The Impact of Brewing Methods on the Quality of a Cup of Coffee
Abstract
1. Introduction
2. Sensory Characteristics of Coffee Brews and Their Evaluation
3. Coffee Brewing Methods
3.1. Description of the Beverage Preparation
- Cold drip and cold brew: These types of preparation involve the use of cold water. In the first case, the water passes through a bed of ground coffee, typically using a very slow flow rate, such as 1 drip per second. In the latter type, the extraction water is left in contact with the ground coffee for a long time, usually 8–24 h. The difference between the two preparations is that cold drip requires a shorter time, e.g., 2 h, as the extraction is based on a continuous ‘washing out’ of the bioactive constituents over time instead of slowly reaching an equilibrium between the solid and liquid phase. Cold brews have lower content of total titratable acids and lower antioxidant activity compared to hot brews, as well as lower content of total solids and caffeine. The equilibrium for some compounds is reached after several hours. For example, when comparing the content of 3-chlorogenic acid in cold brew and French press, it was shown that the equilibrium is reached between 6 and 7 h, reaching a concentration similar to the French press coffee [15]. In terms of sensory description, coffee brews have stronger intensity of ‘sweet’, ‘fruity’, and ‘floral’ aroma attributes, and a creamy body [16]. This brewing method leads to an aromatic coffee that retains some volatile compounds, which are typically lost at high temperatures.
- Boiled coffee: This method involves boiling water and ground coffee for relatively long times, even up to 10 min. It has been reported to have higher concentrations of cafestol and kahweol.
- Turkish/Greek coffee: It is prepared using a special pot called ibrik, in which the brew is prepared by using very finely ground coffee. This is necessary so that coffee can sediment easily, but still part of the coffee powder ends up in the coffee cup; therefore, the customer has to be careful not to drink the whole cup. Also, the customer has to request whether they prefer sweet coffee as sugar is added together with the ground coffee to the water before boiling it. The maximum brewing time should not exceed 3 min; however, after a first boiling phase, the pot is put back to boil so that a thick foam is obtained. Traditionally, for Turkish coffee, there are four levels of sweetness to define the brew, and sometimes, milk is added [17].
- Plunger coffee/French press: Ground coffee and hot water are added to a cylindrical container, then left to rest for a few minutes, and finally, the top of the container holding a metal filter is pressed so that it retains the solid particles. The plunger is pushed down manually; therefore, the pressure applied is typically 0.5 bar, and the contact time is typically 2–5 min, depending on the consumer’s preference.
- Filter coffee/Americano: It is basically a filtration process in which hot water is added to a filter containing ground coffee. The paper filter allows water to pass through the ground coffee only once, and this process is important in retaining some compounds, such as lipids, together with the solids. The coffee maker adds water at the correct temperature, then waits about a minute or two so that some foam is produced due to the retained CO2 in the ground coffee, and then, the final amount of water is added until the desired final volume.
- Percolator coffee: It uses a device designed to allow recirculation of the hot water through a bed of ground coffee held in a percolated chamber. Given the recirculation of water, the extraction is very intense, giving even a strong astringent sensation to the over-extraction, together with the loss of many volatile compounds.
- Soluble coffee: The powder of soluble coffee is dissolved in hot water. This review will not extensively report on this kind of preparation due to its strong differences.
- Single-serving coffees: This method uses pods or capsules, which can be made of different materials and contain defined amounts of ground coffee. The basic process, however, does not differ dramatically from the Espresso coffee concept.
- Special coffees with additional ingredients: There is a wide range of niche products or specialty coffees that make this part complicated for comprehensive reporting. Suffice to say that the most common additional ingredients or ‘additives’ to coffees are milk or cream; however, many more can be used to add special flavors.
- Moka: A lower part of the coffee pod contains water, which, when it reaches the boiling point, passes through a bed of compressed ground coffee into the upper part of the moka machine, where it is stored until consumed. For the extraction process, the water typically must reach 110 °C, which corresponds to about 0.5 bar of pressure. Excessive temperatures or extraction times can, however, leave an unpleasant “burnt” flavor. It is normally suggested not to leave the pod too much on the heat source at the end of the extraction process, to avoid this burnt flavor. An additional variable might arise from the amount of ground coffee added to the pod, which influences the level of compression and then the hydraulic resistance.
- Napoletana/Neapolitan coffee: It is also known as “flip drip pot” because of its design. It is an ancient pod, already used commonly in Naples (Italy) before 1800. It produces a brew stronger than filter coffee but not as strong as Espresso. It is based on heating the lower part of the pod containing water, then flipping it when it is boiling, such that hot water can percolate through a beam of coffee powder entrapped between the upper and lower parts of the pod. The coffee is somehow similar to filter coffee but stronger, due to the wetting of the ground coffee during the water heating and a slightly higher water temperature during the extraction.
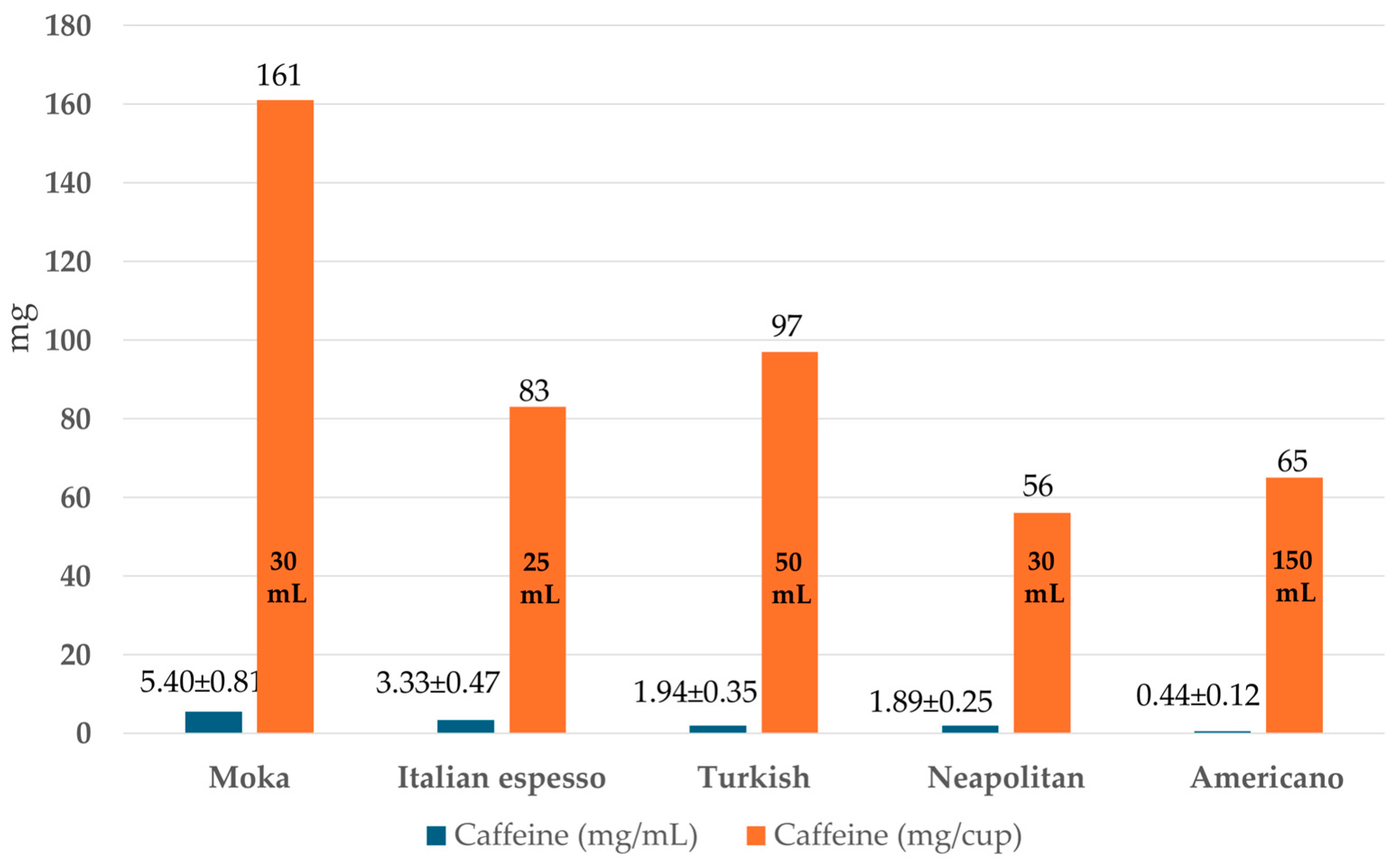
- Espresso: Espresso coffee is a relatively new coffee brew, born from an Italian innovation. It is based on an extraction at high pressure, in a rapid manner, which is also one of the meanings of the word “Espresso” (the other being “made at the moment”, “made explicitly at the customer’s request”). In addition to this, the final coffee of the Espresso brew is typically smaller compared to other preparations. This is particularly true for the Italian Espresso and for the “ristretto” type. The percolation time is considered ideal at about 30 s, with typically 9 bar of pressure and 90 °C for water temperature. The Espresso machine is quite complicated when compared to all other coffee brewing techniques, and the reader can refer elsewhere for details on its engineering properties [2]. Espresso coffee also differs significantly from other coffee brews from a physical point of view, as its viscosity is about double that of other coffees and it has a stronger “body”. Espresso is also characterized by its “crema”, which is a thick and quite stable foam. This is influenced by polysaccharides, while its persistence depends on the protein fraction. Soluble carbohydrates are usually 8 g/L in Espresso, which is approximately 15% of the total solids. Caffeine ranges between 1.2 and 4 mg/mL, depending on the coffee and the cup size. For in-depth information about foam formation, stability, and the chemistry behind these processes, the reader can refer to a chapter by Folmer, Blank, and Hofmann [18]. The type of ground coffee is coarse or medium-coarse and typically has a bimodal distribution of the particle size so that the larger ones will entrap smaller ones and “self-filtrate” during the percolation process.
