1. Introduction
Beer is the most widely consumed alcoholic beverage in the world and, along with tea and coffee, one of the most popular [
1]. Traditionally, beer is brewed from four natural ingredients: malted cereals (mainly barley), hops, yeast and water. In addition, adjuncts such as other cereals or sugar sources can be added. Beer is a dynamic food matrix that undergoes continuous chemical reactions during storage [
2]. The impact of the molecules derived from these reactions will depend on both the style of beer and the storage conditions [
3,
4]. Changes include sensorial properties, flavour deterioration, colour increase and decrease in bitterness perception [
2].
The quality and shelf life of beer are mainly determined by its appearance, flavour, aroma, and texture, and are conditioned by its flavour, microbiological, and colloidal stability [
5]. To achieve and maintain beer stability, it is necessary to eliminate any form of biological contamination in the brewing process [
6]. With regard to aroma, various chemical reactions can be affected during its shelf life, potentially leading to a decrease in sensory quality [
5]. Generally, beer aging is related to the development of carbonyl compounds, sulphur compounds, pyridines, furans, etc. [
2]. The appearance of these typical aging flavours during beer storage has been linked to oxidation of higher alcohols, oxidative degradation of hop-derived bitter acids, Strecker degradation of amino acids, oxidation of unsaturated fatty acids and aldol condensations [
7]. Various factors, both external (e.g., oxygen content, pH, raw material and brewing techniques) and internal (e.g., temperature, light and packaging), influence the rate of chemical reactions that occur during beer storage [
8,
9].
On the other hand, beer is considered a microbiologically stable product. Due to the high concentration of ethanol, CO
2 and relatively low pH (3.8–4.7), it is a rather hostile medium for the growth of microorganisms. This effect is enhanced by the addition of hops, as iso-α-acids have inhibitory properties on Gram-positive bacteria [
10,
11]. In addition, the pasteurization process, which conventional beer undergoes, also helps to maintain the microbiological stability of the final product. However, in craft breweries, this process is not usually carried out, which makes craft beer more susceptible to microbial contamination and spoilage.
Beer may contain microbial contaminants originating from several sources. Microbial contamination of beer can derive from the raw materials and the brew house vessels (primary contaminants), while the secondary contaminants occur during bottling, canning, or kegging [
12]. Although beer does not develop microbiota with health effects, there are certain microorganisms that can grow under these inhospitable conditions and alter its organoleptic properties. Literature highlights that half of the microbiological troubles of both industrial and craft beers are caused by the secondary contamination [
13].
Due to the increase in beer exports coupled with growing consumer concern for product quality, the brewing industry needs to offer a product capable of maintaining its stability and best organoleptic characteristics throughout its shelf life [
5].
On the other hand, food waste is a complex phenomenon occurring worldwide and is gradually receiving attention from the scientific and professional communities, as well as from policy makers due to the large quantities produced worldwide and the associated environmental, social and economic impacts [
14,
15].
Bread is considered to be one of the most wasteful food categories. Due to its own characteristics, bread is a product highly susceptible to microbial attack, resulting in a food with a short shelf life. This, together with consumer preference for freshly baked products, is the main reason for the high levels of bread waste [
16].
Despite the potential use of this bread waste for beer brewing, there are currently few studies on the matter. Almeida et al. [
17] in 2018, brewed a craft beer with waste bread and concluded that the resulting beer had a 20% lower carbon footprint compared to the control craft beer. Subsequently, Brancoli et al. [
18] investigated the use of leftover bread as a substitute for malted barley in brewing (25–28% by weight) and determined that the Global Warming Potential (GWP) decreased by 0.46 kg CO
2 eq. per kg of wasted bread used in brewing. In 2021, Narisetty et al. [
19] showed that a maximum of 25% bread can replace barley due to the need for enzymes. Years later, McDonagh et al. [
20] studied the feasibility of using waste bread to brew beer, investigating the impact on alcohol content and the environmental implications of this substitution. The results showed that beer brewed with up to 60% malted barley by weight, replaced by bread, had sufficient fermentability to produce the required volume of alcohol. In a previous study, the possible use of different types of bread (wheat bread, rye bread, whole wheat bread and corn bread) for beer production was evaluated. The results showed the possibility of replacing up to 50% of the malt with stale bread, which represents a significant saving for the brewing industry. In addition, beers brewed with whole wheat bread were found to contain a higher total polyphenol content and antioxidant capacity; therefore, the reuse of waste whole wheat bread in beer brewing could result in obtaining a final product with healthier properties than conventional beer [
21].
In this work, we continued our previous investigations into the use of whole wheat bread as a partial substitute for malt in craft beer brews. The main objective of this study was to evaluate the shelf life of bread-enriched beers stored under standard brewery conditions (15 °C) over a period of 12 months, in order to assess their nutritional, sensory, physicochemical, and microbiological stability at the end of the storage period. We evaluated the performance of the production system across different brewing styles, like American Lager, Indian Pale Ale (IPA), and Bavarian Weiss Ale. This was achieved by analyzing key physicochemical characteristics such as turbidity, pH, acidity, alcohol by volume (ABV), dry extract, colour, protein content, polyphenol content, antioxidant activity, and microbiological stability. Additionally, sensory evaluations were conducted to explore how the inclusion of bread influences the visual, aromatic, and taste profiles of these beers. The results provide important information on the potential use of whole wheat bread to improve beer quality and extend shelf life, while supporting sustainable brewing practices aligned with circular economy principles.
2. Materials and Methods
2.1. Reagents and Standards
Methanol, Folin–Ciocalteu and Gallic Acid reagents were obtained from Merck Millipore (Madrid, Spain). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was supplied by Sigma-Aldrich Química S.A. (Madrid, Spain). Sodium chloride (NaCl), sodium hydroxide (0.01 M), anhydrous D(+)-glucose for ACS analysis, Coomassie blue G-250 (CBBG) and Bradford reagent were sourced from Panreac (Castellar del Vallés, Spain). All solutions were prepared with analytical-grade reagents and distilled water. Finally, Agar Raka-Rai wasacquired from Scharlab (Sentmenat, Spain).
