Abstract
The present study investigates the hypothesis that green plantain peel (GPP) could be used as a functional ingredient to enrich fermented milk, thus improving its nutritional profile and bioactive content. The objective of the present study was to develop a fermented milk product with added GPP and to evaluate its physical–chemical, technological, microbiological, and sensory characteristics, as well as the bioaccessibility of bioactive compounds after in vitro digestion (INFOGEST). The methodological strategy involved the formulation of four treatments: one control (Fermented Milk Control, FMC) and three with different concentrations of cooked plantain peel (5%—FM5, 10%—FM10, and 20%—FM20). The results demonstrated that the incorporation of peel had a significant impact on the technological properties, resulting in increased syneresis and color change. In contrast, pH and acidity were more influenced by storage duration. Lactic acid bacteria demonstrated viability at probiotic concentrations (≥106 CFU/mL) for a duration of up to 11 days. The incorporation of GPP resulted in a substantial increase in the phenolic compound content and antioxidant activity of the product, with the FM20 treatment showing the highest antioxidant activity (DPPH: 1555 ± 16 µmol TE/mL, ABTS: 576 ± 29 µmol TE/mL, FRAP: 2427 ± 58 µmol Fe2+/mL) compared to FMC. Sensory analysis revealed that texture and color were the most influential attributes, with formulations FM5 and FM10 being the most accepted, as indicated by an acceptability index above 82%. The simulated in vitro digestion led to an increase in the measurable phenolic content and a corresponding enhancement of antioxidant activity. This suggests that the digestive process enhances the release of these compounds from the food matrix, thereby increasing their bioaccessibility.
1. Introduction
Plantain (Musa spp.) is among the most popular crops, especially in tropical and sub-tropical zones [1]. Musa paradisiaca, the plantain variety, is considered one of the largest in this group, with fruits weighing up to 500 g each [2]. In Brazil, the Northeast and North regions have the most extensive plantations of plantains, particularly the states of Alagoas and Bahia [3]. Plantains contain substantial starch content (around 20–30%), which supports their suitability for specific processing approaches, are less sweet, and often require cooking, enabling them to be utilized in various savory dishes through frying, boiling, or baking [4].
According to the United Nations Environment Programme [5], in 2022, approximately 1.05 billion tons of food waste (including inedible parts) were generated globally, totaling 132 kg per capita—nearly one-fifth of all food available to consumers. Bananas are among the fruits that experience the most significant post-harvest losses, and the peel is often discarded due to cultural preferences. The peel (a by-product) accounts for approximately 40% of the total weight of the plantain. These rejected plantains and peels are used as animal feed and, to a limited extent, in the production of chips, flakes, and powders- products of lower value [6,7]. In this context, one approach to reducing waste is to utilize bananas fully [8]. This by-product has been used as part of vegetarian and vegan diets as a meat substitute. However, its application can go beyond this, such as in the production of fermented beverages, which can enhance aroma, flavor, and nutritional value [9].
Therefore, the peel, which is usually discarded, has the potential to serve as a nutrient source in fermentation and as a functional ingredient to enrich foods. This is due to its high content of total sugar (35%), starch (12.8%), protein (8.6%) [1], dietary fiber (43–49%), polyunsaturated fatty acids (linoleic acid and α-linolenic acid), pectin, essential amino acids (leucine, valine, phenylalanine and tyrosine), micronutrients (K, P, Ca and Mg) and bioactive compounds such as phenolic compounds. Incorporating these components into food products can minimize waste while improving nutritional profiles. It can also enhance the quality of the resulting product [10].
Furthermore, using fruit peels in food production helps to reduce waste, promotes sustainability, and fosters innovation, while supporting the circular economy and aligning with the UN’s Sustainable Development Goals (SDGs). Therefore, developing fermented milk enriched with bioactive compounds from green plantain peel, a by-product typically discarded during plantain chip production, could be an innovative product with high phenolic content [11].
Fermented milk can be produced using traditional starter cultures such as Lactobacillus acidophilus and Streptococcus thermophilus, which may incorporate supplemental probiotic strains from Bifidobacterium spp. and Lactobacillus spp. to yield a functional dairy product known as probiotic fermented milk [12]. According to Food and Agriculture Organization of the United Nations [13], probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”. These probiotics have been added as supplementary fermentative microorganisms in dairy production due to their demonstrated advantages in enhancing texture, flavor profiles, nutritional value, and health benefits, such as modulation of gastrointestinal health, as well as functional properties [12,14].
To understand the true functional potential of fermented beverages in the body, it is essential to quantify the content of phenolic compounds and antioxidant activity after in vitro digestion. INFOGEST (International Network of Excellence on the Fate of Food in the Gastrointestinal Tract) is a widely used in vitro digestion technique in research on the digestibility and bioaccessibility of nutrients and bioactive compounds in various foods, including dairy products [15]. The objective of this study was to develop fermented milk with added plantain peel and evaluate its physical, chemical, and technological characteristics, as well as microbiological viability during storage. The beverage was also evaluated for its nutritional composition and bioactive compounds, as well as the viability of phenolic compounds and antioxidant activity after in vitro digestion.
2. Materials and Methods
2.1. Regents and Materials
The DPPH (2,2-diphenyl-1-picryl-hydrazine-hydrate), gallic acid standard, Folin–Ciocalteu’s phenol reagent, and ABTS (2,2′azino-bis (ethylbenzthiazoline-6-sulfonic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used in the experiments, including ethanol, potassium persulfate, sodium carbonate, ferric chloride, NaOH, and HCl, were of analytical grade. Deionized water was used throughout. De Man, Rogosa and Sharpe agar (MRS, Oxoid, Hampshire, UK) was employed to enumerating Lactobacillus.
The green plantain peel (GPP) was donated by T.A. Alimentos Bananas Chips, a company based in Cuiabá, Mato Grosso, Brazil, that produces plantain chips. Other materials, including Ultra High Temperature (UHT) whole milk (sterilized and standardized milk with 3% of fat) (Piracanjuba, Brazil), crystal sugar, and starter culture for fermented milk (Bio Rich®), were purchased from local shops in Cuiabá, Brazil.
2.2. Preparation of Plantain Peel
Fresh green plantain peel samples (Figure 1) at the initial stage of ripening, subgroup 2-light green of Von Loesecke ripeness scale [16], were selected, washed in running water, and sanitized by immersion in a solution of 1% sodium hypochlorite (NaClO) for 15 min [17]. After this process, the peels were cut into approximately 4 cm2 pieces, ground in a blender, and cooked in drinking water (60% w/w). They were then maintained at a temperature above 80 °C for 10 min. The resulting by-product was then cooled to below 60 °C and stored at −18 °C in 50 g disposable plastic containers until fermentation.

Figure 1.
Images of green plantain peels after decoking and fermented milk added with decoction plantain peel.