3.2. Influence of Key Parameters
4. Effects of the Brewing Methods on Contents of Key Compounds in Coffee Brews
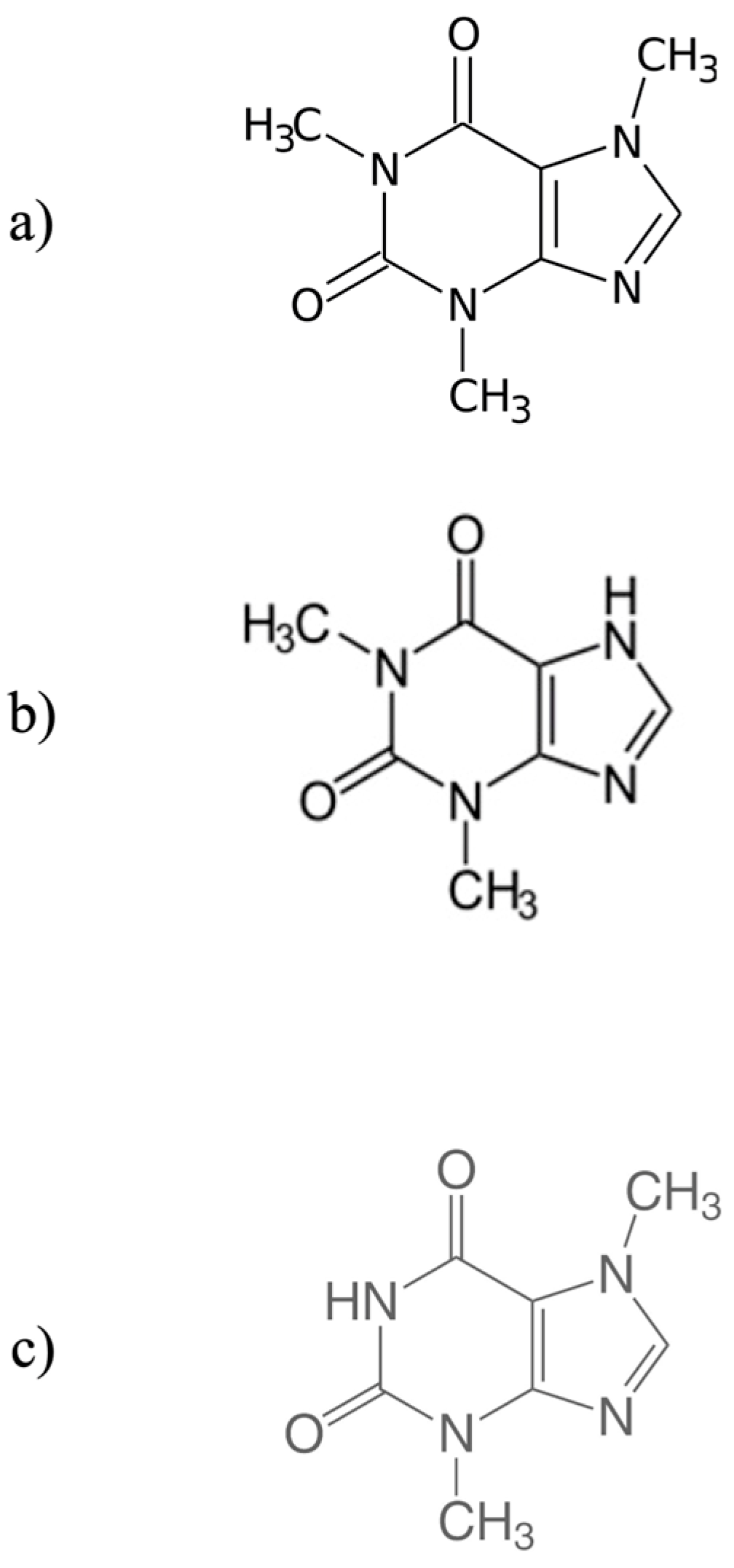
4.1. Caffeine and Other Methylxanthines
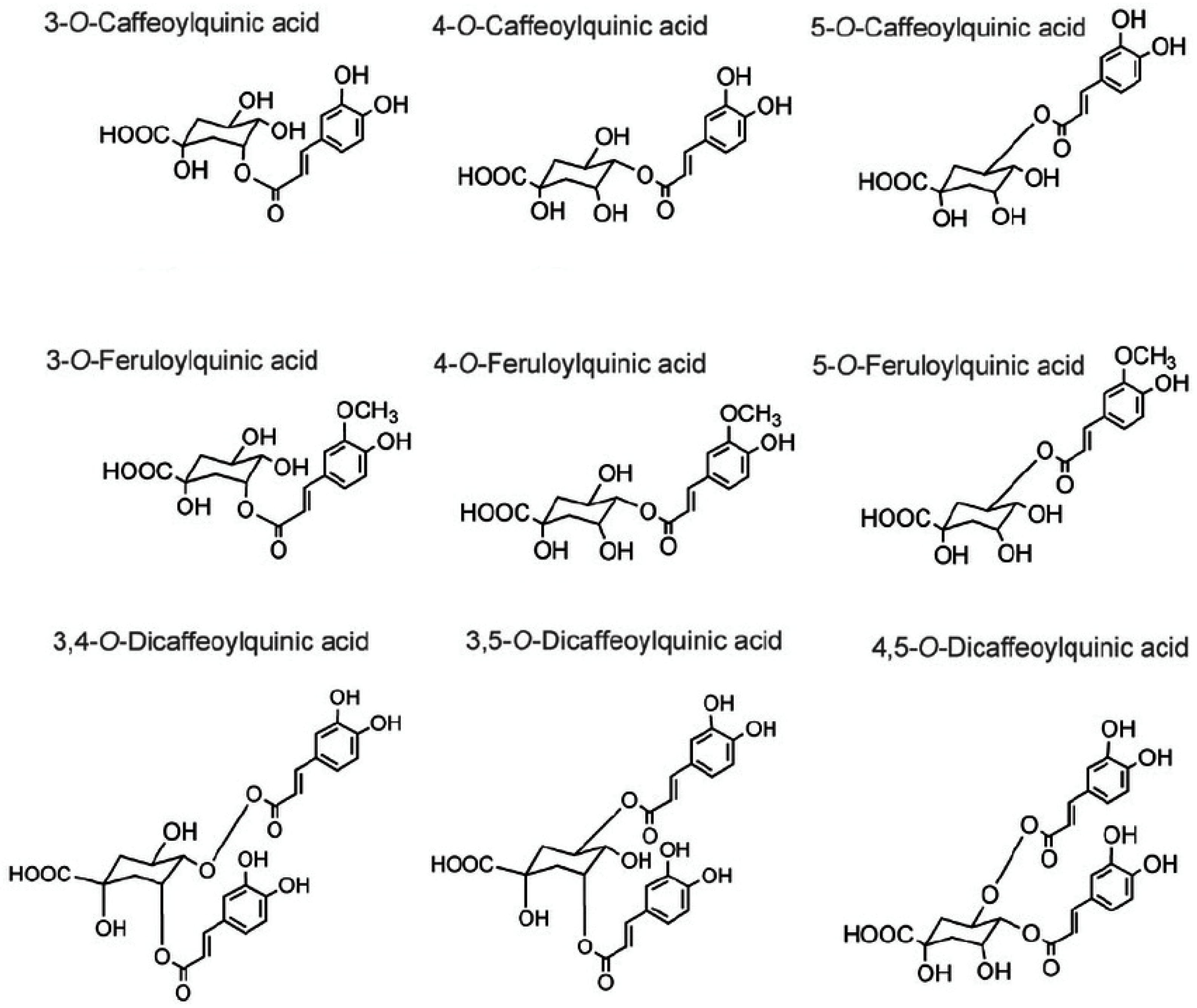
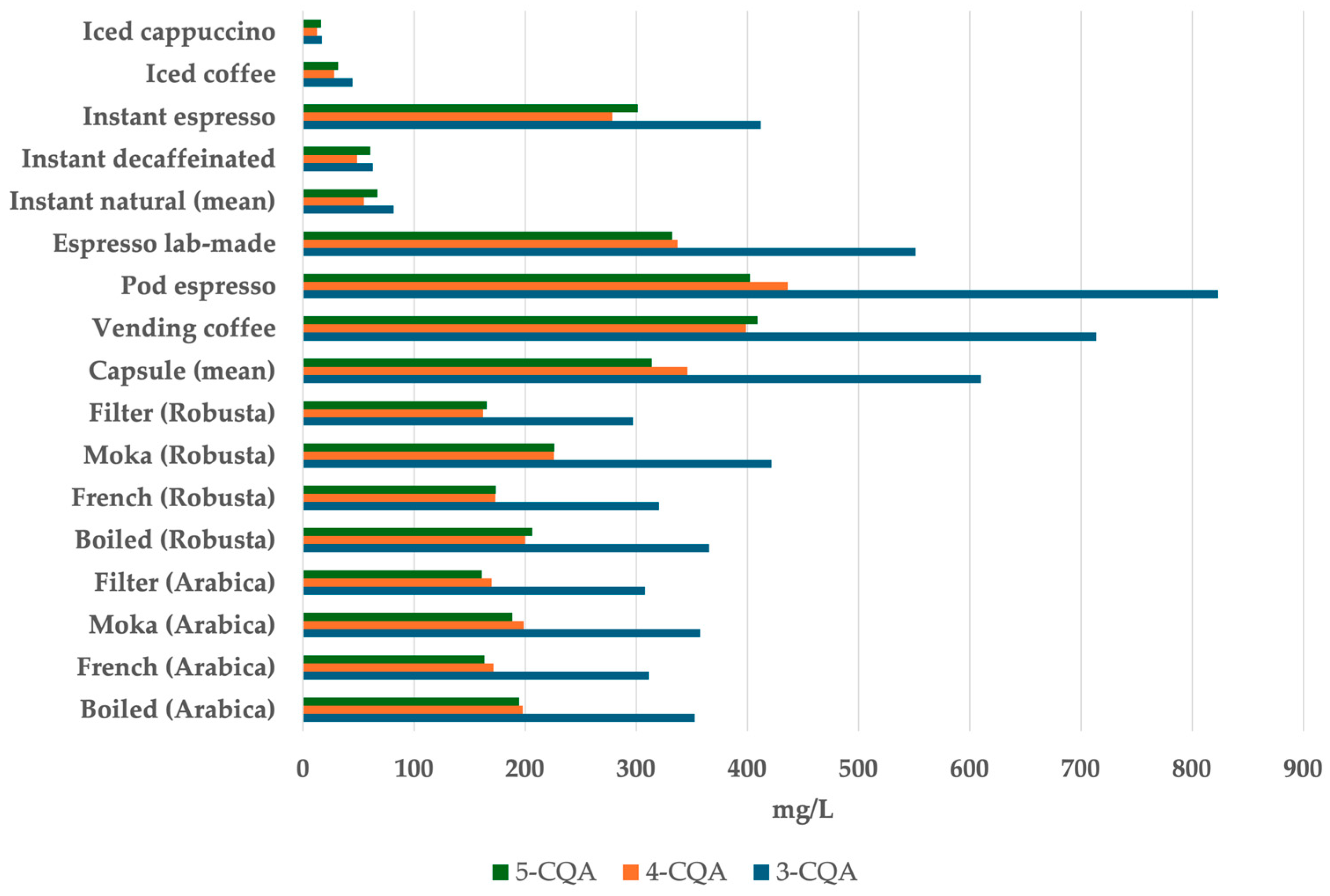
4.2. Phenolic Compounds
4.3. Melanoidins
4.4. Minor Compounds
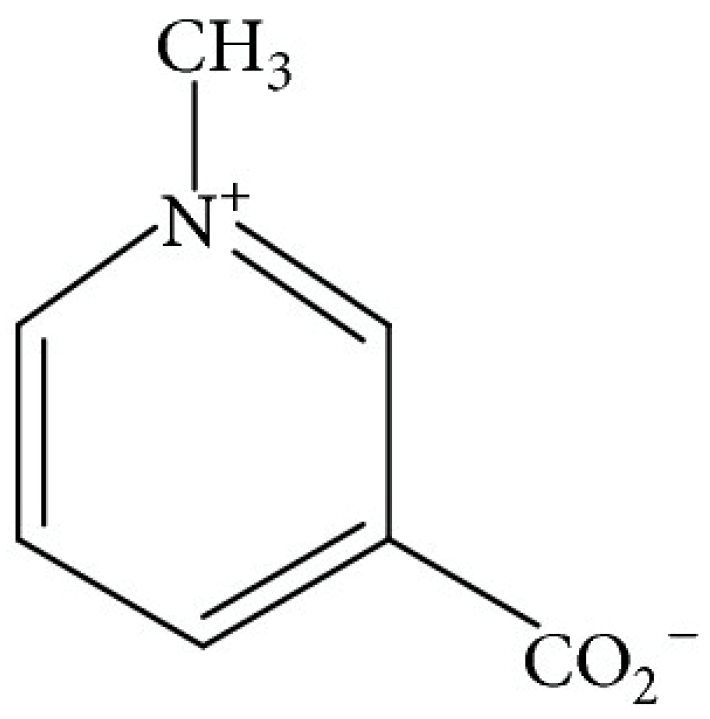
4.4.1. Trigonelline
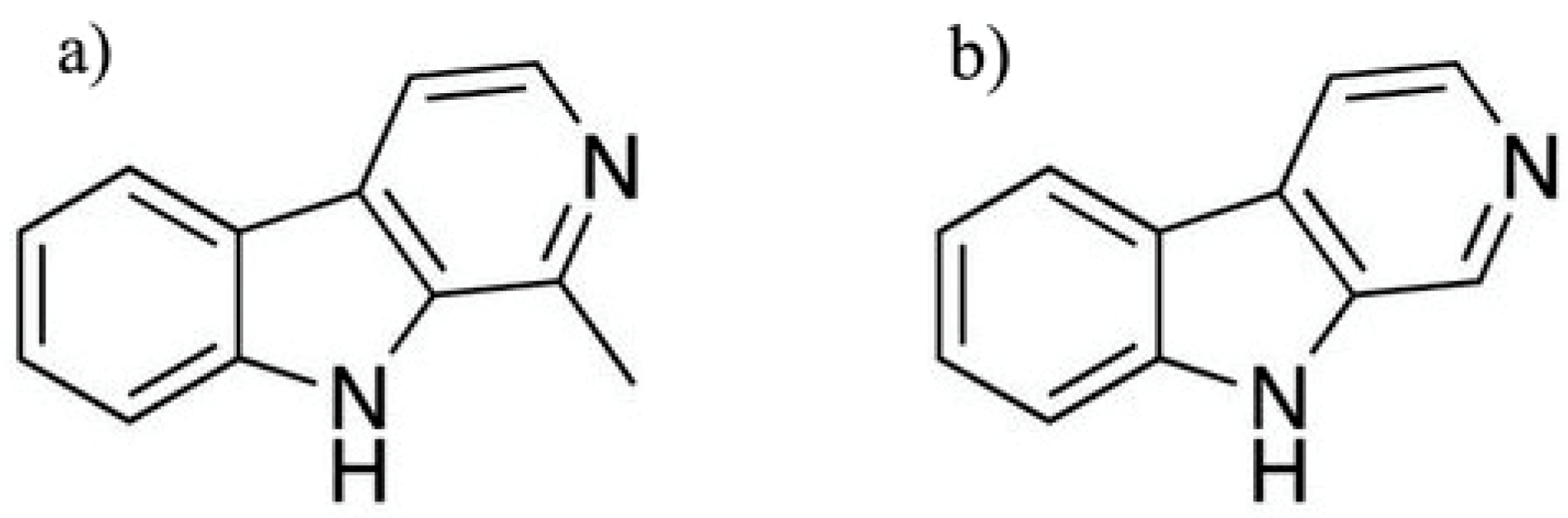
4.4.2. β-Carbolines
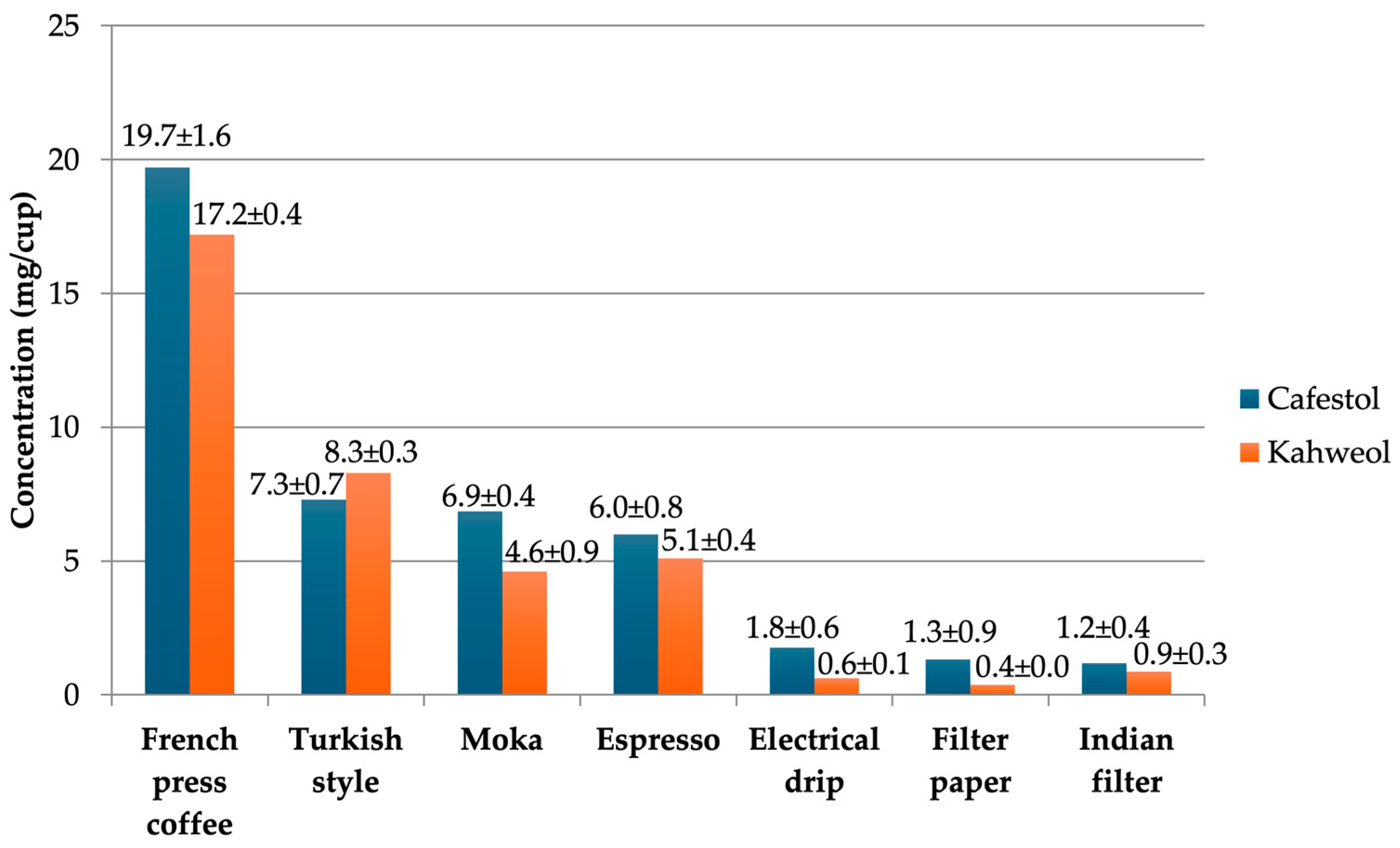
4.4.3. Cafestol and Kahweol
5. Effects of the Brewing Methods on the Extraction of Undesirable Compounds from Coffee Powder and Coffee Brews
5.1. Ochratoxin A (OTA)
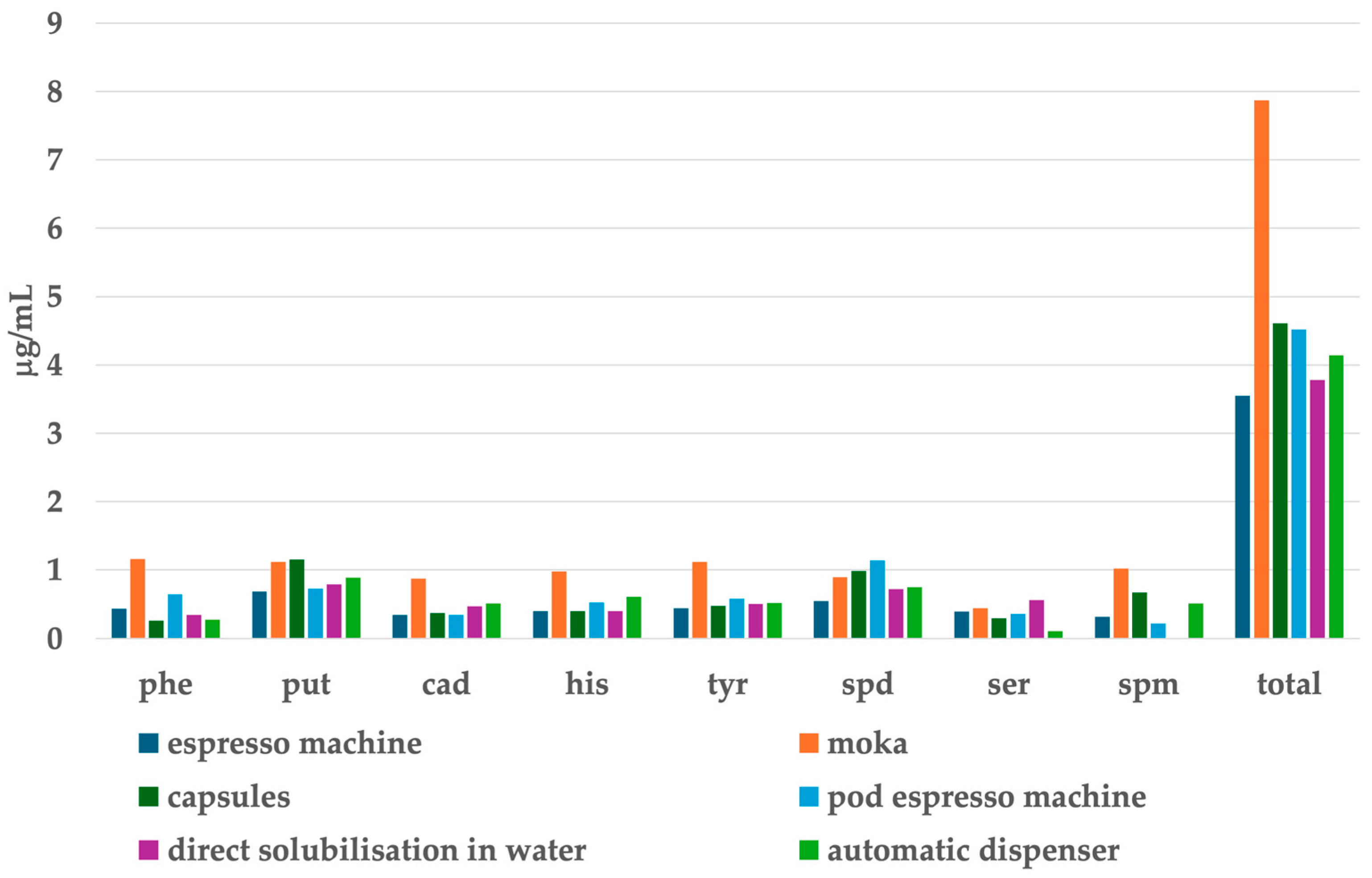
5.2. Biogenic Amines
5.3. Polycyclic Aromatic Hydrocarbons (PAHs)
5.4. Acrylamide
6. Coffee Brew Volatile Compounds: Generation and Impact on Coffee Aroma
| Chemical Class | Compound | Odour Description | Odor Threshold (ppb) |
|---|---|---|---|
| Acid | 2-methylbutanoic acid | Pungent, acidic-like, sour, fermented pineapple, sweaty | 10 |
| 3-methylbutanoic acid | Pungent, acidic-like, sour, sweaty | 540 | |
| 4-methylbutanoic acid | Sweet, acid | ||
| acetic acid | Sour, acid, vinegar | ||
| butanoic acid | Butter rancid | 240 | |
| Aldehydes | 2-methylpropanal | Malty, fruity, buttery, oily, roasted cocoa | 0.7 |
| 2-methylbutanal | Buttery | 1.3 | |
| 3-methylbutanal | Buttery, malty | 0.35 | |
| 4-methylbutanal | Buttery | ||
| hexanal | Fatty, green, grassy, butter rancid | 4.5 | |
| (E)-2-nonenal | Fatty, waxy, cucumber, buttery | 0.08 | |
| acetaldehyde | Fruity | 10 | |
| propanal | Roasted, fruity | 10 | |
| (E)-2-methyl-2-butenal | Sweet, fruity, floral, honey | ||
| E,E-2,4-decadienal | Citrus, fatty, green | 0.07 | |
| Ethers | 3-methyl-1-butanol formate | Sweet, plum, fruity, black currant, apple | 2.5 |
| Esters | ethyl-2-methylbutyrate | Fruity | 0.5 |
| ethyl-3-methylbutyrate | Fruity, apple-like | 0.6 | |
| Furans | 2-furanemethanol acetate | ||
| 2-methylfuran | |||
| 5-methyl-2-furancarboxyaldehyde | 6000 | ||
| furfurylmethyl ether | |||
| furfurylformate | |||
