 58, 01016. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Cardner, P.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.; Lister, C.; Matthews, D.; Maclean, M.; Lean, M.; et al. Relationshipamong antioxidant activity, vasodilation capacity and phenolic content of red wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- Kilmartin, P.; Zou, H.; Waterhouse, A. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Frankel, E.; Waterhouse, A.; Teissedre, P. Principal phenolic phitochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S. Wine phenolic compounds: Chemistry, functionality and health benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Orgiu, F.; Frigerio, G.; Regazzoni, L.; Sousa, L.; Bavaresco, L.; Bosso, A.; Aldini, G.; et al. Phenolic profile and antioxidant activity of different grape (Vitis vinifera L.) varieties. BIO Web Conf. 2019, 12, 04005. [Google Scholar] [CrossRef]
- Radoeva, R.; Yankova, I.; Enchev, B.; Karsheva, M.; Ivanova, E.; Iliev, I. Poluphenols of grape pomace from local Bulgarian variety Mavrud. Antioxidant and antitumor effect against breast cancer. J. Chem. Technol. Metall. 2022, 57, 508–521. [Google Scholar]
- Harbrone, J.; Williams, C. Anthocyanidins and other flavonoids. Nat. Prod. Rep. 2004, 4, 539–573. [Google Scholar]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.; Pan, Q.; Wang, J.; Reeves, M.; Duan, C. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Yoncheva, T.; Kostov, G.; Spasov, H. Content of total phenolic compounds, anthocyanins and spectral characteristics of Gamza red wines depending on the alcohol fermentation conditions. Bulg. J. Agric. Sci. 2023, 29, 159–170. [Google Scholar]
- George, F.; Figueiredo, P.; Toki, K.; Tatsuzawa, F.; Saito, N.; Brouillard, R. Influence of trans-cis isomerization of coumaric acid substituents on colour variance and stabilization in anthocyanins. Phitochemistry 2001, 57, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.; Hermosin-Gutierrez, I.; Piccardo, D.; Gomez-Alonso, S.; Gonzalez-Neves, G. Selectivity of pigments extraction from grapes and their partial retention in the pomace during red-winemaking. Food Chem. 2019, 277, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Malien-Aubert, C.; Dangles, O.; Amiot, M. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effect by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef]
- Burns, J.; Mullen, W.; Landrault, N.; Teissedre, P.; Lean, M.; Crozier, A. Variation in the profile and content of anthocyanins in wines made from Cabernet sauvignon and hybrid grapes. J. Agric. Food Chem. 2002, 50, 4096–4102. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in grape and grape products. Critecal Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Singleton, V.; Trousdale, E. Anthocyanin-tannin interaction expalaining differences in polymeric phenols between white and red wines. Am. J. Enol. Vitic. 1992, 43, 63–70. [Google Scholar] [CrossRef]
- Benmeziane, F.; Cadot, Y.; Djamai, R.; Djermoun, L. Determination of major anthocyanin pigments and flavonols in red grape skin of some table grape varieties (Vitis vinifer sp.) by high-performance liquid chromatography—Photodiode array detection (HPLC-DAD). Oeno One 2016, 50, 125–135. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ortega, A.; Lopez-Roca, J.; Fernandez, J.; Gomez-Plaza, E. Differences in anthocyanins extractability from grapes to wines according to variety. Am. J. Enol. Vitic. 2005, 56, 212–219. [Google Scholar] [CrossRef]