2.3. Fermentation Process
Four fermented milk treatments were prepared, with three genuine replicates each. The fermented milk (FM) was produced using UHT milk, starter culture (Bio Rich®) with Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium cultures, crystal sugar (8%), and different concentrations of decocted GPP (5%—FM5; 10%—FM10; 20%—FM20; and a control treatment—FMC). Fermentation was carried out at 40 °C in a water bath for 5 h. After fermentation, the added treatments of plantain peel were filtered through a stainless-steel sieve to remove the coarse pieces of peel (retention of around 80 ± 5% of peel). The samples were stored in sealed plastic containers (150 mL) for 21 days until they were analyzed. Measurements of pH, acidity, water retention capacity, and syneresis were carried out in triplicate for each treatment on days 1, 4, 8, 14, and 21 of storage.
2.4. Physical Chemical Evaluation (pH, Acidity)
The pH of the fermented milk was determined using a digital pH meter (MS Tecnopon, MPA 210, Piracicaba, Brazil) on 10 g of samples, calibrated with pH 7 and 4 buffer solutions. Titratable acidity was determined by titration with a 0.1 M sodium hydroxide solution (NaOH) using a pH meter, up to a pH of 8.0–8.3. The results were expressed as a percentage (%) v/w, equivalent to lactic acid.
2.5. Syneresis and Water Retention Capacity (WRC)
The evaluation of syneresis and water retention capacity (WRC) was conducted according to the method described by Ahmed et al. [18] during storage. Syneresis was defined as the percentage of whey weight relative to the original weight of the fermented milk sample, Equation (1). At the same time, WRC was calculated as the percentage of the drained gel (precipitate) weight to the original sample weight, Equation (2).
2.6. Texture
The texture of the fermented milk was determined in terms of the following parameters: firmness, consistency, and cohesiveness as described by Meena et al. [19], with modifications. The fermented milk was bottled and stored in sealed plastic jars with lids at refrigerator temperatures (2–5 °C) until testing. The analysis was performed using the TA-XTplus Stable Micro System (Piracicaba, Brazil), with the following settings: pre-test speed of 1 mm/s; test speed of 1 mm/s; post-test speed of 5 mm/s; analysis distance of 10 mm an acrylic cylinder probe with a diameter of 20 mm; trigger force of 10 g; sample volume of 50 mL; and temperature of 4 ± 2 °C. Three texture measurements were taken for each treatment on day 1 and day 21 of storage.
2.7. Color
The instrumental color parameters were directly read in a colorimeter (Hunterlab, Colorquest, São Paulo, Brazil), in the CIE L*a*b* system (L* a* b*): luminosity (L*), and chromaticity coordinates a* (−100 (green) to +100 (red)) and b* (−100 (blue) to +100 (yellow)). The values of L*, a*, and b* were used to calculate total color difference (∆E*), chroma (C*) and Hue angle (H°), using Equations (3), (4) and (5), respectively. Three texture measurements were taken for each treatment, on days 1 and 21 of storage.
2.8. Viability of Lactic Acid Bacteria (LAB) During Storage
For LAB analysis, 1 mL of fermented milk was diluted with 9 mL of sterile peptone water (0.1 g · 100 mL−1) to create a 10−1 dilution, followed by serial dilutions with peptone water (1:10) up to 10−7. Then, 1 mL of each dilution was placed on Man, Rogosa and Sharpe (MRS) agar, and the plates were incubated under anaerobic conditions at 37 °C for 48 h. The analysis results were obtained as logarithms of the number of colony-forming units mL−1 of fermented milk (log CFU mL−1). The analysis was carried out in duplicate on days 1, 11, and 21 of storage of fermented milk.
2.9. Proximal Composition
The moisture content (AOAC 925.45b) was determined using an oven (SOLAB, SL-102, Piracicaba, Brazil) at 105 °C. Total nitrogen was quantified by the micro-Kjeldahl method (N × 6.25) (AOAC 960.52) in a nitrogen distiller (Tecnal TE-0364, Piracicaba, Brazil). Ash content (AOAC 923.03) was determined by incineration in a muffle furnace (Magnus, 200 F, São Vicente, Brazil). All procedures followed the AOAC guidelines [20]. The lipid content was assessed according to the Bligh-Dyer methodology [21]. The sample was homogenized with a mixture of chloroform, methanol, and water (1:2:0.8, v/v/v). After phase separation, the lower layer (chloroform), containing the lipids, was collected and the solvent evaporated. Total lipid content was determined gravimetrically.
The Total Carbohydrate content was estimated by difference Equation (6). All analyses were performed in triplicate. The total energy value of the formulated products was calculated using 4 kcal·g−1 for proteins and carbohydrates, and 9 kcal·g−1 for lipids.
2.10. Total Phenolic Compounds
Total phenolic compounds contents were determined by using the Folin–Ciocalteu method [22]. The absorbance was measured at 765 nm using a spectrophotometer (Biochrom, Libra S32, Cambridge, UK) and was analyzed in quadruplicate. Gallic acid was used as the standard for a calibration curve, and the results were expressed as mg of gallic acid equivalents per dry weight of fermented milk (mg GAE g−1).
2.11. Antioxidant Activity
The antioxidant capacity was determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay, according to the method of Brand-Williams et al. [23]. Briefly, 100 µL of the sample was added to 3.9 mL of a 0.06 mM DPPH solution in light-protected tubes. After a 30 min incubation period, the absorbance was read at 517 nm using a spectrophotometer (Biochrom, Libra S32, Cambridge, UK). The antioxidant activity was calculated based on the standard Trolox curve, and the results were expressed in antioxidant activity equivalent to Trolox (μM Trolox g−1 sample).
Antioxidant capacity based on the method of ABTS radical [2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)] reduction was made as described by Re et al. [24]. The radical ABTS was formed by reacting ABTS at 7 mM and potassium persulfate at 140 mM. In tubes, 30 μL of the sample was transferred, and 3 mL of the ABTS radical solution was added. After 6 min, the absorbance at 734 nm (Biochrom, Libra S32, Cambridge, UK) was analyzed in quadruplicate. The results were expressed as μmol Trolox equivalent (TE) g−1 dry sample.
The Ferric Reducing Antioxidant Power (FRAP), the ability of antioxidants to reduce ferric ion to ferrous ion, was estimated according to the methodology reported by Pulido et al. [25]. In tubes, 90 μL of samples were transferred 270 μL of distilled water, 2.7 mL reagent FRAP (the reagent was prepared extemporaneously by mixing: (A) 300 mM acetate buffer (pH 3.6); (B) a 10 mM solution of TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine) in 40 mM HCl; and (C) a 20 mM solution of iron (III) chloride. Solutions A, B, and C were combined in a 10:1:1 (v/v/v) ratio, respectively. The samples were homogenized in a tube shaker (Phoenix-AP56, São Paulo, Brazil) and then incubated in a water bath (572-Fisatam, São Paulo, Brazil) at 37 °C. After 30 min, the absorbance at 595 nm (Biochrom, Libra S32, Cambridge, UK) was analyzed in quadruplicate. The results were expressed as μmol ferrous sulfate equivalent g−1 dry sample.
2.12. Microbiological Analysis
The analysis to assess the microbiological safety of the fermented milk was carried out before sensory tests. Salmonella/25 mL [26], Escherichia coli mL−1 [27] and Molds and Yeasts mL−1 [28] were analyzed. The results were compared with the Brazilian standards established by Normative Instruction—NI No. 161, of 1 July 2022 [29].