| furfuryldisulfide | |||
| 2-(methylthio-methyl)furan | Smoky, roasted | ||
| 2-furancarboxaldehyde (furfural) | Sweet, burnt, bready, nutty, caramel | 280 | |
| 2-furfurylthiol | Coffee, roasted | 0.01 | |
| 2-methyl-3-furanthiol | Meaty, fishy, roasted, chicken-like | 0.007 | |
| 1-(2-furanyl)-2-butanone | |||
| Furanones | dihydro-2-methyl-3(2H)-furanone | 0.005 | |
| 4-hydroxy-2,5-dimethyl- 3(2H)-furanone (furaneol) | Caramel, sweet | 10 | |
| 2-ethyl-4-hydroxy-5- methyl-3(2H)-furanone (homofuraneol) | Caramel, sweet | 1.15 | |
| 3-hydroxy-4,5-dimethyl- 2(5H)-furanone (sotolon) | Spicy, sweet, caramel-like | 20 | |
| 5-ethyl-3-hydroxy-4-methyl-2(5H)-furanone (abhexon) | Spicy, seasoning-like, caramel-like | 7.5 | |
| Indole | 3-methylindole | Coconut, phenolic | |
| Ketones | 1-octen-3-one | Mushroom | 0.0036 |
| 2,3-hexadione | |||
| 3,4-dimethyl-2-cyclopentenol-1-one | Caramel-like, sweet | ||
| 4-(4′-hydroxyphenyl)-2-butanone (raspberry ketone) | Sweet fruity | 1–10 | |
| 2,3-butanedione | Buttery, oily | 15 | |
| 2,3-pentanedione | Buttery, fermented dairy, creamy, sweet, oily | 30 | |
| 2-hydroxy-3-methyl-2-ciclopenten-1-one (Cycloten) | Sweet, caramel | 300 | |
| Norisoprenoids | (E)-β-damascenone) | Cooked apple, sweet, fruity, honey-like | 0.00075 |
| Phenols | phenylethanal | Floral, sweet, fruity | 4 |
| vanillin | Vanilla | 25 | |
| 2-methoxyphenol (Guaiacol) | Phenolic, roasted, burnt | 2.5 | |
| 4-methoxyphenol | Phenolic | 68 | |
| 4-ethylguaiacol | Phenolic, spicy | 25 | |
| 4-ethenyl-guaiacol | Phenolic | ||
| 4-vinylguaiacol | Phenolic, clove, spicy | 0.75 | |
| p-anisaldehyde | Minty | 27 | |
| Pyrazines | 2-methoxy-3,5-dimethylpyrazine | Earthy | 0.006 |
| 2-ethyl-3,5-dimethylpyrazine | Earth, nutty-roast, hazelnut, roasted | 0.04 | |
| 3-ethyl-2,5-dimethylpyrazine | Earthy | 43,000 | |
| 3-isopropyl-2-methoxypyrazine | Earthy, roasty | 0.002 | |
| 2-(sec-butyl)-3-methoxypyrazine | Green-earthy | 0.001 | |
| 2-ethenyl-3-ethyl-5-methylpyrazine | Earthy | 0.000014 | |
| 2-isobutyl-3-methoxypyrazine | Earthy, peasy | 0.002 | |