- Kennedy, J.; Matthews, M.; Waterhous, A. Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Vitic. 2002, 53, 268–274. [Google Scholar] [CrossRef]
- Roggero, J.; Coen, S.; Ragonnet, B. High performance liquid chromatography survey on changes in pigment content in ripening prapes of Syrah. An approach to anthocyanin metabolism. Am. J. Enol. Vitic. 1986, 37, 77–83. [Google Scholar] [CrossRef]
- Gonzalez-San Jose, M.; Barron, L.; Diez, C. Evolution of anthocyanins during maturation of Tempranillo grape variety (Vitis vinifera) using polynominal regression models. J. Sci. Food Agric. 1990, 51, 337–343. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordão, A.; Mendes-Faia, A. Anthocyanins profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine region. J. Int. Sci. Vigne Du Vin 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grape. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar] [CrossRef]
- Yan, Y.; Song, C.; Falginella, L.; Castellarin, S. Day temperature has a stronger effect than night temperature on anthocyanin and flavonol accumulation in Merlot (Vitis vinifera L.) grape during ripening. Front. Plant Sci. 2020, 11, 1095. [Google Scholar] [CrossRef]
- Martinez-Moreno, A.; Perez-Alvarez, E.; Lopez-Urrea, R.; Paladinez-Quezada, D.; Moreno-Olivares, J.; Intrigliolo, D.; Gil-Munoz, R. Effect of deficit irrigation with saline water on wine color and polyphenolic composition of Vitis vinifera L. cv. Monastrell. Sci. Hortic. 2021, 283, 110085. [Google Scholar] [CrossRef]
- Theocharis, S.; Nikolau, N.; Zioziou, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Koundouras, S. Effect of post-veraison irrigation on the phenolic composition of Vitis vinifera L., cv. Xinomavaro grapes. Oeno One 2021, 55, 173–189. [Google Scholar] [CrossRef]
- Torres, N.; Martinez-Luscher, J.; Porte, E.; Yu, R.; Kurtural, S. Impact of leaf removal and shoot thinning on cumulative daily light intensity and thermal time and their cascading effect of grapevine (Vitis vinifera L.) berry and wine chemistry in warm climates. Food Chem. 2021, 343, 128447. [Google Scholar] [CrossRef]
- Saucier, C.; Little, D.; Glories, Y. First evidence of acetaldehyde-flavonol condensation products in red wine. Am. J. Enol. Vitic. 1997, 48, 370–373. [Google Scholar] [CrossRef]
- Somers, T. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Somers, T. Interactions of color composition in young red wines. Vitis 1978, 17, 161–167. [Google Scholar]
- Somers, T.; Evans, M. Evolution of red wines. An assessment of the role of acetaldehyde. Vitis 1986, 25, 31–39. [Google Scholar]
- Cheng, S.; Wu, T.; Gao, J.; Han, X.; Huang, W.; You, Y.; Zhan, J. Color myth: Anthocyanins reactions and enological approaches achieving their stabilization in the aging process of red wine. Food Inov. Adv. 2023, 2, 255–271. [Google Scholar] [CrossRef]