2.13. Sensory Analysis
The sensory analysis of the fermented milk involved affective acceptance tests using a 9-point hedonic scale, followed by a purchase intention test using a 5-point hedonic scale. The volunteer tasters (n = 114) were individuals aged between 18 and 60 years, of both sexes (62% female, 38% male). The tests were conducted in the Sensory Analysis Laboratory at FANUT/UFMT, in isolated booths with white lighting, after signing a consent form. During the test, participants received product formulations in portions of approximately 15 mL at a temperature of 4 ± 2 °C, coded with three-digit numbers, along with a glass of water. They were instructed to drink the water between samples. The attributes evaluated included overall acceptability, taste, texture and appearance. The following formula was used to calculate the product’s Acceptability Index (AI): AI (%) = (A/B) × 100, where A is the average score obtained for the product, and B is the maximum score possible. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Federal University of Mato Grosso (protocol code 6.768.397 on 16 April 2024).
2.14. In Vitro Digestibility (INFOGEST)
The fermented beverages (FMC and FM10) were subjected to in vitro gastrointestinal digestion in accordance with the INFOGEST 2.0 method [30]. The methodology consists of static simulation of the salivary, gastric, and intestinal phases of the human digestive system. Initially, in the salivary phase, 5 mL of the fermented beverage was diluted in 4 mL of FSS, adding 0.75 mL of α-amylase solution (75 U/mL, Sigma-Aldrich, St. Louis, MO, USA), 25 µL of 0.3 M CaCl2, and 0.225 mL of H2O. Subsequently, the pH of the mixture was adjusted to 7 with 5 M NaOH, following which the mixture was incubated for 2 min at 37 °C and 90 rpm. Subsequently, the salivary phase (10 mL) was diluted in 8 mL of FGS. The following substances are required for the experiment: 0.67 mL of pepsin solution (2000 U/mL, P7000, Sigma-Aldrich, St. Louis, MO, USA), 5 µL of 0.3 M CaCl2, and 0.45 mL of H2O. The pH of the gastric phase was adjusted to 3 by the addition of 5 M HCl.
The mixture was then subjected to an incubation process for a period of two hours at a temperature of 37 °C and a rotational speed of 90 rpm. Finally, the gastric mixture (20 mL) was diluted in 8 mL of FIS and 5 mL of pancreatin solution (100 U mL−1, P3292, Sigma-Aldrich, St. Louis, MO, USA), 3 mL of bile salt solution (10 mM, B8756, Sigma-Aldrich, St. Louis, MO, USA), 40 µL of 0.3 M CaCl2, and 3.16 mL of H2O. The pH of the intestinal phase was adjusted to 7 with 5 M NaOH and the mixture was then incubated for 2 h at 37 °C and 90 rpm. Following the conclusion of in vitro digestion, samples were collected and evaluated for phenolic content and antioxidant activity.
2.15. Statistical Evaluation
XLSTAT software (2025 version) was used for statistical evaluation. The data was evaluated for normality (Levene test), followed by ANOVA, regression and principal component analysis (PCA) to evaluate the effect of fermentation type, plantain peel concentration and storage time. The PCA was constructed based on Pearson’s coefficient. The mean comparison test used was Tukey (p < 0.05).
3. Results and Discussion
3.1. pH, Acidity, Syneresis and Water Retention Capacity
Over the 21-day storage period, there was a significant decrease (p < 0.0001) (Table 1) in pH (from 4.65 to 4.40) across all fermented milk samples (Figure 2A). This decline was due to lactic acid production by the LAB during refrigerated storage. A lower pH is crucial for the microbiological safety of the product, as a pH below 4.5 inhibits the growth of pathogenic microorganisms [31]. Similarly, Feitosa et al. [17], observed a gradual and significant reduction (p < 0.05) in pH values over 21 days in yogurts sweetened with Apis mellifera L. honey, indicating comparable physicochemical stability.

Table 1.
ANOVA table for pH, acidity, syneresis and WRC during storage of fermented milk added plantain peel.
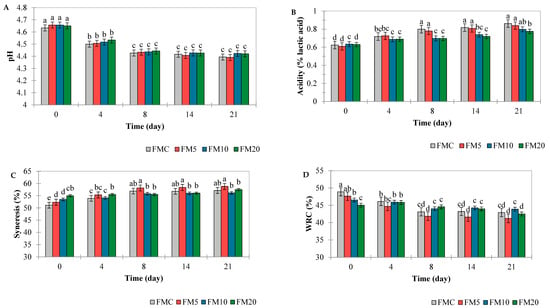
Figure 2.
Ph (A), acidity (B), syneresis (C) and water retention capacity (WRC) (D) of FMC: fermented milk control treatment, FM5—fermented milk with 5% plantain peel added, FM10—fermented milk with 10% plantain peel added, and FM20—fermented milk with 20% plantain peel added. Different letters (a, b, c, d) indicate a statistical difference (Tukey test, p < 0.05) between all means over time.
The acidity values ranged from 0.6% on the first day to 0.9% on the 21st day of storage for all samples, a significant increase over time (Figure 2B). Therefore, all fermented milk samples fell within the acidity range of 0.6% and 1.5%, as recommended by Brazilian Normative Instruction No. 1170 of 26 August 2024 [32]. The acidity values behaved inversely to the pH values, that is, they increased during storage (p < 0.001) (Table 1). This result aligns with the literature, since a decrease in pH implies an increase in acidity, due to the rise in organic acids in the beverage. This pattern was also demonstrated by de Morais Silva et al. [33], who evaluated yogurt with a natural colorant made from anthocyanins extracted from Melinis minutiflora P. Beauv. The decrease in pH during storage is related to lactose consumption and the production of lactic acid, driven by the metabolic activity of lactic acid bacteria.
The syneresis of the fermented milk was influenced by the addition of plantain peel and storage time (p < 0.001) (Table 1), with treatments FM10 and FM20 showing the highest values on the first day of storage, 53% and 55%, respectively (Figure 2C). However, at the end of the 21 days, FM10 and FM20 and the control showed results statistically equal (57%) (p > 0.05) (Figure 2C). In the study of Wang et al. [34], the authors found that the addition of apple pulp, which contains a significant amount of insoluble dietary fiber, disrupts the continuity of the gel structure and increases syneresis.
In addition, the increase in syneresis of most dairy products during storage is due to changes in the gel network, which is produced by attractive forces between the casein particles. These changes can lead to additional intermolecular bonds, and consequently, the spontaneous release of water from the gel, which results in gel contraction [35]. Therefore, the addition of plantain peel increased the syneresis of the fermented milks, possibly due to the presence of dietary fibers. Additionally, syneresis is promoted by changes in temperature, increased acidity, pH, and mechanical factors. At the end of the storage time, the pH of the fermented milks supplemented with plantain peel was similar to that of the control product (p > 0.05) (Figure 2A), indicating that the fermented milks remained stable during storage did not undergo phase separation in the products.