| 2-ethenyl-3,5-dimethylpyrazine | Earthy, nutty | 0.000012 | |
| 2,3-dimethylpyrazine | Hazelnut, roasted | 800 | |
| 2,5-dimethylpyrazine | Hazelnut, roasted | 80 | |
| 2-ethylpyrazine | Peanuts, roasted | 62 | |
| 2,3,5-trimethylpyrazine | Roasted, nutty | 9 | |
| 2-ethyl-3,6-dimethylpyrazine | Burnt, coffee, nutty-roast | 8.6 | |
| 2-ethyl-6-methylpyrazine | Peanuts, roasted | ||
| 6,7-dihydro-5H-cyclopentapyrazine | Hazelnut, roasted | 4000 | |
| 6,7-dihydro-5-methyl-5H-ciclopentapyrazine | Hazelnut, nutty-roast | 6000 | |
| 2,3-diethyl-5-methylpyrazine | Hazelnut, roasted, nutty-roast | 0.09 | |
| Pyranones | 3-hydroxy-2-methyl-4-pyran-4-one (maltol) | Candy, caramel | 20,000 |
| Pyridine | pyridine | 77 | |
| Pyrroles | 1-methyl pyrrole | Negative notes–defective beans | |
| (2-acetyl-1-pyrrolidine) | Nutty-roast | ||
| Sulphur compounds | dimethyl trisulfide | Cabbage-like | 0.001 |
| bis(2-methyl-3-furyl)disulphide | Meaty | 0.00076 | |
| 3-(methylthiol)propanal (methional) | Boiled potato, soy sauce | 0.2 | |
| 2-(methylthiol)propanal | Soy sauce | ||
| Thiols | 3-mercapto-3-methylbutylformate | Green blackcurrant | 0.0035 |
| 3-mercapto-3-methylbutylacetate | Roasty | ||
| 3-methyl-2-buten-1-thiol | Smoke, roasted, amine-like | 0.0003 | |
| 3-mercapto-3-methylbutanol | Hazelnut, roasted | 0.0035 | |
| methanethiol | Cooked potato | 0.2 | |
| 3-mercapto-3-methylbutyl formate | Cassis, cat, green | 0.0035 | |
| Terpenes | linalool | Flowery | 0.17 |
| limonene | 4 | ||
| geraniol | 1.1 | ||
| Thiazoles | 2,4-dimethyl-5-ethylthiazole | Earthy, roasty | |
| 2-acetyl-2-thiazoline | Roasted | ||
| Thiophene | 3-methylthiophene | ||
| 3,5-dihydro-4(2H)-thiophenone | Smoke, roasted |
7. Effects of the Brewing Methods on the Aroma Composition of Coffee Brew
7.1. Filtered Coffee
7.2. Espresso Coffee
7.3. Turkish Coffee
7.4. French Press Coffee
7.5. Moka Coffee
7.6. Neapolitan Coffee
7.7. Cold Drip and Cold Brew Coffee
8. Exploring the Impact of Tasting and Serving Modalities on Coffee Aroma Perception
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1H NMR | Proton Nuclear Magnetic Resonance |