2.2. Brewing Process
Three different craft beer styles were selected based on the most widely consumed types globally: one Lager beer (American Lager) and two Ale beers (IPA and Bavarian Weiss).
Beer samples were designated according to the codification presented in
Table 1.
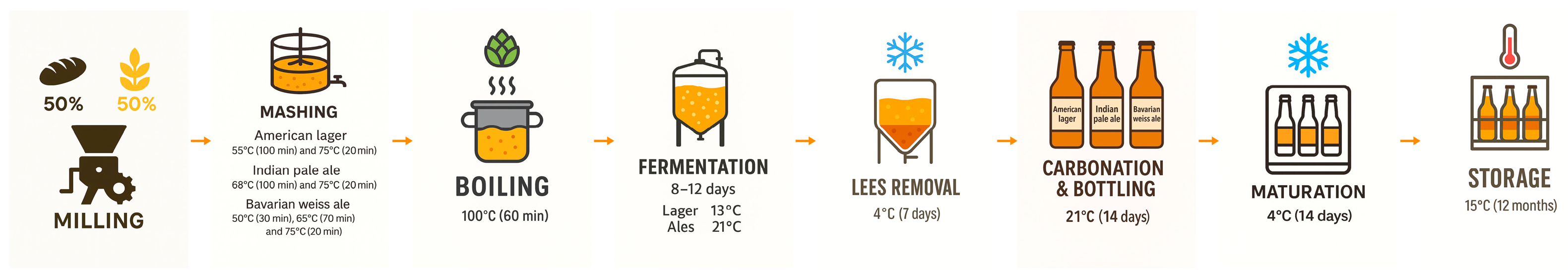
All trials were carried out in the pilot brewery of the University of Valladolid (Palencia, Spain). For each beer style, a reference wort was produced with 100% malted grain/flakes, using 2.381 kg for American lager (Castle Malting, Beloeil, Belgium), 2.650 kg for IPA (Weyermann, Bamberg, Germany) and 2.170 kg (Weyermann, Bamberg, Germany) for Bavarian wheat ale per 10 L of mineral water (Carbónicas Navalpotro S.A, Almazán, Spain). Experimental beers were prepared in the same way but with 50% of the malt mass replaced by an equal mass of stale whole wheat bread from La Tahona de Sahagún (Palencia, Spain). Each formulation was brewed in duplicate as independent 10 L pilot batches, and these duplicate brews were treated as the experimental units for statistical analysis.
A comprehensive description of the procedure optimized by our group and recently reported [
22] is summarised in
Figure 1. Briefly, malt and bread were milled separately with a 1 mm two-roll gap immediately before mashing. Mineral water was pre-heated to 40 °C, the grist (or grist + bread) was added and stirred for 20 min, and the mash was then stepped through the temperature programme shown in
Figure 1.
After a 24 h room-temperature rest (to maximize enzymatic conversion), the wort was lautered, and the spent grains were sparged with 80 °C water to restore the 10 L volume. Each wort was boiled for 60 min at 100 °C.
Hopping rates were 18 IBU with Saaz for Lager; 50 IBU for IPA (Cascade 35 IBU at 0 min and Citra 15 IBU at 30 min); and 15 IBU with Magnum for Weissbier. All hops were purchased from Laguilhoat (Fuenlabrada, Spain). On flame-out the trub was removed and the wort was cooled in a −25 °C chamber to the specific fermentation temperature (13 °C for lager; 21 °C for ales). Prior to pitching, oxygenation was achieved by 2 min of vigorous shaking.
Dry yeasts (SafLager S-23, SafAle US-05 and SafAle WB-06 (Fermentis, Marcq-en-Baroeul, France)) were rehydrated in sterile water (25 °C, 1 g yeast in 10 mL) for 15 min and added at 0.5 g L−1. Primary fermentation proceeded for 8–12 days in 10 L stainless steel tanks fitted with over-pressure valves. When final attenuation was reached, the beers were cooled to 4 °C for 7 days to enhance sedimentation, the lees were removed and the beers were tempered back to 21 °C. Bottling was performed in 330 mL glass bottles with dextrose priming (4 g L−1 bar−1) and SafAle F-2 to target CO2 pressures of 2.0 bar (Lager), 2.5 bar (IPA) and 3.0 bar (Weissbier). Bottles were conditioned for 14 days at 21 °C; internal pressure was checked with a crown-cap aphrometer.
Subsequently, the beers were held at 4 °C for a further two-week maturation and later stored at 15 °C for up to 12 months. Samples were collected immediately after maturation (S0) and after 12 months of storage (S12) for analytical comparisons.
2.3. Physicochemical Analysis
For most tests, unfiltered samples were used, except for those requiring spectrophotometric analysis (Colour, Total Polyphenol Content, Antioxidant Capacity and Protein Content), which were pre-processed by centrifugation (Bunsen, model KOCH 1460, Humanes de Madrid, Spain) at 4000 rpm for 5 min, followed by filtration through a vacuum filter (model: Kitasato) and 0.45-micron Millipore filters (Merck Millipore, Darmstadt, Germany). All filtered samples were analyzed using a spectrophotometer (ThermoFisher Scientific, model Genesys 20 UV-Vis, Madrid, Spain).
pH: pH values were measured using a calibrated pH meter (HACH-LANGE, sensiON™ + pH3 model, Hospitalet, Spain).
Acidity: Continuous pH measurements were performed, and acidity was assessed by an acid–base titration until the sample reached a pH of 7. Results were reported as the percentage of lactic acid.
Turbidity: Turbidity was determined using a turbidimeter (Hanna Instruments, model HI 98703, Eibar, Spain). Each beer sample was placed in a transparent glass container with a lid, which was then inserted into the turbidimeter to obtain turbidity values in NTU (Nephelometric Turbidity Units).