The water retention capacity (WRC) of the fermented milk was influenced by two factors: the addition of plantain peel and storage time, as well as the interaction between these factors (p< 0.05) (Table 1). The addition of plantain peel reduced WRC from 49 to 44%, while storage time reduced WRC by 6–12% for all treatments, statistically significant (Figure 2D). These results align with the data obtained in the evaluation of syneresis in fermented beverages, as the increase in syneresis is a result of the reduction in the casein gel’s capacity to hold water molecules within its structure. Similar results to our study were found by de Morais Silva et al. [33] in yogurt added with fatty grass extract (Melinis minutiflora P. Beauv.), in which it was found that the addition of the plant extract reduced the WRC of the yogurt. This fact was attributed to the lower pH of the enriched yogurt. It should be noted here that the reduction in WRC is associated with both the reduction in pH during storage and the presence of fibers from the plantain peel, which disrupts the continuity of the casein gel.
3.2. Texture and Color
In evaluating the texture parameters of the fermented milks, it was observed that the addition of plantain peel significantly altered the firmness of the product only when the addition was 20% (Table 2).

Table 2.
Instrumental texture of control fermented milk and fermented milk with plantain peel added during storage.
The firmness of the fermented milk with 20% of plantain peel was the lowest, compared to the other treatments and the control. As for the other parameters evaluated (cohesiveness, consistency, and viscosity index), the addition of plantain peel did not present a significant influence. The values found for the cohesiveness of fermented milk were similar to those found in the study by Meena et al. [19], (control equal to −6.09 g), which characterizes the product as having a smooth structure. Studies that added banana fiber, plantain peel fiber, and plantain puree to fermented milk beverages observed an improvement in texture and viscosity parameters, mainly due to the high dietary fiber content and its interaction with the milk protein matrix during fermentation [36,37]. The method of preparing the fermented milk with the addition of plantain peel likely contributed to maintaining the product’s characteristics close to those of the control, as part of the cooked peel was retained during the filtering stage. Therefore, the amount of fiber added to the product was not sufficient to change the texture.
On the other hand, with storage time, there was an increase in firmness, consistency, and viscosity index in all treatments. This increase in firmness during storage is in line with the results found for syneresis and WRC, where the increase in acidity of the fermented beverage during storage alters the structure of the gel, leading to shrinkage and, as a result, an increase in gel strength is observed [19]. Storage time did not change the cohesiveness of fermented milk. Meena et al. [19] also did not observe any change in the cohesiveness of yogurt during storage.
In the evaluation of the color parameters, it was observed that the higher the concentration of plantain peel, the lower the luminosity (L*). This occurs because the plantain peel has a high content of dark pigments [38]. Chroma (C*) expresses the saturation or intensity of the color, while the hue angle (H°) indicates the observable color [39]. Thus, the fermented milks presented a very pale greenish-yellow coloration (H° of 160–170) (C* < 10), with the FM10 and FM20 samples tending to have a similar pale greenish-yellow coloration (Table 3).

Table 3.
Color parameters of control fermented milk and fermented milk with plantain peel added during storage.
Color variation (∆E*) (Table 3) represents an estimate of the change in color of the samples over storage time with the control sample serving as a reference for comparation. Thus, when compared to the color of the first day of all treatments, samples FM10 and FM20 showed a significant difference (∆E > 4) [40], indicating a noticeable change in color variation. Storage time did not affect the color (p > 0.05), indicating a product with a stable color throughout storage. In the multivariate analysis, it was observed that over the course of storage time (factor 1, 57.75%), there was a reduction in pH, WRC, and L, and an increase in syneresis, consistency, firmness, and viscosity index (Figure 3A). In addition, there is a separation of samples by storage time (on the right: samples on day 21 of storage; on the left: samples on day 1). Furthermore, the FMC and FM5 formulations are grouped (bottom) (Figure 3B), as are the FM10 and FM20 formulations (top).
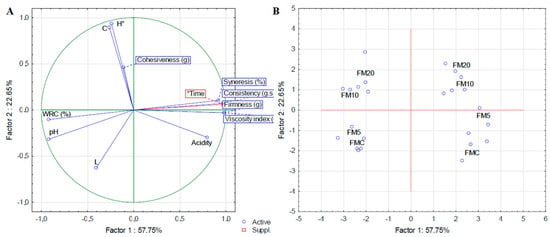
Figure 3.
Principal component analysis of the physical chemical, texture and color characteristics of fermented milk added with green plantain peel. (A): Biplot for variables and (B): Biplot for cases.
3.3. Viability of LAB and Microbiological Safety
For counting lactic acid bacteria (LAB), it can be observed that on the first day of storage, all fermented milk formulations with added plantain peels presented a count in the order of 108 CFU mL−1 (Table 4). According to literature, fruit peels are rich in dietary fibers and bioactive compounds that act as prebiotics, providing nutrients that promote the growth and survival of lactobacilli and other probiotic bacteria during storage [41,42].

Table 4.
Lactic acid bacteria (LAB) count (CFU mL−1) of fermented milk with plantain peel.
During refrigerated storage, FM10 consistently shows higher LAB counts compared to FM5 and FM20. This pattern is explained by the interplay between nutrient availability and potential inhibitory effects of banana peel components [43]. Despite phenolic and flavonoid compounds in plantain peel exhibiting antibacterial activity against several pathogenic bacteria [44], there is no direct evidence that these compounds have an effect against beneficial bacteria at low doses in fermented milk [45,46].
Safdari et al. [37] found that adding plantain fiber and plantain peel fiber to symbiotic yogurt made from camel milk improves probiotic bacteria survival. Similar results are described by Sah et al. [41], who supplemented fermented milk with pineapple peels, and found that the supplementation maintains or even increases the viable counts of lactobacilli and other probiotic cultures throughout storage, resulting in higher probiotic counts compared to control samples without peel addition.
To be considered a probiotic, fermented milk should contain at least 6 log CFU mL−1 (1,000,000 colony-forming units per milliliter) of viable lactobacilli at the time of consumption. Many products aim for higher counts, such as 7–9 log CFU mL−1, to ensure sufficient probiotic levels throughout storage [47,48]. Thus, the fermented milk with plantain peel may be considered probiotic until the eleventh day of storage (Table 4). As fermentation progresses and during storage, lactic acid and other organic acids accumulate, resulting in a lower pH. This increasingly acidic environment can stress or kill lactobacilli, reducing their viability over time [47,49]. Additionally, over time, the available nutrients in the milk decrease, limiting the resources necessary for lactobacilli survival and growth [49]. These factors justify the reduction in viability of LAB on the twenty-first day of storage.