| AEDA | Aroma Dilution Extract Analysis |
| ATR | Attenuated Total Reflectance |
| Bia | Espresso—Bialetti |
| Bo | Lungo—French Press |
| CFQA | Caffeoylferuloylquinic Acid |
| CHARM | Combined Hedonic Aroma Response Measurement |
| CoA | Coenzyme A |
| CQA | Caffeoylquinic Acid |
| CQI | Coffee Quality Institute |
| DAD | Diode Array Detector |
| DART | Direct Analysis in Real-Time Ionization |
| DE | Espresso from semi-automatic machine |
| diCQA | diCaffeoylquinic Acid |
| DL | Lungo from semi-automatic machine |
| F | Lungo—Filter Coffee |
| FQA | Feruloylquinic Acid |
| FTIR | Fourier Transform Infrared |
| GC | Gas Chromatography |
| GC-O | Gas Chromatography-Olfactometry |
| GC–FID | Gas Chromatography Flame Ionization Detector |
| GC–MS | Gas Chromatography Mass Spectrometry |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode Array Detection |
| HPLC-MS | High-Performance Liquid Chromatography Mass Spectrometry |
| HPLC-PDA | High Performance Liquid Chromatography with Photodiode Array Detection |
| HPLC-TOF | High Performance Liquid Chromatography Time of Flight |
| HPLC-UV | High-Performance Liquid Chromatography Ultra-Violet |
| ICO | International Coffee Organization |
| KK | Lungo—Karlsbader Kanne |
| LC–MS | Liquid Chromatography Mass Spectrometry |
| LDL | Low-Density Lipoprotein |
| MEKC | Micellar Electrokinetic Chromatography |
| MS-Nose | Mass Spectrometry-Nose |
| NE | Espresso—NEspresso |
| OAV | Odor Activity Value |
| OTA | Ochratoxin A |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| pCoQA | p-Coumaroylquinic Acid |
| RP-HPLC | Reverse Phase High-Performance Liquid Chromatography |
| SCA | Specialty Coffee Association |
| SCAA | Specialty Coffee Association of America |
| SCI | Specialty Coffee Institute |
| SE | Espresso from fully automatic machine |
| SL | Lungo from fully automatic machine |
| SPE | Solid Phase Extraction |
| SPME-GC/MS | Solid Phase Microextraction–Gas Chromatography/Mass Spectrometry |
| TDS | Temporal Dominance of Sensations |
| TPC | Total Phenolic Content |