Dry Extract: Dry extract content was assessed with a thermobalance (Gibertini Eurotherm, Novate Milanese, Italy). A 1 g sample of each beer was weighed, evaporated, and the remaining solid was measured to determine the dry extract percentage by difference.
Alcohol By Volume (ABV): ABV was determined using an ebulliometer (GAB system, model 1010006, Moja, Spain), calibrated with distilled water. The boiling points of the water standard and the beer sample were compared, and the ABV was calculated to a precision of 0.1% using a ruler scale.
Protein Content: Protein content was determined by the Bradford method [
22], based on the binding of Coomassie blue G-250 dye with proteins in the beer sample. Each analysis used 3140 μL of distilled water, 200 μL of Bradford reagent, and 60 μL of the beer sample in a test tube. A calibration curve from 1 to 40 μL was prepared using serum albumin (0.1 μg/μL). Samples were then measured at 595 nm.
Colour (EBC): A 3 mL beer sample was placed in a standard 1 cm glass cuvette. Absorbance at 430 nm was recorded, with distilled water as a blank. The absorbance value was converted to the European Brewery Convention (EBC) colour scale by multiplying by 25.
Total Polyphenol Content (TPC): TPC was quantified using the Folin–Ciocalteu method, with absorbance measured at 760 nm [
23].
Antioxidant Activity (DPPH): Antioxidant activity was measured by the DPPH method following Abderrahim et al. [
24].
2.4. Microbiological Analysis
Two microbiological methods were employed to assess potential contamination in the beer samples:
Lactobacillus spp. Count:
Lactobacillus spp. was quantified through surface plating on selective media. In this technique, a small volume of sample was spread evenly on the surface of agar plates specific to
Lactobacillus spp., which were then incubated under conditions optimal for the growth of these bacteria. Colony-forming units (CFU) were counted after incubation, with a detection limit of <100 CFU/mL. This approach enables a reliable assessment of
Lactobacillus spp. presence and growth, which is critical given their role in beer spoilage [
25].
Enterobacteriaceae Detection: Enterobacteriaceae presence was determined via membrane filtration. In this method, the beer samples were filtered through 0.45 micron Millipore membranes (Merck Millipore, Darmstadt, Germany). The membranes were subsequently placed on selective agar plates, designed for Enterobacteriaceae growth and incubated to facilitate colony formation. Colonies were then counted, with a detection limit of <10 CFU/mL. Membrane filtration is a widely used method in the brewing industry for detecting low levels of Enterobacteriaceae, enhancing quality control measures. This technique involves passing beer samples through a membrane filter that captures bacteria, which are then cultured on selective media to identify contaminants. Studies have demonstrated the effectiveness of membrane filtration in detecting microbial trace contaminations in beer, including Enterobacteriaceae, thereby ensuring product safety and quality [
26].
2.5. Sensory Analysis
Eight professional beer tasters (five men and three women) were trained following ISO 8586:2012 standards. The training process included six two-hour tasting sessions, in which panellists learned to identify various beer descriptors using a presence–absence scale and a discontinuous three-point scale. During the first two sessions, tasters focused on descriptor identification through a free choice profile technique paired with a control identification test. In the remaining four sessions, a standardized tasting sheet was developed, incorporating the most relevant descriptors selected through a qualitative focus group technique. Criteria for potential removal were set, with panellists scoring below 70% on the control test subject to elimination. This process resulted in the exclusion of two tasters, yielding a final panel of six highly qualified judges.
The sensory evaluation took place in a single session at 5:00 p.m. Before beginning, tasters were briefed on the project objectives and the specific beers to be evaluated. Each beer was tested in duplicate, with twelve samples presented in total. After the first six samples, a short break was given. Each beer was served at 6 °C in glasses conforming to ISO 3591:1977 standards, with a unique three-digit random code assigned to each sample, and a randomized order of presentation for each taster. The analysis was conducted in a controlled tasting room that met UNE-EN ISO 8589:2010 guidelines. Panellists spent approximately two minutes on each sample, using a descriptive approach aligned with the tasting sheet developed during training. This sheet included descriptors to facilitate the sensory profiling of the beers across three distinct categories:
Visual: Intensity, tonality, limpidity, froth colour, and CO2 bubbles.
Aroma: Maltiness, cereal malt, ripe fruit malt, hoppy, exotic fruit, citric fruit, herbaceous, yeast, tropical fruit yeast, spicy yeast, bread yeast, toasted, coffee, licorice, and caramel, along with defect descriptors such as oxidized, cider, vinegar, musty, stable, and soapy.
Taste: Acidity, CO2, bitterness, body, and persistence.
These descriptors encompassed most of the sensory categories, including several primary terms represented in the beer sensory wheel.
2.6. Statistical Analysis
All analyses were conducted in triplicate. Statistical analyses were conducted utilizing XLSTAT v.2023.3.1 software (Addinsoft, Paris, France). For each sampling time (S0 and S12), all physicochemical variables were first assessed with a two-way MANOVA (fixed factors: Style and Bread); multivariate significance was evaluated using Wilks’ lambda (Λ), which ranges from 0 to 1 (Λ ≈ 0 indicates strong group differences; Λ ≈ 1 indicates no multivariate effect). This statistic tests the null hypothesis that there are no significant multivariate differences among the beer types. Statistical significance was determined based on the associated p-value < 0.05, considered significant. Each variable was then analyzed with a two-way ANOVA (Style, Bread, Style × Bread) and, within the same sampling time, re-analyzed by a one-way ANOVA (12 beer batches as levels). Pair-wise differences were located with Tukey’s HSD (p < 0.05). Additionally, the R Pearson correlation coefficients, calculated at p < 0.05, were used to determine relationships among the physicochemical and sensory variables examined.