3.4. Fermented Milk Composition
Table 5 presents the data on the proximal composition of cooked plantain peel (light green, Von Loesecke stage 2), and of fermented milks with added plantain peel and control sample. Regarding the ash content of plantain peel, the value found was 1.48%, close to that found in the study by Neris et al. [50] (1.73%) for raw plantain peel. The main minerals found in plantain peel were described by Uzairu and Kano [51], who reported that plantain peels have considerable levels of Ca (176.30 ± 8.77 mg/100 g), Na (47.37 ± 5.82 mg/100 g), K (787.70 ± 6.20 mg/100 g), Mg (81.60 ± 0.12 mg/100 g), and Fe (40.95 ± 15.61 mg/100 g). The protein content in decocted plantain peel was 6.22 g/100 g, a value close to that reported by Happi Emaga et al. [52] (8–11%). The authors also highlighted that no significant variation was found in the composition of plantain varieties. Additionally, leucine, valine, phenylalanine, and threonine were essential amino acids in significant quantities [52].

Table 5.
Proximal composition of decocted plantain peel and fermented milk with added plantain peel.
The content of lipids was 0.34 g 100 g−1 of decocted plantain peel. Happi Emaga et al. [52] highlight that the lipid content from plantain peel is rich in polyunsaturated fatty acids, particularly linoleic acid and α-linolenic acid. The lipid content of the fermented milk remained unchanged regardless of the amount of plantain peel added to the product. On the other hand, the addition of 10% and 20% plantain peel to the fermented milk had a dilution effect on the product, resulting in an increase in moisture content and a reduction in protein content. The addition of 5% did not significantly influence the final composition of the fermented milk.
3.5. Total Phenolic Compounds and Antioxidant Activity
Regarding Total Phenolic Compounds (TPC) and antioxidant activity, it was observed that there was a gradual and linear increase in the treatments added with green plantain peel. This result can be attributed to the presence of bioactive compounds in the plantain peel added to the fermented milk (Table 6).

Table 6.
Total phenolic compounds (TPC) and antioxidant activity (DPPH, ABTS and FRAP) per mL of fermented milk with added plantain peel.
Plantain peels contain substantial amounts of phytochemicals (alkaloids (3.53 ± 0.64 g/100 g), flavonoids (0.16 ± 0.05 g/100 g), tannins (2.18 ± 0.63 g/100 g), and terpenoids (1.88 ± 0.24 g/100 g) in unripe plantain peels) [51]. Additionally, according to the literature, plantain peels are rich sources of phenolics, potentially contributing to their health benefits, which differ from those of dessert bananas and pure plantains. The phenolic compounds presented in plantain peels are predominantly composed of flavonol glycosides, with rutin being the most abundant [53]. The presence of these phytochemical compounds explains the high TPC values found in the peel, as well as its antioxidant potential measured by DPPH and ABTS radicals. On the other hand, the extract of plantain peel does not have a good capacity to oxidize metal ions, such as Fe, since no antioxidant power was detected in the FRAP method (Table 6). This result is due to the fact that plantain peel is rich in minerals such as Zn and Cu, which are negatively correlated with FRAP results [54].
The considerable increase in antioxidant activity observed in fermented milk (approximately 3 times in FM10 and 5 times in FM20) (Table 6) is due to the phytochemicals present in the plantain peel, and to the release of phytochemicals by microorganisms during the fermentation process. Lactic acid bacteria (LAB) play a key role in increasing the phenolic content and antioxidant activity of fermented milk products with added fruit parts. LAB achieved this through the biotransformation of fruit-derived phenolics and by enhancing the release and bioaccessibility of antioxidant compounds during fermentation.
LAB can break down complex phenolic compounds in fruit parts into simpler, more bioactive forms. This process increases the total phenolic content and changes the phenolic profile, often resulting in higher levels of specific phenolic acids and flavonoids with potent antioxidant properties [55,56]. Also, LAB fermentation can disrupt the plant cell walls and protein-phenolic complexes, releasing bound phenolics into the milk matrix. This increases the availability of phenolic compounds and enhances their bioaccessibility [57].
Additionally, microorganisms that produce proteases, including certain lactic acid bacteria, could break down milk proteins, resulting in the formation of peptides with antioxidant properties [58]. A study that examined the production of bioactive peptides following milk fermentation with kefir grains identified 49 peptide types before fermentation. In contrast, after fermentation, this number increased to 624, which likely contributes to the enhanced antioxidant properties of the fermented products. As a result, the rise in phenolic content directly boosts the antioxidant activity of the fermented product. LAB fermentation has been shown to significantly enhance free radical scavenging abilities (measured by DPPH, ABTS, and FRAP assays) in milk and fruit-based beverages [55,57].
3.6. Sensorial Evaluation
In the assessment of microbiological safety, all tests were within the standards recommended by legislation (Salmonella-absence/25 mL, E. coli < 10 CFU mL−1 and Molds and Yeasts < 10 CFU mL−1), therefore the products were considered microbiologically safe for sensory analysis.
There were 114 participants in the sensory acceptance test, comprising 62% females and 38% males. Among the judges, at least 52.94% reported consuming yogurt or fermented milk at least once a week, 44.11% reported consuming fermented milk less than once a week, and only 2.94% stated they did not consume fermented milk. In the evaluation of sensory attributes (Overall Evaluation, Flavor, Texture, Aroma and Appearance) by the tasters, using the 9-point hedonic scale, only the FM20 treatment received average scores below 7.0 and had the lowest Acceptability Index (AI) (72.1%) (Table 7). Additionally, FM20 showed the most significant difference from the control across all acceptance test attributes. Conversely, the FM5 and FM10 treatments were statistically equivalent in terms of taste, texture, and aroma, with both treatments also matching the control in terms of aroma. Regarding appearance, FM5 was more acceptable than FM10 and FM20 and were statistically like the control. Therefore, based on the AI, the fermented milks with 5 and 10% plantain peel addition were better accepted by consumers, with scores of 82.2% and 82.5%, respectively, compared to FM20.

Table 7.
Result of the sensorial acceptance test of the control fermented milk and the milk fermented with plantain peel.
The relationship between the sensory attributes (taste, texture, and appearance) and the physicochemical parameters (pH, acidity, color, and instrumental texture) in the multivariate analysis indicates that the factors with the most significant influence on sample acceptance were texture parameters (firmness and consistency), pH, acidity and brightness (color). Factor 1, which explains 51.31% of the response variability, divides the samples into two groups: the positive areas are represented by FMC and FM5, while the negative areas are represented by FM10 and FM20 formulations (Figure 4). Factor 2, accounting for 15.52% of the variability, places the FM20 sample in the positive area, with higher a* values (more towards green). This indicates that the rind color was a key factor influencing the preference for the other samples over the one with 20% plantain peel (Figure 4). Nonetheless, it is essential to note that all the products were accepted by more than 70%, which is the minimum threshold approving of a new product [59].
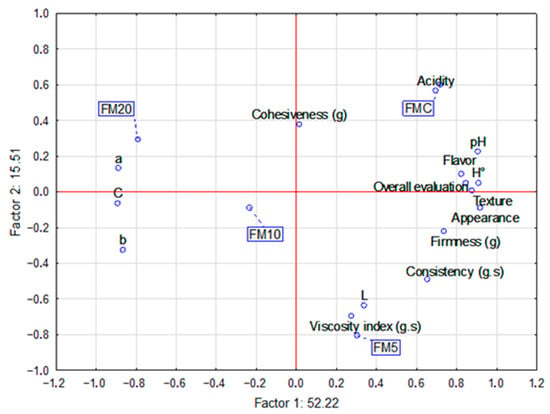
Figure 4.