| UHPLC-DAD-ESI-MS | Ultra-High-Performance Liquid Chromatography coupled with Diode Array Detection and Electrospray Ionization Mass Spectrometry |
| UHPLC-MS/MS | Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry |
| UV | Ultra-Violet |
References
- Van Hilten, H.J.; Fisher, P.J.; Wheeler, M.A. The Coffee Exporter’s Guide, 3rd ed.; International Trade Centre UNCTAD/GATT: Geneva, Switzerland, 2011. [Google Scholar]
- Petracco, M. Technology IV: Beverage preparation: Brewing trends for the new millennium. In Coffee Recent Developments; Blackwell Science Ltd.: London, UK, 2001; pp. 140–164. [Google Scholar]
- Chu, Y.F. Coffee: Emerging Health Effects and Disease Prevention, 1st ed.; John Wiley & Sons: Ames, IA, USA, 2012. [Google Scholar]
- Salamanca, C.A.; Fiol, N.; González, C.; Saez, M.; Villaescusa, I. Extraction of espresso coffee by using gradient of temperature. Effect on physicochemical and sensorial characteristics of espresso. Food Chem. 2017, 214, 622–630. [Google Scholar] [CrossRef]
- Illy, E.; Navarini, L. Neglected food bubbles: The espresso coffee foam. Food Biophys. 2011, 6, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Ferrari, M.; Liverani, F.S.; Liggieri, L.; Ravera, F. Dynamic tensiometric characterization of espresso coffee beverage. Food Hydrocoll. 2004, 18, 387–393. [Google Scholar] [CrossRef]
- Illy, A.; Viani, R. Espresso Coffee: The Science of Quality, 2nd ed.; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Parenti, A.; Guerrini, L.; Masella, P.; Spinelli, S.; Calamai, L.; Spugnoli, P. Comparison of espresso coffee brewing techniques. J. Food Eng. 2014, 121, 112–117. [Google Scholar] [CrossRef]
- Bhumiratana, N.; Adhikari, K.; Chambers, E., IV. Evolution of sensory aroma attributes from coffee beans to brewed coffee. LWT-Food Sci. Technol. 2011, 44, 2185–2192. [Google Scholar] [CrossRef]
- ISO 6668:2018; Green Coffee, Preparation of Samples for Use in Sensory Analysis. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 18794:2018; Coffee-Sensory Analysis—Vocabulary. International Organization for Standardization: Geneva, Switzerland, 2018.