- Rio Segade, S.; Torchio, F.; Giacosa, S.; Aimonino, D.; Gay, P.; Lambri, M.; Dordoni, R.; Gerbi, V.; Rolle, L. Impact os several pre-treatments on the extraction of phenolic compounds in winegrape varieties with different anthocyanin profiles and skin mechanical properties. J. Agric. Food Chem. 2014, 62, 8437–8451. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Singleton, V. Effects of seed tannins on enzymatic decolorization of wine pigment in the presence of oxidizable phenols. Am. J. Enol. Vitic. 2001, 52, 93–100. [Google Scholar] [CrossRef]
- Gil-Munoz, R.; Gomez-Plaza, E.; Martinez, A.; Lopez-Roca, J.M. Evolution of phenolic compounds during wine fermentation and post-fermentation: Influence of grape temperature. J. Food Compos. Anal. 1999, 12, 259–272. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Yeast interaction with anthocyanins during red wine fermentation. Am. J. Enol. Vitic. 2005, 56, 104–109. [Google Scholar] [CrossRef]
- Wenzel, K. Die selection einer hefemutante zur verminderung der farstoffverluste wahreng der rotweingarung. Vitis 1989, 28, 111–120. [Google Scholar]
- Escott, C.; Morata, A.; Zamora, F.; Loira, I.; Manuel del Fresno, J.; Saurez-Lepe, J. Study of the interaction of anthocyanins with phenolic aldehydes in a model wine solution. ACS Omega 2018, 3, 15575–15581. [Google Scholar] [CrossRef]
- Marquez, A.; Serratosa, M.; Merida, J. Pyranoanthocyanin derived pigments in wine: Structure and formation during winemaking. J. Chem. 2013, 2013, 713028. [Google Scholar] [CrossRef]
- Sheridan, M.; Elias, R. Reaction of acetaldehyde with wine flavonoids in the presence of sulfur dioxide. J. Agric. Food Chem. 2016, 3, 8615–8624. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Cai, Y.; Haslam, E. Polyphenol interactions. Anthocyanins: Copigmentation and colour changes in red wines. J. Sci. Food Agric. 1992, 59, 299–305. [Google Scholar] [CrossRef]
- Asen, S.; Stewart, R.; Norris, K. Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1145. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, Y.; Chen, Z.; Wen, Z.; Wei, F.; Zheng, Q.; Wang, C.; Xiao, X. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Davies, A.; Mazza, G. Copigmentation of simple and acylated anthocyanins with colorless phenolic compounds. J. Agric. Food Chem. 1993, 41, 716–720. [Google Scholar] [CrossRef]
- Mazza, G.; Brouillard, R. The mechanism of co-pigmentation of anthocyanins in aqueous solution. Phytochemistry 1990, 29, 1097–1102. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical reviews. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar] [CrossRef]
- Haslam, E. In vino veritas. Oligomeric procyanidins and the ageing of red wines. Phytochemistry 1980, 19, 2577–2582. [Google Scholar] [CrossRef]
- Chen, L.; Hrazdina, G. Structural properties of anthocyanins-flavonoid complex formation and its role in plant copor. Phytochemistry 1981, 20, 297–302. [Google Scholar] [CrossRef]
- Mirabel, M.; Saucier, C.; Guerra, C.; Glorie, Y. Copigmentation in model wine solutions: Occurrence and relation to wine ageing. Am. J. Enol. Vitic. 1999, 50, 211–218. [Google Scholar] [CrossRef]
- Baranac, J.; Petranovic, N.; Dimitric-Marcovic, J. Spectrophotometric study of anthocyanin copigmentation reactions. J. Agric. Food Chem. 1996, 44, 1333–1336. [Google Scholar] [CrossRef]
- Markovic, J.; Petranovic, N.; Baranac, J. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acid. J. Agric. Food Chem. 2000, 48, 5530–5536. [Google Scholar] [CrossRef] [PubMed]