3. Results and Discussion
3.1. Physicochemical Analysis
A preliminary two-way MANOVA (Style × Bread) was applied separately to S0 and S12 for turbidity, pH, colour, dry extract, acidity and % ABV. For fresh beers (S0), multivariate effects were significant for Style (Λ = 0.006,
p < 0.001), Bread (Λ = 0.32,
p = 0.015) and their interaction (Λ = 0.08,
p = 0.002). After 12 months of storage (S12), the differences were even stronger: Style (Λ = 0.002,
p < 0.001), Bread (Λ = 0.27,
p = 0.008) and Style × Bread (Λ = 0.04,
p < 0.001). As expected, beer style emerged as the main source of multivariate variation, yet the bread adjunct also altered the overall physicochemical fingerprint. The addition of bread did not affect all beers uniformly; its impact differed depending on whether the base style was lager, IPA, or Weiss beer. Consequently, each variable was examined with a two-way ANOVA, and the resulting significance levels are summarized in
Table 2.
As shown, the factor style was significant (
p < 0.05) for every basic physicochemical variable at both sampling times, whereas bread addition was significant for turbidity, dry extract and colour at S0, and for turbidity, dry extract and acidity at S12. The style × bread interaction was significant for turbidity, pH, colour and dry extract at S0, and for turbidity, pH and dry extract at S12, indicating that the impact of bread differs among beer styles. Because this interaction prevents a single overarching conclusion, each variable was subsequently analyzed with a one-way ANOVA within each sampling time; the resulting means and Tukey letter codes are presented in
Table 3 (S0) and
Table 4 (S12).
No statistically significant differences in % ABV were observed between bread and control beers within any style after 12 months, although, as expected, % ABV still differed across styles (
Table 3 and
Table 4). By contrast, pH and turbidity exhibited marked style effects: the wheat beers combined the highest turbidity with the lowest pH values (
Table 3 and
Table 4). Consistent with Wang and Ye [
27], lower pH levels favoured the formation of insoluble protein-polyphenol complexes, increasing turbidity. This relationship suggests that beer with a lower pH may present higher turbidity due to the stability of these aggregates in solution. As shown in
Table 4, Weiss-style beer made with 100% malt and matured for 12 months exhibited the highest turbidity values. So, this elevated turbidity is likely attributed to wheat malt’s high levels of soluble proteins, which enhance both foam stability and turbidity in the final product. A recent study confirmed that these proteins significantly contribute to foam properties and turbidity stability in wheat beers [
28].
Over time, turbidity decreases during long-term storage as these colloidal particles precipitate, especially in bread beers, where initial turbidity is higher due to increased protein content. Extended storage promotes the sedimentation of these complexes, resulting in improved clarity.
Furthermore, the data on dry extract content revealed significant differences among the styles. Control beers (e.g., LA1, LA2, W1, and W2) presented the highest dry extract content both immediately after brewing and after 12 months of storage. In contrast, beers produced with the addition of bread (LAB, WB) and IPA formulations exhibited significantly lower values. Additionally, a decrease in dry extract content was observed across all treatments after 12 months of storage. These results align with previous findings, which documented a decrease in dissolved solids due to the sedimentation of proteins, polyphenols, and other colloidal compounds. As these particles aggregate and precipitate over time, they stabilize the beer, resulting in improved clarity [
27,
29].
Beers brewed with bread exhibited even lower dry extract levels, likely due to additional proteins and fermentable compounds introduced by the bread. During long-term storage, these components either metabolize or precipitate, especially in Weiss-style beers with bread, where the high protein levels encourage colloidal sedimentation of solids [
27]. These findings are in agreement with previous studies, indicating that proteins and polyphenols form haze-active complexes that tend to aggregate under lower pH conditions, eventually precipitating and leading to a gradual decrease in extract content over time [
29,
30].
Also, regarding the colour results, beer styles were statistically differentiated, showing significant variation among them. The decrease in beer colour intensity observed during long-term storage could be attributed to the sedimentation and degradation of polyphenols and other colour-contributing compounds. For instance, recent studies have demonstrated that polyphenols, particularly flavonoids, play a significant role in beer colour. Their gradual reduction over time, often due to precipitation, contributes to a lighter appearance in beers. During storage, compounds like catechin and epicatechin can decrease significantly, especially in the first few weeks, which contributes to the gradual loss of colour intensity in stored beers [
1,
30].
According to Šibalić et al. [
31], the phenolic compounds in beer derived from raw materials and the brewing process can undergo oxidation during storage, contributing to colour darkening. Based on this, we could conclude that no signs of oxidation were observed, as beer coloration did not increase after 12 months of storage.
3.2. Bioactive Compounds
A separate two-way MANOVA (Style × Bread) was applied to the three bio-active variables—protein, total polyphenols (TPC) and antioxidant activity (DPPH)—at each sampling time. For fresh beers (S0), Style produced a pronounced multivariate effect (Λ = 0.073,
p < 0.001); Bread was also significant (Λ = 0.331,
p = 0.004), as was the Style × Bread interaction (Λ = 0.345,
p = 0.008). After 12 months (S12), all effects intensified: Style (Λ = 0.040,
p < 0.001), Bread (Λ = 0.230,
p = 0.003) and Style × Bread (Λ = 0.016 (
p < 0.001). Thus, while beer style again accounted for most multivariate variation, the bread adjunct significantly modified the joint protein-phenol-antioxidant profile, and its influence differed by style—especially at the end of storage. Therefore, each variable was examined with a two-way ANOVA, and the resulting significance levels are summarized in
Table 5.
As
Table 5 indicates, Style was a significant factor for protein, total polyphenols and antioxidant activity at both sampling times. Bread addition was significant for total polyphenols at S0 and S12 and became significant for both protein and antioxidant activity only at S12. The Style × Bread interaction appeared for antioxidant activity at S0 and S12 and for protein content at S12, showing that the effect of bread varies with beer style and is more pronounced at the end of storage.
Given these style-specific patterns, the three bio-active variables are discussed separately in the following subsections.
3.2.1. Protein Content
In freshly brewed beers (S0), protein content varied considerably across styles, with average values ranging from 0.56 to 1.95 mg
(
Figure 2). After 12 months of storage, protein content decreased in the lager and Weiss beers—especially in the bread variants. The decline in protein levels likely resulted from the precipitation of protein compounds during the 12-month storage period, an expected process observed in bottled beer over time. Other studies were consistent with our findings, particularly regarding wheat beers. Research has shown that wheat proteins tend to precipitate over time, especially in environments with lower polyphenol concentrations, resulting in reduced protein content during storage [
30].