- Revi, I. Coffee Cupping: Evaluation of Green Coffee Quality. In Coffee: Production, Quality and Chemistry; Farah, A., Farah, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 335–360. [Google Scholar]
- Lingle, T.R.; Menon, S.N. Cupping and grading—Discovering character and quality. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: London, UK, 2017; pp. 181–203. [Google Scholar]
- Spencer, M.; Sage, E.; Velez, M.; Guinard, J.X. Using single free sorting and multivariate exploratory methods to design a new coffee taster’s flavor wheel. J. Food Sci. 2016, 81, S2997–S3005. [Google Scholar] [CrossRef]
- Fuller, M.; Rao, N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavor of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Yuksel, A.N.; Bayram, M. New Trends In Turkish Coffee. In The Most Recent Studies in Science and Art, 1st ed.; Gece Publishing: Ankara, Türkiye, 2018; pp. 1924–1930. [Google Scholar]
- Folmer, B.; Blank, I.; Hofmann, T. Crema—Formation, Stabilization, and Sensation. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: London, UK, 2016; pp. 399–417. [Google Scholar]
- Petracco, M. The cup. In Espresso Coffee: The Science of Quality, 2nd ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 290–315. [Google Scholar]
- Mestdagh, F.; Glabasnia, A.; Giuliano, P. The Brew—Extracting for Excellence. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: London, UK, 2017; pp. 355–380. [Google Scholar]
- Severini, C.; Derossi, A.; Fiore, A.G.; De Pilli, T.; Alessandrino, O.; Del Mastro, A. How the variance of some extraction variables may affect the quality of espresso coffees served in coffee shops. J. Sci. Food Agric. 2016, 96, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Severini, C.; Ricci, I.; Marone, M.; Derossi, A.; De Pilli, T. Changes in the aromatic profile of espresso coffee as a function of the grinding grade and extraction time: A study by the electronic nose system. J. Agric. Food Chem. 2015, 63, 2321–2327. [Google Scholar] [CrossRef]
- Andueza, S.; De Peña, M.P.; Cid, C. Chemical and sensorial characteristics of espresso coffee as affected by grinding and torrefacto roast. J. Agric. Food Chem. 2003, 51, 7034–7039. [Google Scholar] [CrossRef]
- De Vivo, A.; Tricarico, M.C.; Sarghini, F. Espresso coffee design based on non-monotonic granulometric distribution of aromatic profile. Food Res. Int. 2019, 123, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.-F., Ed.; John Wiley & Sons, Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Batali, M.E.; Ristenpart, W.D.; Guinard, J.-X. Brew temperature, at fixed brew strength and extraction, has little impact on the sensory profile of drip brew coffee. Sci. Rep. 2020, 10, 16450. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, E.E. The Soluble Solids in Beverage Coffee as an Index to Up Quality; Coffee Brewing Institute: New York, NY, USA, 1957. [Google Scholar]
- Andueza, S.; Maeztu, L.; Dean, B.; de Pena, M.P.; Bello, J.; Cid, C. Influence of water pressure on the final quality of arabica espresso coffee. Application of multivariate analysis. J. Agric. Food Chem. 2002, 50, 7426–7431. [Google Scholar] [CrossRef]
- Moeenfard, M.; Silva, J.A.; Borges, N.; Santos, A.; Alves, A. Diterpenes in espresso coffee: Impact of preparation parameters. Eur. Food Res. Technol. 2015, 240, 763–773. [Google Scholar] [CrossRef]
- De Peña, M.P.; Ludwig, I.A.; Cid, C. Beverage preparation. In Coffee: Production, Quality and Chemistry; Farah, A., Farah, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 272–291. [Google Scholar]
- Hendon, C.H.; Colonna-Dashwood, L.; Colonna-Dashwood, M. The role of dissolved cations in coffee extraction. J. Agric. Food Chem. 2014, 62, 4947–4950. [Google Scholar] [CrossRef]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Asiah, N.; Aqil, M.; Dwiranti, N.S.; David, W.; Ardiansyah, A. Sensory and chemical changes of cold and hot brew arabica coffee at various resting time. Asia Pac. J. Sustain. Agric. Food Energy 2019, 7, 23–26. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.A.; Leake, D.S.; Ames, J.M. In vitro antioxidant activity of coffee compounds and their metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef]
- Mazzafera, P.; Baumann, T.W.; Shimizu, M.M. Decaf and the steeplechase towards decaffito the coffee from caffeine-free Arabica plants. Trop. Plant Biol. 2009, 2, 63–76. [Google Scholar] [CrossRef]
- Schuster, J.; Mitchell, E.S. More than just caffeine: Psychopharmacology of methylxanthine interactions with plant-derived phytochemicals. Prog. Neuro-Psyhopharm. Biol. Psychiatry. 2019, 32, 263–264. [Google Scholar] [CrossRef]
- Zulak, K.G.; Liscome, D.K.; Ashihara, H.; Facchini, P.J. Alkaloids. In Plant Secondary Metabolites: Occurrence Structure, and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2006; pp. 102–136. [Google Scholar]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef]
- Ashihara, H.; Crozier, A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. Adv. Bot. Res. 1999, 30, 118–205. [Google Scholar]
- Flament, I.; Gautschi, F.; Winter, M.; Willhalm, B.; Stoll, M. Les composants furanniques de l’arôme café: Quelques aspects chimiques et spectroscopiques. In Proceedings of the 3rd International Scientific Colloquium on Green and Roasted Coffee Chemistry, Trieste, Italy, 2–9 June 1967; ASIC: Paris, France, 1968; pp. 197–215. [Google Scholar]
- Bicho, N.C.; Lidon, F.C.; Ramalho, J.C.; Leitao, A.E. Quality assessment of Arabica and Robusta green and roasted coffees—A review. Emir. J. Food Agric. 2013, 25, 945–950. [Google Scholar] [CrossRef]
- Silvarola, M.B.; Mazzafera, P.; Fazuoli, L.C. A naturally decaffeinated arabica coffee. Nature 2004, 249, 826. [Google Scholar] [CrossRef] [PubMed]
- Burdan, F. Caffeine in Coffee. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2014; pp. 201–207. [Google Scholar]
- Lee, C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin. Chim. Acta 2000, 295, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.G.; Moraes, R.S.; Perrone, D.; Maia, L.C.; Santos, K.R.N.; Iório, N.L.P.; Farah, A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutans. Food Chem. 2010, 118, 782–788. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grzeskowiak, A.; Grzeskowiak, T. Analytical methods applied for the characterization and the determination of bioactive compounds in coffee. Eur. Food Res. Technol. 2015, 240, 19–31. [Google Scholar] [CrossRef]
- Bravo, J.; Juaniz, I.; Monente, C.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. J. Agric. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef]
- Santini, A.; Ferracane, R.; Mikušová, P.; Eged, Š.; Šrobárová, A.; Meca, G.; Mañes, J.; Ritieni, A. Influence of different coffee drink preparations on ochratoxin A content and evaluation of the antioxidant activity and caffeine variations. Food Control 2011, 22, 1240–1245. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A. Roasting conditions, grinding level and brewing method highly affect the healthy benefits of a coffee cup. Int. J. Clin. Nutr. Diet. 2018, 4, 127. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan coffee brew chemical analysis in comparison to espresso, moka and American brews. Food Res. Int. 2014, 61, 152–160. [Google Scholar] [CrossRef]