- Baranac, J.; Petranovic, N.; Dimitric-Marcovic, J. Spectrophotometric study of anthocyanin copigmentation reactions 2 Malvin and the nonglycosidez flavone quercetin. J. Agric. Food Chem. 1997, 45, 1694–1697. [Google Scholar] [CrossRef]
- De Beer, D.; Harbertson, J.; Kilmartin, P.; Roginsky, V.; Barsukova, T.; Adams, D.; Waterhouse, A. Phenolics: A comparison of diverse analytical methods. Am. J. Enol. Vitic. 2004, 55, 389–400. [Google Scholar] [CrossRef]
- Sacchi, K.; Bisson, L.; Adams, D. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Lempereur, V.; Blateyron-Pic, L.; Labarde, B.; Saucier, C.; Kelebek, H.; Glories, Y. Groupe national de travail sur les tanins oenologiques: Premier resultats. Rev. Fr. DOenologique 2002, 196, 23–29. [Google Scholar]
- Naves, A.; Spranger, M.; Zhao, Y.; Leandro, M.; Sun, B. Effect of addition of commercial grape seed tannins on phenolic composition, chromatic characteristics and antioxidant activity of red wine. J. Agric. Food Chem. 2010, 58, 11775–11782. [Google Scholar] [CrossRef]
- Arnold, R.; Noble, A. Bitternes and astringencyof grape seed phenolics in a model wine solution. Am. J. Enol. Vitic. 1978, 29, 150–152. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different type of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Smith, A.; June, H.; Nobble, A. Effects of viscosity on the bitterness and astringency of grape seed tannin. Food Qual. Prefer. 1996, 7, 161–166. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A. Bitterness and astringency of flavan-3-ol monomers, dimmers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Baxter, N.; Lilley, T.; Haslam, E.; Williamson, M. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef]
- Luck, G.; Liao, H.; Murray, N.; Grimmer, H.; Warminski, E.; Williamson, M.; Lilley, T.; Haslam, E. Polyphenols astringency and prolin-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Gheynier, V.; Moutonet, M. Interaction of grape seed tannins with salivary proteins. J. Agric. Food Chem. 1999, 47, 42–47. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Nephelometric study of salivary protein-tannin aggregates. J. Sci. Food Agric. 2001, 82, 113–119. [Google Scholar] [CrossRef]
- Ricardo-da-Silva, J.; Cheynier, V.; Souquet, J.M.; Moutonet, M.; Cabanis, J.C.; Bourzeix, M. Interaction of grape seed procyanidins with various proteins in relation to wine finning. J. Sci. Food Agric. 1991, 57, 111–125. [Google Scholar] [CrossRef]
- Maury, G.; Sarni-Manchado, P.; Lefebvre, S.; Cheynier, V.; Moutonet, M. Influence of fining with different molecular weight gelatin on procyanidin composition and precipitation of wines. Am. J. Enol. Vitic. 2001, 52, 140–145. [Google Scholar] [CrossRef]
- Jőbstl, E.; O’Connell, J.; Fairclough, P.; Williamson, M. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef]
- Hagerman, A.; Butler, L. The scecificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 259, 4494–4497. [Google Scholar] [CrossRef]
- Oh, H.; Hoff, J.; Armstrong, G.; Haff, L. Hydrophobic interaction in tannin-protein complex. J. Agric. Food Chem. 1980, 28, 394–398. [Google Scholar] [CrossRef]
- Artz, W.; Bishop, P.; Dunker, K.; Schanus, E.; Swanson, B. Interaction of synthetic proanthocyanidin dimmer and trimer with bovine serum albumin and purified bean globulin fraction G-1. J. Agric. Food Chem. 1987, 35, 417–421. [Google Scholar] [CrossRef]