IPA beers’ protein content did not display the reduction seen in other beer styles after long-term storage, suggesting a potential stabilizing effect from hop-derived compounds (
Figure 2). Recent studies confirm that hop polyphenols play a crucial role in haze stability by interacting with proteins. These interactions form non-covalent protein-polyphenol complexes that resist sedimentation, thereby preserving turbidity even during extended storage. This effect is especially notable in hopped IPAs, where the high polyphenol concentration promotes the formation of stable complexes with haze-active proteins, enhancing both the durability and visual consistency of haze in these beers [
27].
3.2.2. Total Polyphenol Content (TPC)
As shown in
Figure 3, fresh beers showed marked differences between styles in total polyphenols, reaching their highest levels in the bread-enriched IPA and, to a lesser extent, in the bread-wheat beers. After 12 months of storage, polyphenol concentrations decreased in most samples; the steepest declines occurred in the 100% malt Lager and in the bread IPAs, while the other styles lost less than 7%.
These outcomes are consistent with the findings of Jongberg et al. [
32], who demonstrated that polyphenols form stable complexes with proteins such as Protein Z and LTP1 during beer storage, often leading to precipitation, particularly under oxidative conditions, which affects haze stability.
After 12 months of storage, all IPA beers maintained higher polyphenol levels compared to other styles, likely due to hop-derived protein polyphenol interactions, which contribute to haze retention [
27].
In contrast, Lager and Weiss beers exhibited a more pronounced decrease in polyphenol content over time. Remarkably, this reduction was less evident in the beers brewed with bread. Bread Lagers retained higher polyphenol levels than their 100% malt counterparts, likely due to antioxidant compounds present in the bread. Similarly, bread Weiss beers demonstrated greater polyphenol retention compared to their malt-only versions, suggesting a comparable antioxidant effect [
30]. These findings support the hypothesis that incorporating bread could enhance polyphenol stability during long-term storage, particularly in beer styles more susceptible to polyphenol degradation.
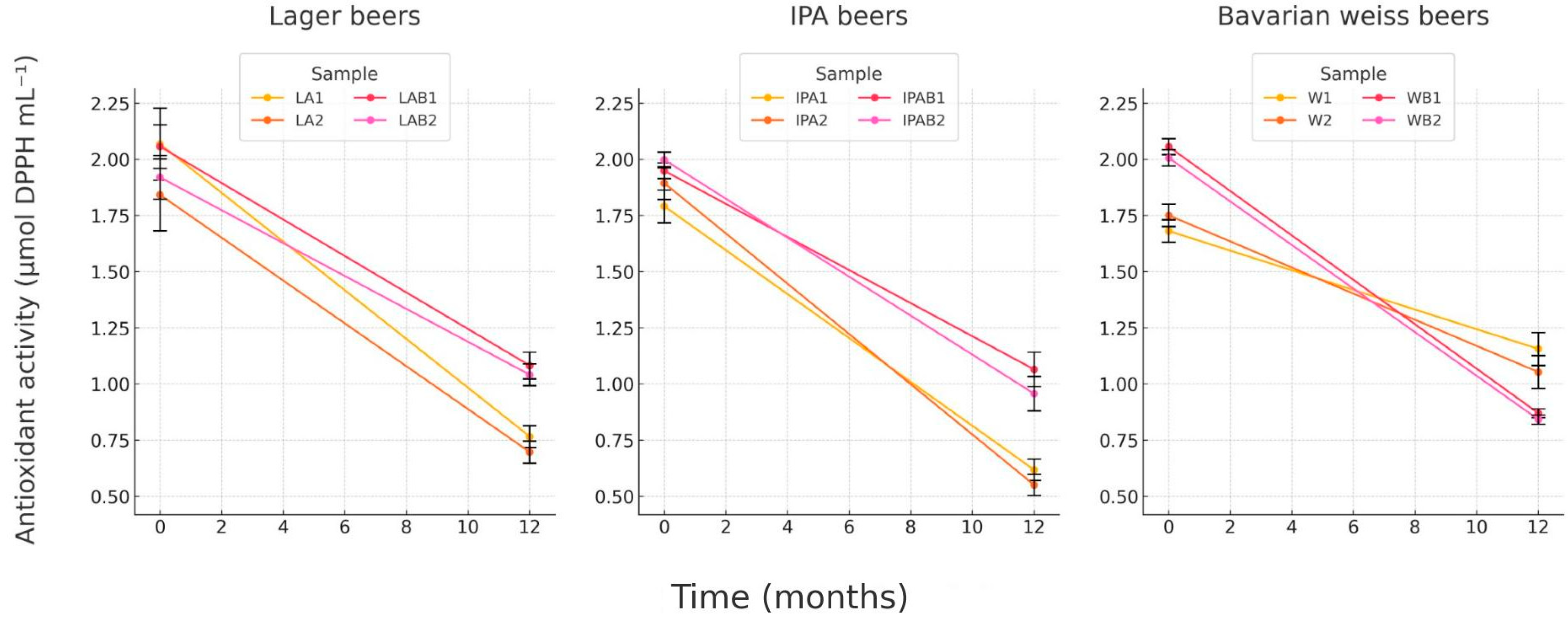
3.2.3. Antioxidant Activity by DPPH
Initial antioxidant activity varied by beer type, with IPAs and Weiss bread beers showing higher values than other styles. After 12 months of storage, a notable decrease in antioxidant activity was observed across most samples, especially in 100% malt Lagers and Weiss beers, suggesting antioxidant depletion over time (
Figure 4).