Multifactorial analysis relating to the sensory acceptance (flavor, texture and appearance) of the control fermented milk and the fermented milks added with plantain co-product with physical–chemical parameters (color, instrumental texture, acidity and pH).
Regarding purchase intention, the developed fermented milk demonstrates high potential for commercialization, with 45% of tasters indicating they would “certainly buy” and an additional 35% suggesting they would “probably buy”. Only 5% of the tasters stated they would “certainly not buy” the fermented milk with added plantain peel (Figure 5).
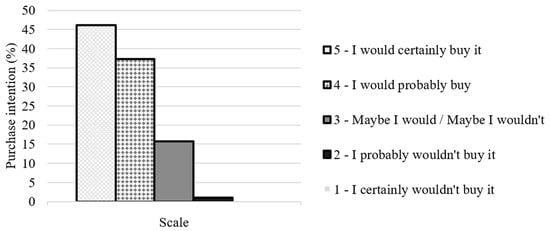
Figure 5.
Intention to purchase fermented milk with added banana peel from participants in the sensory analysis.
3.7. Bioaccessibility of Phenolic Compounds and Antioxidant Activity After In Vitro Digestion (INFOGEST)
The total phenolic compounds and antioxidant capacity of the control fermented milk samples and those with 10% plantain peel (the sample with the best sensorial evaluation) were tested after in vitro digestion according to the INFOGEST protocol. The antioxidant capacity was measured by the ability to “deactivate” a free radical (DPPH) and reduce a metal ion (ferric ion) (FRAP). The amount of TPC in the FMC sample increased by 43% after in vitro digestion. However, there was no statistical difference between the digested (Infg-SD-FMC) and undigested (Infg-SND-FMC) FMC samples (Figure 6). Digestion increased the amount of TPC in FM10 by around 28% (Infg-SD-FM10) compared to the in-nature sample (FM10). This increase may be due to the breakdown of bonds between phenolic compounds and proteins/carbohydrates, which increases the fraction that can be detected [60]. Additionally, the fermented milk matrix can protect phenolic compounds during digestion, thereby increasing their bioaccessibility [60,61].
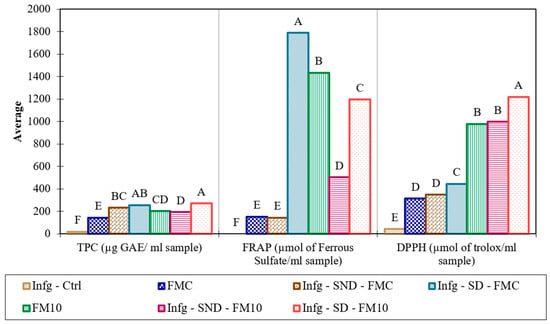
Figure 6.
Total phenolic compounds (TPC) and antioxidant activity (FRAP and DPPH) in control fermented milk samples (FMC) and samples with added plantain peel (FM10), before and after INFOGEST in vitro digestion. Legend: Infg (INFOGEST); Infg-Ctrl (INFOGEST control); Infg-SND (sample of fermented milk subjected to the INFOGEST test, undigested (without the addition of enzymes); Infg-SD (sample of fermented milk subjected to the INFOGEST test, digested (with addition of enzymes)). Different letters (A, B, C, D, E, F) indicate a statistical difference between the means (Tukey test, p < 0.05).
Both samples (FMC and FM10) demonstrated a greater capacity to deactivate free radicals following in vitro digestion, with respective increases of 29% and 19% (Figure 6). This increase is due to the greater availability of antioxidant compounds released from the food matrix [62,63]. This result is consistent with the data obtained in the total phenolic content (TPC) evaluation, suggesting that the fermented milk matrix contributes to maintaining antioxidant capacity. Fermented milk samples (FMC and FM10) showed significantly higher ferric ion reduction activity in digested samples (SD) than in undigested (SND) samples. According to Plank et al. [63] and Pena et al. [62] digestion promotes the hydrolysis of milk proteins, releasing peptides with a high capacity to reduce ferric ions. This increase in the FRAP value in digested samples justifies the observed increase. However, the pure sample (FM10), which was not subjected to in vitro digestion, exhibited a higher capacity to reduce ferric ions than the samples that were. This suggests that some phenolic compounds may be degraded or transformed into other compounds that can alter the profile and antioxidant activity [64].
4. Conclusions
The addition of green plantain peel to fermented milk influenced certain characteristics during storage, including a reduction in syneresis, an increase in water retention capacity (WRC) and an enhancement of color differences. Conversely, parameters like pH and acidity were not significantly affected by the addition of plantain peel. However, storage time had a significant effect, resulting in a decrease in pH, an increase in acidity, and improved firmness and consistency in all beverages. The incorporation of green plantain peel also increased the phenolic compound content and antioxidant activity of the fermented beverage.
The plantain peel promoted the growth of lactic acid bacteria (LAB), although bacterial viability was reduced during storage. Notably, the FM10 sample maintained a similar count to the control at the end of storage. In sensory analysis, fermented milks with 5 and 10% peel (FM5 and FM10) were the most similar to the control, e and both were well accepted by tasters (82.2% and 82.5%, respectively). After in vitro digestion, the phenolic compounds and antioxidant activity in sample FM10 increased. Overall, incorporating green plantain peel into the production process presents a viable technological approach for creating fermented milk enriched with bioactive compounds, thereby contributing to waste reduction and enhancing the nutritional value of the product.
Author Contributions
Conceptualization (A.R.d.S.S., A.P.P., C.P. and J.A.C.B.); Methodology (M.C.M. and J.A.C.B.); Formal analysis (J.A.C.B.); Investigation (A.R.d.S.S., A.P.P. and R.A.d.S.M.); Resources (C.P., K.T.M. and M.C.M.); Writing—Original Draft (A.R.d.S.S.); Writing—Review & Editing (A.P.P., C.P., K.T.M. and J.A.C.B.); Supervision (J.A.C.B.). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the School of Nutrition at the Federal University of Mato Grosso (UFMT-Brazil), protocol code 6.768.397 on 16 April 2024.
Informed Consent Statement
Written informed consent has been obtained from the participants to publish this paper.
Data Availability Statement
Data are available upon request.