- Kawamoto, H.; Nakatsudo, F.; Murakani, K. Stoichometric studies of tannin-protein coprecipitation. Phytochemistry 1996, 41, 1427–1431. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Deleris, A.; Avallone, A.; Cheynier, V.; Moutonet, M. Analysis and characterization of wine condensed tannins precipitated by proteins used as fining agent in Enology. Am. J. Enol. Vitic. 1999, 50, 81–86. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Singleton, V. Interactive precipitation between phenolic fractions and peptides in wine-like model solutions:turbidity, partical size and residual content as influenced by pH, temperature and peptide concentration. Am. J. Enol. Vitic. 1995, 46, 329–338. [Google Scholar] [CrossRef]
- Asquith, T.; Uhlig, J.; Mehansho, H.; Putman, L.; Carlson, D.; Butler, L. Binding of condensed tannins to salivary prolin-rich glycoproteins: The role of carbohydrate. J. Agric. Food Chem. 1987, 35, 331–334. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef]
- Harbertson, J.; Picciotto, E.; Adams, D. Measurement of pigments in grape berry extracts and wines using protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-Da-Silva, J.; Laureano, O. Tannin profiles of Vitis vinifera L. cv. Red grapes growing in Lisboa and from their monovaraietal wines. Food Chem. 2009, 112, 197–204. [Google Scholar] [CrossRef]
- Wimalasiri, P.; Olejar, K.; Harrison, R.; Hider, R.; Tian, B. Whole bunch fermentation and the use of grape stems: Effect on phenolic and volatile aroma composition of Vitis vinifera cv. Pinot noir wine. Aust. J. Grape Wine Res. 2021, 28, 395–406. [Google Scholar] [CrossRef]
- Kennedy, J.; Robinson, S.; Walker, M. Grape and Wine Tannins: Production, Perfection, Perception; Practical Winery and Vineyard: San Rafael, CA, USA, 2007; pp. 57–67. [Google Scholar]
- Kovac, V.; Alonso, E.; Revilla, E. The effect of adding supplementary quantity of seeds during fermentation on the phenolic composition of wines. Am. J. Enol. Vitic. 1995, 46, 363–367. [Google Scholar] [CrossRef]
- Lee, J.; Kennedy, J.; Devlin, C.; Redhead, M.; Rennaker, C. Effect of early seed removal during fermentation on proanthocyanidin extraction in red wine: A commercial production example. Food Chem. 2008, 107, 1270–1273. [Google Scholar] [CrossRef]
- Thorngate, J.; Singleton, V. Localization of procyanidins in grape seeds. Am. J. Enol. Vitic. 1994, 45, 259–262. [Google Scholar] [CrossRef]
- Gillispie, E.; Miller, K.; McElrone, A.; Block, D.; Rippner, D. Red wine fermentation alters grape seed morphology and internal porosity. Am. J. Enol. Vitic. 2023, 74, 0740030. [Google Scholar] [CrossRef]
- Girard, B.; Kopp, T.; Reynolds, A.; Gliff, M. Influence of vinification treatments on aroma constituents and sensory descriptors of Pinot noir wines. Am. J. Enol. Vitic. 1997, 48, 198–206. [Google Scholar] [CrossRef]
- Reynolds, A.; Cliff, M.; Girard, B.; Kopp, T. Influence of fermentation temperature on composition and sensory properties of Semillion and Shiraz wines. Am. J. Enol. Vitic. 2001, 52, 235–240. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Bellworthy, S.; Garcia-Viguera, C.; Reader, H.; Watkins, S. Effect of sulfur dioxide and must extraction on colour, phenolic composition and sensory quality of red table wine. J. Sci. Food Agric. 1998, 78, 297–307. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Ferrandino, A.; Guidoni, S. Influence of wine-grape skin hardness on the kinetics of anthocyanin extraction. Int. J. Food Prop. 2012, 15, 249–261. [Google Scholar] [CrossRef][Green Version]