However, bread Lagers and Bread IPAs maintained higher antioxidant activity than their pure malt counterparts, implying that compounds from bread might contribute to antioxidant stability during extended storage. Similarly, at 12 months of storage, Weiss beers brewed with bread showed comparable values to those of the 100% malt Weiss beers, indicating a potential stabilizing effect from bread-derived antioxidants. This trend was consistent with another investigation by Jongberg et al. [
32], showing that antioxidants in beer could degrade during storage due to oxidative reactions. Antioxidants, particularly polyphenols, interact with proteins and may oxidize, leading to reduced antioxidant activity. As indicated by total polyphenol content data, the incorporation of whole wheat bread likely introduced additional polyphenolic compounds, leading to a more stable antioxidant profile.
3.3. Microbiological Analysis
Microbiological analysis of the beer samples revealed that Lactobacillus spp. levels were undetectable in all beer styles, with counts below 100 CFU·mL−1, except in Weiss beers 100% malt, which exhibited markedly elevated levels (8.5 × 105 CFU·mL−1), indicating a potential risk for sensory alterations and reduced product stability.
The elevated
Lactobacillus levels observed in Weiss beer may be attributed to its higher protein and nutrient content, in combination with a lower hopping rate, which is known to create favourable conditions for lactic acid bacterial growth [
25]. This aligns with previous findings indicating that beer styles with reduced hop content, such as Weiss beers, are more susceptible to
Lactobacillus spp. spoilage [
33], which can lead to undesirable sensory attributes including sourness and turbidity. However, such elevated
Lactobacillus spp. counts were not detected in the bread-enriched versions, suggesting that the addition of bread may have influenced microbial stability, preventing the proliferation of
Lactobacillus spp. seen in traditional wheat formulations.
Additionally, effective sanitation and strict quality control are essential to prevent contamination and ensure beer quality. High levels of
Enterobacteriaceae can indicate fecal contamination or poor sanitation, posing risks of off-flavours and potential health concerns. In this study, no
Enterobacteriaceae were detected in any of the beer samples after 12 months of storage, with counts remaining below the detection limit of 10 CFU·mL
−1. These results suggest that the beers maintained microbiological stability with respect to
Enterobacteriaceae throughout the long-term storage period, reflecting effective hygiene practices and a low risk of contamination [
33].
3.4. Sensory Analysis
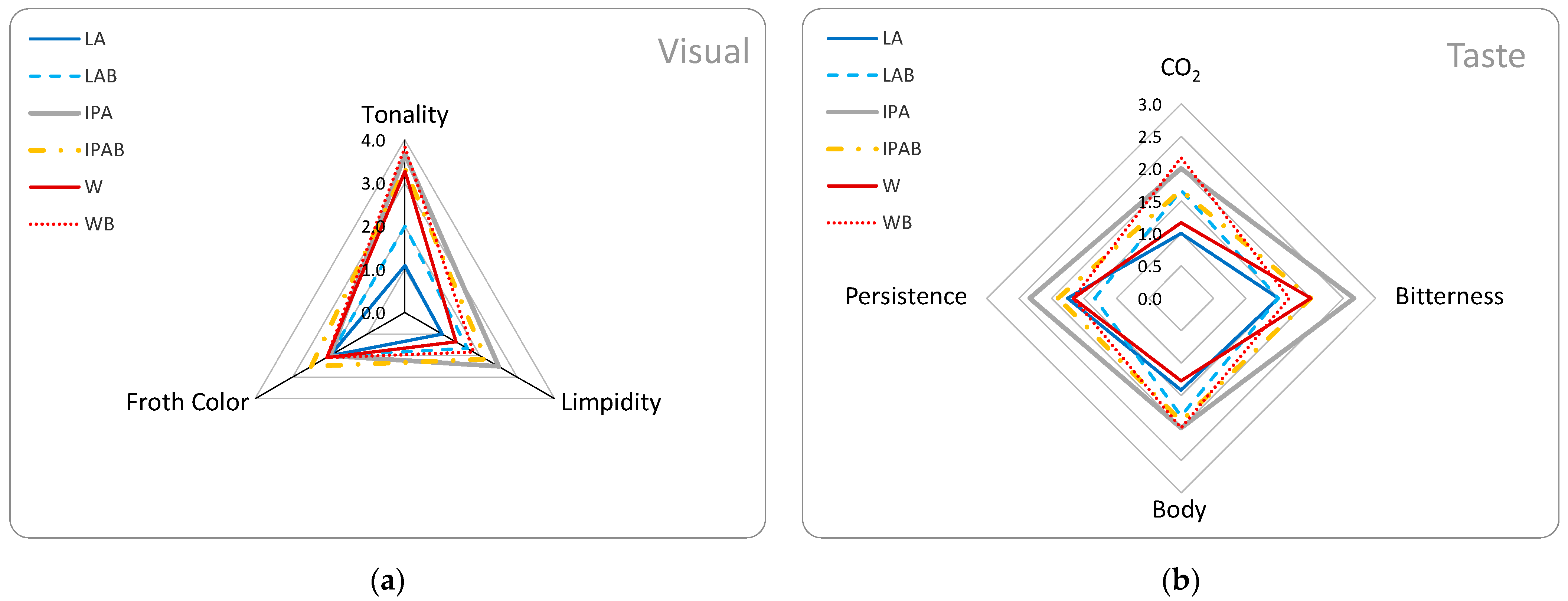
The visual sensory characteristics, collected after 12 months of storage, highlighted key differences influenced by beer styles (
Figure 5a).
Lagers displayed higher limpidity and lighter tonality, reflecting the clearer, paler nature typical of this style. In contrast, IPAs and Weiss beers have higher tonality and lower limpidity, consistent with their known turbidity and deeper colour. The elevated turbidity in IPA and Weiss beers could result from the presence of haze-active proteins and polyphenols, which are more abundant in these beer styles due to their formulations and ingredients, such as wheat in Weiss beers and hop polyphenols in IPAs. However, the addition of bread appears to impact tonality positively in most styles, such as Lagers and Weiss, suggesting that bread contributes to a richer colour profile. This effect was less evident in IPAs, where bread-enriched IPAB showed slightly lower tonality than the standard IPA. This could indicate a different interaction between polyphenols and proteins in the presence of bread additives, possibly influenced by the high polyphenol content from hops.