Acknowledgments
The authors would like to dedicate the article to T.A. Alimentos Bananas Chips, responsible for the donation of the banana peel.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Serna-Jiménez, J.A.; Siles López, J.Á.; de los Ángeles Martín Santos, M.; Chica Pérez, A.F. Exploiting waste derived from Musa spp. processing: Banana and plantain. Biofuels Bioprod. Biorefining 2023, 17, 1046–1067. [Google Scholar] [CrossRef]
- Cunha, L. Registradas as Primeiras Variedades de Banana-da-Terra do Brasil; EMBRAPA: Brasília, Brazil, 2019. [Google Scholar]
- Faria, H.C.d.; Donato, S.L.R.; Pereira, M.C.T.; Silva, S.d.O. Avaliação fitotecnica de bananeiras tipo Terra sob irrigação em condições semi-áridas. Ciência E Agrotecnologia 2010, 34, 830–836. [Google Scholar] [CrossRef]
- Kumalasari, R.; Mayasti, N.K.I.; Surahman, D.N.; Ekafitri, R.; Wahyuni, A.S.; Desnilasari, D. Functional properties of ripe plantain (Musa spp) flour from different varieties. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012018. [Google Scholar] [CrossRef]
- FAO. Food Waste Index Report; FAO: Rome, Italy, 2024. [Google Scholar]
- Amini Khoozani, A.; Birch, J.; Bekhit, A.E.-D.A. Production, application and health effects of banana pulp and peel flour in the food industry. J. Food Sci. Technol. 2019, 56, 548–559. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-Chemical Characterization of Culled Plantain Pulp Starch, Peel Starch, and Flour. Int. J. Food Prop. 2015, 18, 165–177. [Google Scholar] [CrossRef]
- Storck, C.R.; Nunes, G.L.; Oliveira, B.B.D.; Basso, C. Folhas, talos, cascas e sementes de vegetais: Composição nutricional, aproveitamento na alimentação e análise sensorial de preparações. Ciência Rural 2013, 43, 537–543. [Google Scholar] [CrossRef]
- Oliveira, M.C.F.; Pandolfi, M.A.C. ESTUDO BIBLIOGRÁFICO: Aproveitamento integral na elaboração de subprodutos na indústria alimentícia. Rev. Interface Tecnológica 2020, 17, 797–806. [Google Scholar] [CrossRef]
- Akinyemi, O.; Akinbomi, J.; Abbey, D. Comparative characterization of plantain peel, pawpaw peel and watermelon rind using FTIR. Eng. Technol. Res. J. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Méndez-Galarraga, M.; Pirovani, M.; García-Cayuela, T.; Van De Velde, F. Fruit and Vegetable Beverages Fermented with Probiotic Strains: Impact on the Content, Bioaccessibility, and Bioavailability of Phenolic Compounds and the Antioxidant Capacity. Curr. Food Sci. Technol. Rep. 2025, 3, 4. [Google Scholar] [CrossRef]
- Mukherjee, A.; Breselge, S.; Dimidi, E.; Marco, M.L.; Cotter, P.D. Fermented foods and gastrointestinal health: Underlying mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 248–266. [Google Scholar] [CrossRef]
- FAO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. In Prevention; Food and Agriculture Organization of the United Nations: Córdoba, Argentina, 2001; Volume 5, pp. 1–10. [Google Scholar]
- Ugai, S.; Liu, L.; Kosumi, K.; Kawamura, H.; Hamada, T.; Mima, K.; Arima, K.; Okadome, K.; Yao, Q.; Matsuda, K.; et al. Long-term yogurt intake and colorectal cancer incidence subclassified by Bifidobacterium abundance in tumor. Gut Microbes 2025, 17, 2452237. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D. Applications of the INFOGEST In Vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2022, 14, 135–156. [Google Scholar] [CrossRef]
- Loesecke, V.; Willard, H. Bananas—Chemistry, Physiology and Technology; Interscience Publishers: New York, NY, USA, 1949. [Google Scholar]
- Feitosa, V.B.D.; Oliveira, E.N.A.D.; Souza, R.L.A.d.; Feitosa, B.F.; Feitosa, R.M. Estabilidade físico-química de iogurtes adoçados com mel de abelha Apis mellifera L. Ciência Anim. Bras. 2020, 21, e-50923. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Alqah, H.A.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical quality attributes and antioxidant properties of set-type yogurt fortified with argel (Solenostemma argel Hayne) leaf extract. LWT-Food Sci. Technol. 2021, 137, 110389. [Google Scholar] [CrossRef]
- Meena, L.; Neog, R.; Yashini, M.; Sunil, C. Pineapple pomace powder (freeze-dried): Effect on the texture and rheological properties of set-type yogurt. Food Chem. Adv. 2022, 1, 100101. [Google Scholar] [CrossRef]
- AOAC. Official methods of analysis of the Association of Official Analytical Chemists. In Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Colombia, MD, USA, 2012. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Methods in Enzymology; Academic Press: New York, NY, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- ISO 6579-1; Microbiology of the Food Chain—Horizontal Method for Detection, Enumeration and Serotyping of Salmonella—Part 1: Method for Detection of Salmonella spp. International Organization for Standardization: Rio de Janeiro, Brazil, 2017.
- ISO 16649-2; Microbiology of Food and Animal Feed—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli—Part 2: Colony Counting Technique at 44 °C Using 5-Bromo-4-chloro-3-indolyl-β-D-glucuronide. International Organization for Standardization: Rio de Janeiro, Brazil, 2020.
- ISO 21527-2; Microbiology of Food and Animal Feed—Horizontal Method for the Enumeration of Molds and Yeasts—Part 2: Colony Counting Technique in Products with Water Activity Less Than or Equal to 0.95. International Organization for Standardization: Rio de Janeiro, Brazil, 2008.
- Ministry of Health/National Health Surveillance Agency. Normative Instruction no. 161, July 1, 2022. Establishes Microbiological Standards for Foods. In Official Journal of the Federative Republic of Brazil; Ministry of Health/National Health Surveillance Agency: Brasília, Brazil, 2022. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Schoustra, S.; van der Zon, C.; Groenenboom, A.; Moonga, H.B.; Shindano, J.; Smid, E.J.; Hazeleger, W. Microbiological safety of traditionally processed fermented foods based on raw milk, the case of Mabisi from Zambia. LWT 2022, 171, 113997. [Google Scholar] [CrossRef]
- Ministério da Agricultura e Pecuária, MAPA. PORTARIA SDA/MAPA Nº 1.170, de 26 de Agosto de 2024: Regulamento Técnico de Identidade e Qualidade de Composto lácteo, Destinado ao Consumo Humano; Ministério da Agricultura e Pecuária, MAPA: Brasília, Brazil, 2024.