- Budić-Leto, I.; Lovric, T.; Pezo, I.; Kljusuric, J. Study of dynamics of polyphenol extraction during traditional and advanced maceration processes of the Babic grape variety. Food Technol. Biotechnol. 2005, 43, 47–53. [Google Scholar]
- Gomez-Plaza, E.; Gil-Munoz, R.; Lopez-Roca, J.; Martinez-Cutillas, A.; Fernandez, J. Phenolic compounds and color stability of red wines: Effect of skin maceration time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Sims, C.; Baters, R. Effect of skin fermentation time on the phenols, anthocyanins, ellagic acid sediment, and sensory characteristics of a red vitis rotundifolia wine. Am. J. Enol. Vitic. 1994, 45, 56–62. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Sato, M.; Ueno, N.; Singleton, V. Colour and sensory characteristics of Merlot red wines caused by prolonged pomace contact. J. Wine Res. 2001, 11, 7–18. [Google Scholar] [CrossRef]
- Scudamore-Smith, P.; Hooper, R.; McLaren, E. Color and phenolic changes of Cabernet sauvignon wine made by simultaneous yeast/bacterial fermentation and extended pomace contact. Am. J. Enol. Vitic. 1990, 41, 57–67. [Google Scholar] [CrossRef]
- Stoyanov, N.; Mitev, P.; Tagareva, S.; Kemilev, S. Influence of the process of cold maceration and non-Saccharomyces yeast application in red winemaking. J. Mt. Agric. Balk. 2017, 20, 79–94. [Google Scholar]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A. Changes in anthocyanins and color characteristics of Pinot noir wines during different vinification processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Xia, N.; Liu, A.; Qi, M.; Zhang, H.; Huang, Y.; He, F.; Duan, C.; Pan, Q. Enhancing the color and astringency of red wines through white grape seeds addition: Repurposing wine production byproducts. Food Chem. 2024, 23, 101700. [Google Scholar] [CrossRef]
- Zimman, A.; Waterhouse, A. Incorporation of malvidiv-3-glucoside into high molecular weight polyphenols during fermentation and wine ageing. Am. Juornal Enol. Vitic. 2004, 55, 139–146. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Zeppa, G.; Gerbi, V. Anthocyanin extractability assessment of grape skins by texture analysis. J. Int. Sci. Vigne Du Vin 2008, 42, 157–162. [Google Scholar]
- Giacosa, S.; Ferrero, L.; Paissoni, M.A.; Rio Segade, S.; Gerbi, V.; Rolle, L. Grape skin anthocyanin extraction from red varieties during simulated maceration: Influence of grape seeds and pigments adsorption on their surface. Food Chem. 2023, 424, 136463. [Google Scholar] [CrossRef]
- Mateus, N.; Pinto, R.; Ruao, P.; De Freitas, V. Influence of the addition of grape seed procyanidins to Port wines in the resulting reactivity with human salivary proteins. Food Chem. 2004, 84, 195–200. [Google Scholar] [CrossRef]