In addition,
Figure 5b showed how CO
2 levels, bitterness, body, and persistence varied across different beer samples in long-term storage, highlighting the impact of bread addition, increasing CO
2 taste and mouthfeel body across styles, although it slightly reduces bitterness and persistence, especially in IPAs. All descriptors shown in
Figure 5a,b were found to differ significantly (
p < 0.05) except for froth colour.
Regarding the aroma profile of beers, all descriptors represented in
Figure 6a,b exhibited statistically significant differences across the beer samples (
p < 0.05), and no off-flavour descriptors such as oxidized, cider, vinegar, musty, stale, or soapy were detected, indicating that beers did not exhibit any sensory aromatic defects at the end of their studied shelf life. The results presented in
Figure 6a showed variations in sensory attributes such as “Yeast,” “Toasted,” “Ripe fruit malt,” and “Maltiness”, differentiating the sensory profiles across the beer samples.
The incorporation of bread into beer formulations appears to selectively enhance specific sensory attributes, particularly toasted and malty notes, which were more pronounced in bread beers. This effect is likely attributed to the presence of Maillard reaction products and additional fermentable compounds introduced by the bread, contributing to greater aromatic complexity. In contrast, the ‘Yeast’ and ‘Ripe fruit malt’ aromas exhibited less noticeable variation. These findings are consistent with our previous research, which suggested that bread can contribute complex aroma and flavour compounds associated with roasting and Maillard-type browning reactions [
21].
Finally, the hop aromas were evaluated (
Figure 6b) and, as expected, were most pronounced in all IPA samples, consistent with their traditionally higher hop content. However, after 12 months of storage, the addition of bread appeared to alter specific hop-derived aromatic notes, particularly in IPAs. The observed reduction in exotic fruity aromas in bread-enriched beers may be attributed to interactions between bread components and volatile hop esters, which could become less stable in the presence of additional proteins or carbohydrates introduced by the bread [
21]. On the other hand, the stronger herbaceous aroma observed in bread IPA beers suggested that bread may enhance green notes, possibly by retaining specific hop compounds or reducing the evaporation of these esters over time.
3.5. Correlations Analysis Between Sensory Attributes and Physicochemical Properties in Beer Samples
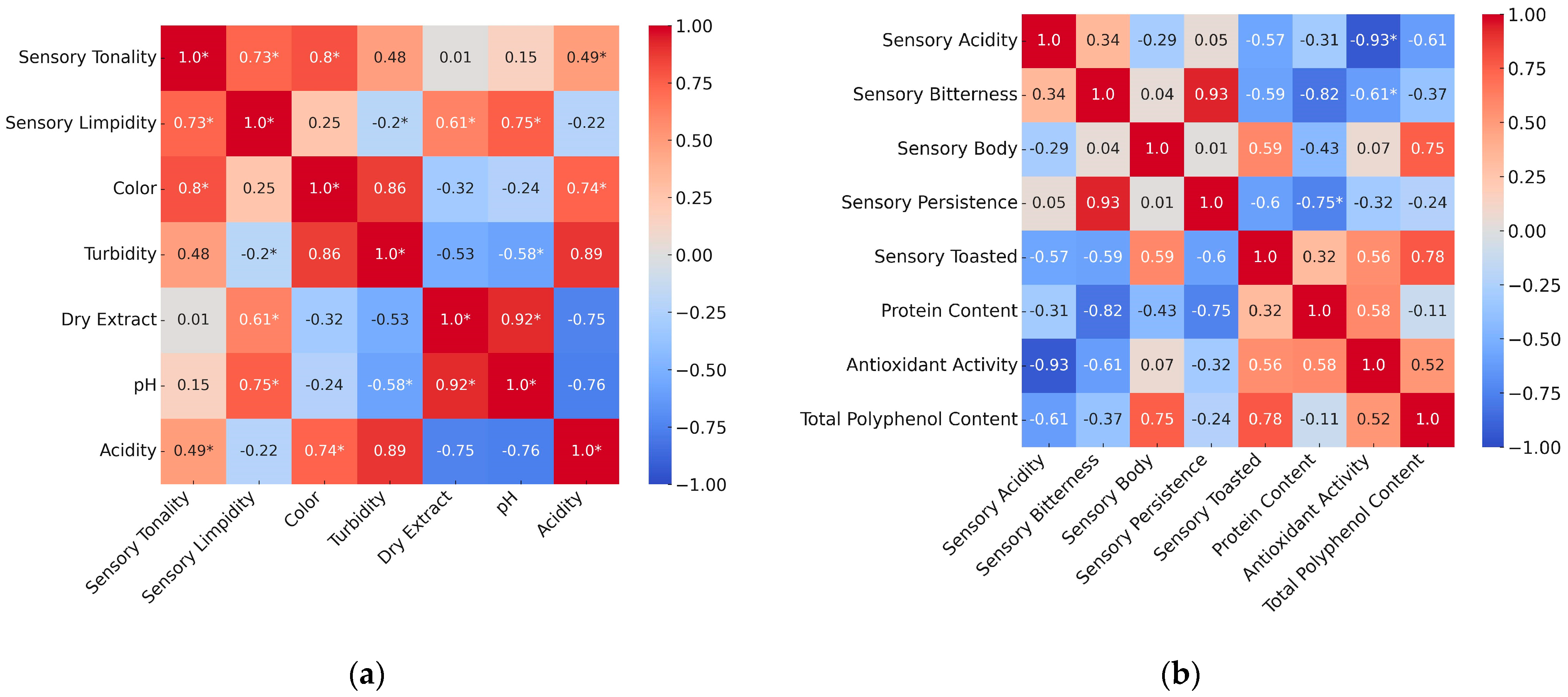
A correlation matrix heatmap between all physicochemical parameters and visual sensory attributes was analyzed. Strong positive (+1) and negative (−1) correlations are highlighted in red and blue, respectively. The variables included in
Figure 7a were selected based on the most relevant pairing combinations, particularly those involving Colour, Turbidity, Dry extract, pH, and Acidity in relation to Tonality and Limpidity, allowing for a clearer interpretation of the data.