- de Morais Silva, I.; Rocha, L.d.O.F.; Schmiele, M.; de Andrade Neves, N. Obtenção de corante natural de antocianinas extraídas de capim-gordura (Melinis minutiflora P. Beauv.) e estudo da aplicação em iogurtes. Res. Soc. Dev. 2022, 11, e9811326230. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- de Lima, S.C.G.; de Oliveira, P.D.; Júnior, J.D.B.L.; Rodrigues, L.S.; de Sousa Neres, L. Efeito da adição de diferentes sólidos na textura, sinérese e característica sensorial de iogurte firme. Rev. Do Inst. De Laticínios Cândido Tostes 2011, 66, 32–39. [Google Scholar]
- Forena, R.; Nisa, F.; Prasmita, H. The effect of carboxymethyl cellulose (CMC) and banana puree (Musa acuminata Colla) addition on the characteristics and microstructure of goat milk yogurt. BIO Web Conf. 2024, 90, 01004. [Google Scholar] [CrossRef]
- Safdari, Y.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. The Effect of Banana Fiber and Banana Peel Fiber on the Chemical and Rheological Properties of Symbiotic Yogurt Made from Camel Milk. Int. J. Food Sci. 2021, 2021, 5230882. [Google Scholar] [CrossRef]
- Barros, S.L.; Santos, N.C.; Almeida, R.D.; de Alcântara Silva, V.M.; Almeida, R.L.J.; Nascimento, A.P.S. Comportamento reológico e perfil de textura de iogurte integral com polpa de achachairu (Garcinia humilis). Rev. Principia 2019, 47, 145–152. [Google Scholar] [CrossRef]
- Gaya, L.D.G.; Ferraz, J.B.S. Aspectos genético-quantitativos da qualidade da carne em frangos. Ciência Rural 2006, 36, 349–356. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Ribeiro, P.R.V.; Bassinello, P.Z.; Brito, E.S.d.; Zocollo, G.J.; Caliari, M.; Soares Júnior, M.S. Phenolic and saponin profile in grains of carioca beans during storage. LWT-Food Sci. Technol. 2021, 139, 110599. [Google Scholar] [CrossRef]
- Sah, B.; Vasiljevic, T.; McKechnie, S.; Donkor, O. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J. Dairy Sci. 2015, 98 9, 5905–5916. [Google Scholar] [CrossRef]
- Toupal, S.; Coşansu, S. Effects of Freeze-Dried Banana and Watermelon Peel Powders on Bile Salt Resistance, Growth Kinetics, and Survival of Probiotic Bacteria. Probiotics Antimicrob. Proteins 2023, 16, 1762–1772. [Google Scholar] [CrossRef]
- Vogado, C.D.; Leandro, E.D.; Zandonadi, R.P.; De Alencar, E.R.; Ginani, V.C.; Nakano, E.Y.; Habú, S.; Aguiar, P.A. Enrichment of Probiotic Fermented Milk with Green Banana Pulp: Characterization Microbiological, Physicochemical and Sensory. Nutrients 2018, 10, 427. [Google Scholar] [CrossRef]
- Imade, E.E.; Ajiboye, T.O.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Green synthesis of zinc oxide nanoparticles using plantain peel extracts and the evaluation of their antibacterial activity. Sci. Afr. 2022, 16, e01152. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Abd El Ghani, S. Production of Functional Fermented Milk Beverages Supplemented with Pomegranate Peel Extract and Probiotic Lactic Acid Bacteria. J. Food Qual. 2020, 2020, 4710273. [Google Scholar] [CrossRef]
- Chan, C.-L.; Gan, R.-y.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Luz, C.; Calpe, J.; Quiles, J.; Torrijos, R.; Vento, M.; Gormáz, M.; Mañes, J.; Meca, G. Probiotic characterization of Lactobacillus strains isolated from breast milk and employment for the elaboration of a fermented milk product. J. Funct. Foods 2021, 84, 104599. [Google Scholar] [CrossRef]
- Casarotti, S.; Monteiro, D.; Moretti, M.; Penna, A. Influence of the combination of probiotic cultures during fermentation and storage of fermented milk. Food Res. Int. 2014, 59, 67–75. [Google Scholar] [CrossRef]
- Neris, T.S.; Sousa, S.; Loss, R.A.; Carvalho, J.W.P.; Guedes, S.F. Avaliação físico-química da casca da banana (Musa spp.) in natura e desidratada em diferentes estádios de maturação. Ciência E Sustentabilidade 2018, 4, 5–21. [Google Scholar] [CrossRef]
- Uzairu, S.M.; Kano, M.A. Assessment of phytochemical and mineral composition of unripe and ripe plantain (Musa paradisiaca) peels. Afr. J. Food Sci. 2021, 15, 107–112. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Tsamo, C.V.P.; Hérent, M.; Tomekpé, K.; Emaga, T.H.; Quetin-Leclercq, J.; Rogez, H.; Larondelle, Y.; Andre, C. Phenolic profiling in the pulp and peel of nine plantain cultivars (Musa sp.). Food Chem. 2015, 167, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, D.E.; Adeyemi, O.S. Comparative evaluation of the antioxidant activity, trace elements, and phytochemical analysis of the extracts of unripe plantain whole fruit and pulp. Karbala Int. J. Mod. Sci. 2023, 9, 2. [Google Scholar] [CrossRef]
- De Assis, B.B.T.; Pimentel, T.; Dantas, A.; Lima, M.D.S.; Da Silva Campelo Borges, G.; Magnani, M. Biotransformation of the Brazilian Caatinga fruit-derived phenolics by Lactobacillus acidophilus La-5 and Lacticaseibacillus casei 01 impacts bioaccessibility and antioxidant activity. Food Res. Int. 2021, 146, 110435. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Trossolo, E.; Tonini, S.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Fermented Whey Ewe’s Milk-Based Fruit Smoothies: Bio-Recycling and Enrichment of Phenolic Compounds and Improvement of Protein Digestibility and Antioxidant Activity. Antioxidants 2023, 12, 1091. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; de Castro, R.J.S. Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Świąder, K.; Marczewska, M. Trends of using sensory evaluation in new product development in the food industry in countries that belong to the EIT regional innovation scheme. Foods 2021, 10, 446. [Google Scholar] [CrossRef]
- Helal, A.; Cattivelli, A.; Conte, A.; Tagliazucchi, D. In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt. Molecules 2022, 27, 6843. [Google Scholar] [CrossRef]
- Prestes, A.A.; Verruck, S.; Vargas, M.O.; Canella, M.H.M.; Silva, C.C.; da Silva Barros, E.L.; Dantas, A.; de Oliveira, L.V.A.; Maran, B.M.; Matos, M.; et al. Influence of guabiroba pulp (campomanesia xanthocarpa o. berg) added to fermented milk on probiotic survival under in vitro simulated gastrointestinal conditions. Food Res. Int. 2021, 141, 110135. [Google Scholar] [CrossRef]
- Pena, F.L.; Souza, M.C.; Valle, M.C.P.R.; Bezerra, R.M.N.; Rostagno, M.A.; Antunes, A.E.C. Probiotic fermented milk with high content of polyphenols: Study of viability and bioaccessibility after simulated digestion. Int. J. Dairy Technol. 2021, 74, 170–180. [Google Scholar] [CrossRef]
- Plank, B.C.A.; Guergoletto, K.B.; Rocha, T.S. Improved Bacterial Survival and Antioxidant Activity After In Vitro Digestion of Fermented Dairy Beverages by Lacticaseibacillus casei LC-01 and Lactiplantibacillus plantarum BG-112 Containing Yacon. Probiotics Antimicrob. Proteins 2025, 17, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.d.S.; de Oliveira Raphaelli, C.; Massaut, K.B.; Camargo, T.M.; Radünz, M.; Hoffmann, J.F.; Vizzotto, M.; Pieniz, S.; Fiorentini, Â.M. Probiotic Yogurt Supplemented with Lactococcus lactis R7 and Red Guava Extract: Bioaccessibility of Phenolic Compounds and Influence in Antioxidant Activity and Action of Alpha-amylase and Alpha-glucosidase Enzymes. Plant Foods Hum. Nutr. 2024, 79, 219–224. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).