- Barnavon, L.; Doco, T.; Terrier, T.; Ageorges, A.; Romieu, C.; Pellerin, P. Involvement of pectin methyl-esterase during the ripening of grape berries: Partial cDNA isolation, transcript expression and changes in the degree of methyl-esterification of cell wall pectins. Phytochemistry 2001, 58, 693–701. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-Garcia, J.M.; Bautista-Ortin, A.B.; Lopez-Roca, J.M.; Gomez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vonifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio FZeppa, G.; Gerbi, V. Relationship between skin break force and anthocyanin extractability at different ripening stage. Am. J. Enol. Vitic. 2009, 60, 93–97. [Google Scholar] [CrossRef]
- Boulet, J.; Vernhet, A.; Poncet-Legrand, C.; Cheynier, V.; Doco, T. Exploring the role of grape cell wall and yeast polysaccharides in the extraction and stabilization of anthocyanins and tannins in red wines. OENO One 2024, 58, 7793. [Google Scholar]
- Leszczuk, A.; Zajac, A.; Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Investigation of changes in the arabinogalactan proteins (AGPs) structure, size and composition during the fruit ripening process. Sci. Rep. 2020, 10, 20621. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.; Haeger, A.; Verhoef, R.; Schols, H.; McCleary, B.; McKee, L. Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 2009, 59, 413–425. [Google Scholar] [CrossRef]
- Garrido-Banuelos, G.; Buica, A.; Du Toit, W. Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grape and red wines. A current state-of-art review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7743–7759. [Google Scholar] [CrossRef]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2020, 58, 9–21. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Lee, E.; Nomura, N.; Patil, B.; Yoo, K. Measurement of total phenolic content in wine using an automatic Folin-Ciocalteu assay method. Int. J. Food Sci. Technol. 2014, 49, 2364–2372. [Google Scholar] [CrossRef]
- Somers, T.; Evans, M. Spectral evaluation of young red wines: Anthocyanin equilibria, total phenolics, free and molecular SO2, “chemical age”. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- Kafkas, N.; Kosar, M.; Oz, A.; Mitchell, A. Advanced analytical methods for phenolics in fruits. J. Food Qual. 2018, 2018, 3836064. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Mattivi, F.; Waterhouse, A. Analysis of red wine phenolics: Comparison of HPLC and spectrophotometric methods. Vitis 2001, 40, 87–91. [Google Scholar]
- Labarde, B.; Cheynier, V.; Brossaud, F.; Souquet, J.M.; Moutounet, M. Quantitative fractionation of grape proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1999, 47, 2719–2723. [Google Scholar] [CrossRef]
- Souquet, J.M.; Labarde, B.; Le Guerneve, C.; Cheynie, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Hagerman, A.; Rice, M.; Richard, N. Mecchanism of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16 (4→8) catechin (procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Fragoso, S.; Acena, L.; Guasch, J.; Mesters, M.; Busto, O. Quantification of phenolic compouds during red winemaking using FT-NIR spectroscopy and PLS-regression. J. Agric. Food Chem. 2011, 59, 10795–10802. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Lorrain, B.; Giacosa, S.; Rio Segade, S.; Cagnasso, E.; Gerbi, V.; Teissedre, P. Rapis methods for the evaluation of total phenol content and extractability in intact grape seeds of Cabernet sauvignon: Instrumental mechanical properties and FT_NIR spectrum. J. Int. Sci. Vigne Du Vin 2012, 46, 29–40. [Google Scholar]