Turbidity showed a strong and statistically significant positive correlation with acidity, suggesting that beers with higher turbidity also tend to exhibit greater acidity. Although turbidity also displayed a high correlation coefficient with colour, this relationship was not statistically significant at p < 0.05.
A strong and significant correlation was observed between sensory tonality and sensory limpidity, indicating perceptual alignment in visual sensory descriptors. The strongest correlation found was between colour and sensory tonality (r = 0.80, p < 0.05), suggesting that the physical appearance of the beer strongly influences its perceived visual tonality.
Furthermore, beers with higher dry extract content tended to exhibit higher pH values (r = 0.92,
p < 0.05). This relationship may be attributed to the presence of unfermented carbohydrates, dextrins, and particularly protein-derived compounds, which contribute significantly to the buffering capacity in beers [
34]. These proteins and peptides, mainly from malt, help resist pH reduction during fermentation and storage, thereby stabilizing the final pH of the product.
Using the same criteria,
Figure 7b presents the comparison between sensory aroma, taste attributes and physicochemical parameters, which showed the strongest correlations.
The high indirect correlation between sensory acidity and antioxidant activity in beers (r = −0.93,
p = 0.0001) revealed a statistically significant relationship, indicating that higher antioxidant levels may lead to a perception of reduced acidity. This phenomenon could be attributed to antioxidants neutralizing acidic compounds, thereby modifying sensory perception. Previous research has indicated that polyphenolic antioxidants can interact with organic acids, potentially diminishing their sensory impact and contributing to a smoother taste profile [
35]. Additionally, elevated antioxidant activity could inhibit microbial growth, preventing the production of organic acids that would otherwise increase acidity. For example, polyphenols in beer possess antimicrobial properties that reduce the proliferation of acid-producing bacteria, thus maintaining a more stable pH during storage [
36].
Another inverse relationship was observed between protein content and sensory bitterness (r = −0.82,
p = 0.0015), which was also statistically significant. This finding supports the notion that proteins can bind with bitter substances, reducing their solubility and availability in the oral cavity and thereby diminishing bitterness perception. This phenomenon is in line with observations by Gonçalves et al. [
37], who reported that proteins in beer, particularly those derived from malt, can form complexes with bitter compounds, leading to reduced bitterness intensity.
Additionally, a negative correlation was observed between protein content and sensory body (r = −0.43,
p = 0.14), which, although not statistically significant, suggested a possible trend where higher protein levels might contribute to a fuller mouthfeel and balance bitter flavours. This effect could be due to proteins’ ability to increase viscosity and interact with bitter compounds, reducing their perceived intensity. A recent study also reported that higher protein content enhances mouthfeel and reduces perceived bitterness in beer [
38].
The correlation between total polyphenol content and sensory body (r = 0.75,
p = 0.052) did not reach statistical significance, but still indicated a notable trend. This association suggests that higher polyphenol levels may contribute to a fuller mouthfeel. Polyphenols can increase viscosity and astringency, thereby enhancing the perception of body in beer. This interpretation aligns with prior studies showing that polyphenols interact with proteins and polysaccharides, leading to a more complex and rich mouthfeel [
39].
Finally, a statistically significant positive correlation was observed between total polyphenol content and toasted flavour (r = 0.78,
p = 0.032), indicating that beers with higher polyphenol levels tend to exhibit stronger toasted sensory characteristics. This relationship is likely influenced by the presence of polyphenols from toasted or roasted malts, as well as contributions from whole wheat components, which enhance the sensory complexity of the beer. These findings are consistent with prior research showing that Maillard reaction products from toasted malt contribute both to antioxidant capacity and to the development of toasted flavour profiles [
31].
4. Conclusions
The results of this study revealed important insights into the evolution of craft beers, particularly those enriched with bread, during a 12-month storage period. Physicochemical analyses demonstrated significant reductions in turbidity and dry extract, especially in bread beer. This is likely due to the additional bioactive compounds as proteins, introduced by whole wheat bread, which contributed to increasing colloidal sedimentation, resulting in clearer beers after extended storage. The decrease in colour intensity aligned with the gradual sedimentation of polyphenols, a key factor in colour stability, which supports previous findings that link polyphenol precipitation with lighter colour profiles in extended storage beers.
Additionally, the bread beers retained higher levels of antioxidant activity and total polyphenol content compared to their 100% malt counterparts, suggesting that bread contributed positively to bioactive compound stability. This was particularly evident in IPAs and Weiss beers made with bread, where antioxidant stability was preserved despite the natural decline over time. These outcomes highlight the role of bread as a stabilizing factor for polyphenols at the end of beer’s long-term storage process, which not only enhanced antioxidant activity, but also contributed to sensory properties, particularly toasted and maltiness aromas. The higher polyphenol content also correlated with a fuller body, adding complexity to the mouthfeel, a feature desirable in craft beer profiles. Moreover, the aroma data suggested that the strategic use of bread in brewing could add unique aromatic nuances, particularly in enhancing malt and toast-related attributes, potentially appealing to consumers seeking richer and more complex flavour profiles in craft beers. In addition, bread contributed to a fuller mouthfeel and increased carbonation taste, potentially altering the sensory profile and drinkability of beer.
The correlation analysis underscored the connection between physical-chemical properties and sensory attributes, revealing a strong association between polyphenol content and toasted flavours, while higher protein content was associated with reduced bitterness. Moreover, bread-enriched beers maintained microbiological stability, with no remarkable spoilage microorganisms detected, affirming that bread can enhance the sensory and bioactive profile, without compromising microbiological quality after 12 months of storage. In the context of a rising bioeconomy, using bread as a partial malt substitute ingredient not only provides a sustainable approach to resource utilization but also improves beer quality. This study supports the potential of bread as an innovative ingredient that complements the traditional brewing process, contributing to a more robust and complex sensory profile while aligning with circular economy goals by valorizing food waste.