- Garcia-Hernandez, C.; Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M. Analysis of red wines using an electronic tongue and infrared spectroscopy. Correlations with phenolic content and color parameters. LWT-Food Sci. Technol. 2020, 118, p108785. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Aleixandre, J.; Du Toi, W. Chemometric compositional analysis of phenolic compounds in fermenting samples and wine using different infrared spectroscopy techniques. Talanta 2018, 176, 526–536. [Google Scholar] [CrossRef]
- Di Egidio, V.; Sinelli, N.; Giovanelli, C.; Moles, A.; Casiraghi, E. NIR and MIR spectroscopy as rapid methods to monitor red wine fermentation. Eur. Food Res. Technol. 2010, 230, 947–955. [Google Scholar] [CrossRef]
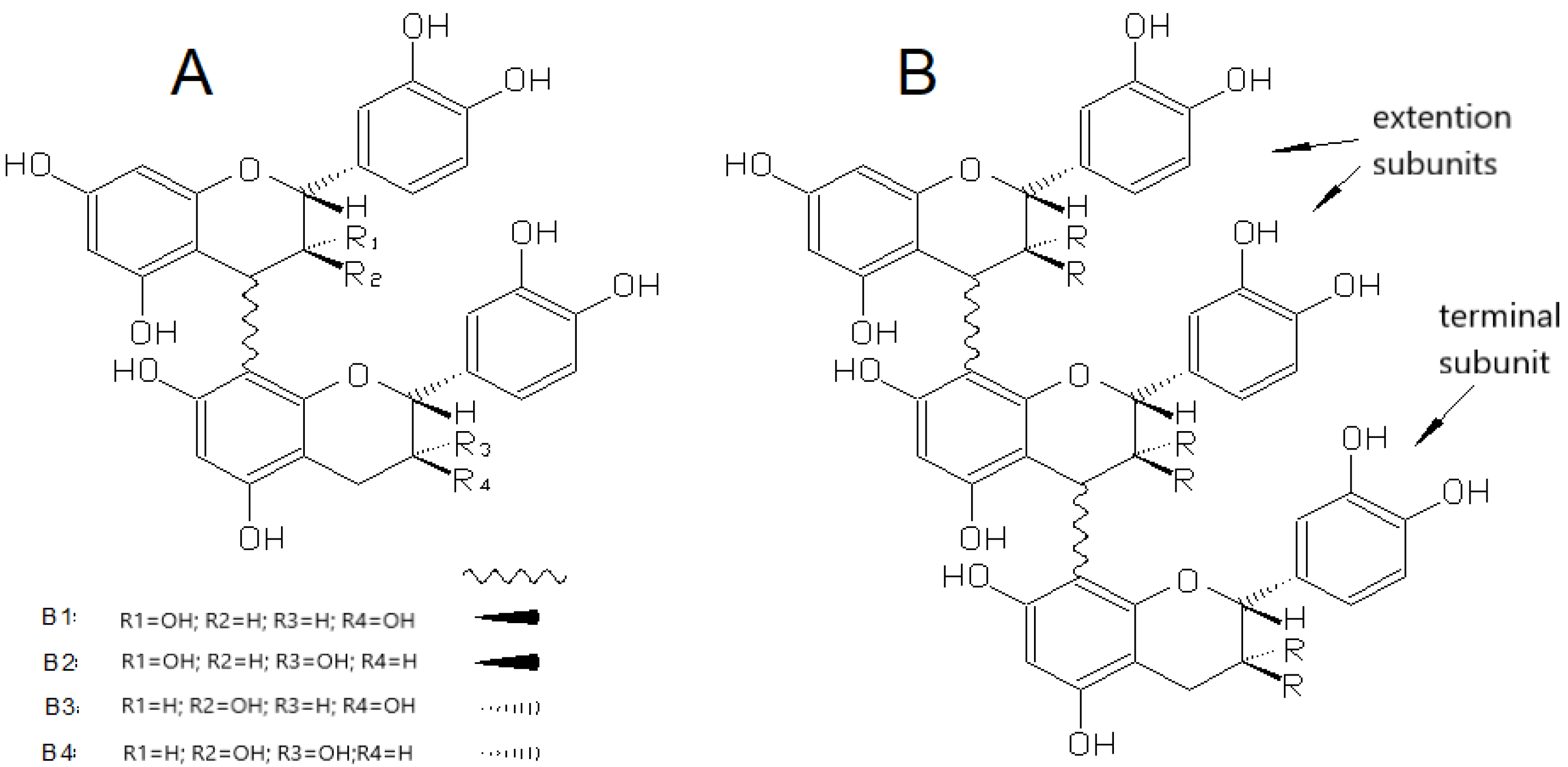

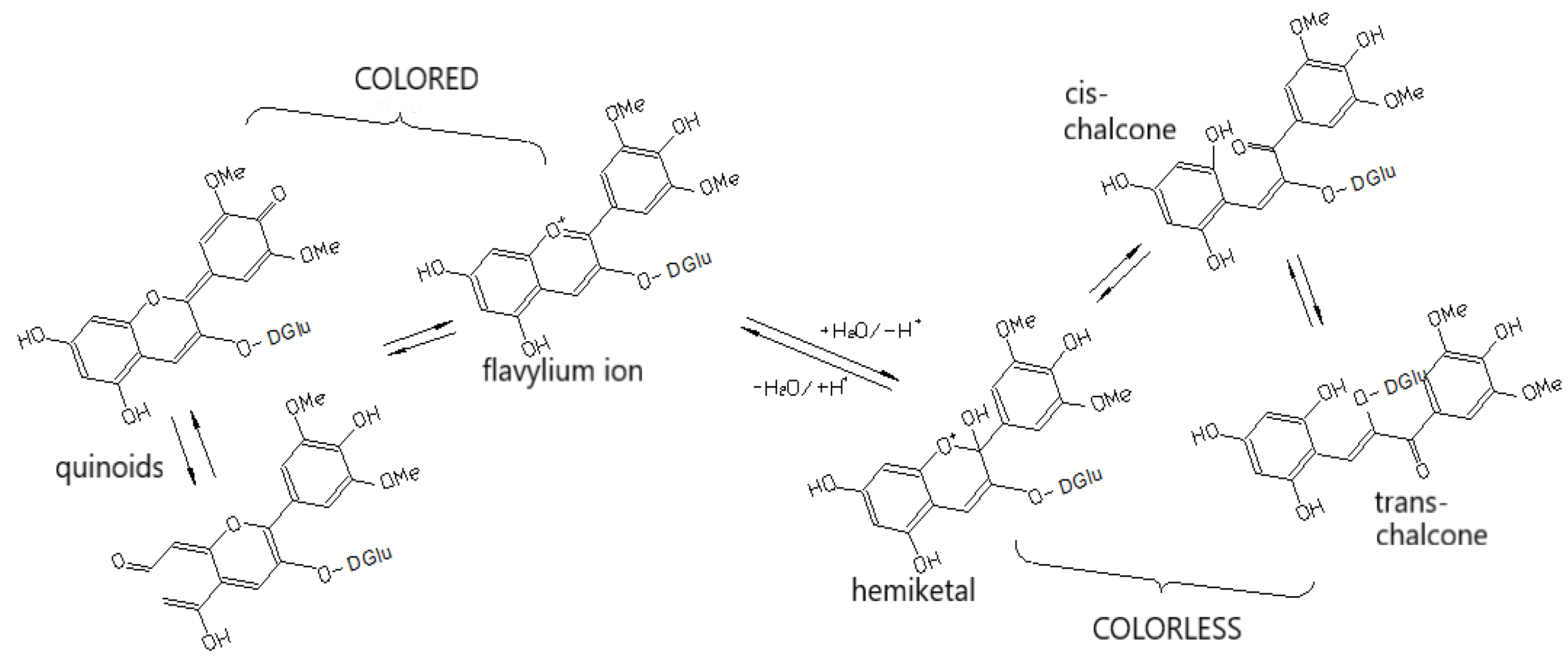
| Phenolic Compounds | Where They are Concentrated | Taste in General | What Happens with Them During Maturation |
|---|---|---|---|
| Hydroxycinnamic acids | Grape pulp and skins | Slightly bitter; mild acidity; more important for oxidation and other processes and less directly related to the taste | Relatively stable during maturation |
| Flavan-3-ols | In grape solid parts; more than 50% are in the seeds | Bitter and astringent when present in higher concentration | Reduced in all solid parts, or the more taste-active epicatechin-gallate reduces by 3 to 7 folds |
| Procyanidins | In grape solid parts; especially rich in grape seeds | Stronger astringency which increases with increasing participation of epicatechin-gallate (degree of galoyllation—DG) | Dimmers from solid parts reduce slightly during ripening; Seed procyanidins—DG in extension units decreases; the degree of polymerization (mDP) decreases Skin procyanidins—mDP increases |
| Flavonols | Predominantly in grape skins; also found in the stems and leaves | Minimal bitterness and astringency contribution; more active as antioxidant | More affected by sunlight exposure |
| Anthocyanins | Grape skins | Slightly affect wine taste and mainly in forms combined with other phenolics | Increase during maturation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanov, N.; Tagareva, S.; Yoncheva, T.; Shopska, V.; Kostov, G. Significance of Grape Phenolic Compounds for Wine Characteristics: Dynamics and Extractability During Fruit Maturation. Beverages 2025, 11, 163. https://doi.org/10.3390/beverages11060163
Stoyanov N, Tagareva S, Yoncheva T, Shopska V, Kostov G. Significance of Grape Phenolic Compounds for Wine Characteristics: Dynamics and Extractability During Fruit Maturation. Beverages. 2025; 11(6):163. https://doi.org/10.3390/beverages11060163
Chicago/Turabian StyleStoyanov, Nikolay, Silviya Tagareva, Tatyana Yoncheva, Vesela Shopska, and Georgi Kostov. 2025. "Significance of Grape Phenolic Compounds for Wine Characteristics: Dynamics and Extractability During Fruit Maturation" Beverages 11, no. 6: 163. https://doi.org/10.3390/beverages11060163
APA StyleStoyanov, N., Tagareva, S., Yoncheva, T., Shopska, V., & Kostov, G. (2025). Significance of Grape Phenolic Compounds for Wine Characteristics: Dynamics and Extractability During Fruit Maturation. Beverages, 11(6), 163. https://doi.org/10.3390/beverages11060163