- McCusker, R.R.; Fuehrlein, B.; Goldberger, B.A.; Gold, M.S.; Cone, E.J. Caffeine content of decaffeinated coffee. J. Anal. Toxicol. 2006, 30, 611–613. [Google Scholar] [CrossRef] [PubMed]
- McCusker, R.R.; Goldberger, B.A.; Cone, E.J. Caffeine content of specialty coffees. J. Anal. Toxicol. 2003, 27, 520–522. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, Y.; Jiang, F.; Jiang, T.; Zhu, J.; Tang, W.; Liu, X.; Zhou, Y.; Yu, L. Comparison of characterization of cold brew and hot brew coffee prepared at various roasting degrees. J. Food Process. Preserv. 2023, 2023, 3175570. [Google Scholar] [CrossRef]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and comparison of cold brew and cold drip coffee extraction methods. J. Sci. Food Agric. 2019, 99, 391–399. [Google Scholar] [CrossRef]
- Olechno, E.; Pucion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of various factors on caffeine content in coffee brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef]
- Merecz, A.; Marusinska, A.; Karwowski, B.T. The content of biologically active substances and antioxidant activity in coffee depending on brewing method. Pol. J. Nat. Sci. 2018, 33, 267–284. [Google Scholar]
- Caprioli, G.; Cortese, M.; Maggi, F.; Minnetti, C.; Odello, L.; Sagratini, G.; Vittori, S. Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: The influence of espresso machines and coffee cultivars. Int. J. Food Sci. Nutr. 2015, 65, 46. [Google Scholar] [CrossRef] [PubMed]
- Prankerd, R.J. Critical compilation of pKa values for pharmaceutical substances. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: London, UK, 2007; Volume 33, pp. 1–33. [Google Scholar]
- Clifford, M.N. Chlorogenic acids and other cinnamates: Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Lee, T.A.; Kempthorn, R.; Hardy, J.K. Compositional changes in brewed coffee as function of brewing time. J. Food Sci. 1992, 57, 1417–1419. [Google Scholar] [CrossRef]
- DIN-10767:1992-05; Analysis of Coffee and Coffee Products: Determination of Chlorogenic Acids Content—HPLC Method. DIN: Berlin, Germany, 1992.
- Upadhyay, R.; Ramalakshmi, K.; Rao, L.J.M. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 2012, 130, 184–188. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Podsedek, A.; Zyzelewicz, D.; Materska, M.; Jankowski, S.; Janda, B. Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur. Food Res. Technol. 2009, 228, 913–922. [Google Scholar] [CrossRef]
- Tfouni, S.A.V.; Carreiro, L.B.; Teles, C.R.A.; Furlani, R.P.Z.; Cipolli, K.M.V.A.B.; Camargo, M.C.R. Caffeine and chlorogenic acids intake from coffee brew: Influence of roasting degree and brewing procedure. Int. J. Food Sci. Technol. 2014, 49, 747–752. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and antioxidant activity of cold brew coffee. Sci. Rep. 2018, 8, 16030. [Google Scholar] [CrossRef]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of caffeoylquinic acids in coffee brews by HPLC-DAD. J. Anal. Meth. Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef]
- Beder-Belkhiria, W.; Zeghichi-Hamri, S.; Kadri, N.; Boulekbache-Makhlouf, L.; Cardoso, S.; Oukhmanou-Bensidhoum, S.; Madani, K. Hydroxycinnamic acids profiling, in vitro evaluation of total phenolic compounds, caffeine and antioxidant properties of coffee imported, roasted and consumed in Algeria. Mediterr. J. Nutr. Metab. 2018, 11, 51–63. [Google Scholar] [CrossRef]
- Kaur, M.; Tyagi, S.; Kundu, N. Effect of Brewing methods and time on secondary metabolites, total flavonoid and phenolic content of green and roasted coffee Coffea arabica, Coffea canephora and Monsooned Malabar. Eur. J. Med. Plants 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Ormaza-Zapata, A.M.; Díaz-Arango, F.O.; Rojano, B.A. The effect of pressure filtration coffee preparation methods (Coffea arabica L. var. Castillo) on antioxidant content and activity, and beverage acceptance. Rev. DYNA 2019, 86, 261–270. [Google Scholar]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Olechno, E.; Pucion-Jakubik, A.; Markiewicz-Zukowska, R.; Socha, K. Impact of brewing methods on total phenolic content (TPC) in various types of coffee. Molecules 2020, 25, 5274. [Google Scholar] [CrossRef] [PubMed]
- Nosal, B.M.; Sakaki, J.R.; Kim, D.-O.; Chun, O.K. Impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to the body’s antioxidant status. Food Sci. Biotechnol. 2022, 31, 1081–1088. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2001, 11, 364–373. [Google Scholar] [CrossRef]
- Oosterveld, A.; Voragen, A.G.J.; Schols, H.A. Effect of roasting on the carbohydrate composition of Coffea arabica beans. Carbohydr. Polym. 2003, 54, 183–192. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S.; Kroll, J. Characterisation of 11S protein fractions and phenolic compounds from green coffee beans under special consideration of their interactions. A review. Dtsch. Lebensm. Rundsch. 2005, 101, 148–160. [Google Scholar]
- Obretenov, C.; Demyttenaere, J.; Tehrani, K.A.; Adams, A.; Kersiene, M.; De Kimpe, N. Flavor release in the presence of melanoidins prepared from L-(+)-ascorbic acid and amino acids. J. Agric. Food Chem. 2002, 50, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J.; Fernandez-Fraguas, C.; Jimenez-Perez, S. Iron-binding ability of melanoidins from food and model systems. Food Chem. 2005, 90, 821–827. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Morales, F.J. Estimation of dietary intake of melanoidins from coffee and bread. Food Funct. 2011, 2, 117–123. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Pérez-Hernández, L.M.; Chávez-Quiroz, K.; Medina-Juárez, L.Á.; Gámez Meza, N. Phenolic characterization, melanoidins, and antioxidant activity of some commercial coffees from Coffea arabica and Coffea canephora. J. Mex. Chem. Soc. 2012, 56, 430–435. [Google Scholar]
- Gigl, M.; Hofmann, T.; Frank, O. NMR-based studies on odorant–melanoidin interactions in coffee beverages. J. Agric. Food Chem. 2021, 69, 15334–15344. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.; Gómez-Domínguez, I.; Hurtado-Ribeira, R.; Martin, D.; Coimbra, M.A.; del Castillo, M.D.; Coreta-Gomes, F. In vitro human colonic fermentation of coffee arabinogalactan and melanoidin-rich fractions. Int. J. Biol. Macromol. 2024, 275, 133740. [Google Scholar] [CrossRef]
- Bicho, N.C.; Leitao, A.E.; Ramalho, J.C.; Lidon, F.C. Identification of chemical clusters discriminators of the roast degree in Arabica and Robusta coffee beans. Eur. Food Res. Technol. 2011, 233, 303–311. [Google Scholar] [CrossRef]
- Farah, A.; Ferreira, T.; Vieira, A.C. Trigonelline and derivatives. In Coffee: Production, Quality and Chemistry; Farah, A., Farah, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 627–640. [Google Scholar]
- Ky, C.L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Heo, J.; Adhikari, K.; Choi, K.S.; Lee, J. Analysis of caffeine, chlorogenic acid, trigonelline, and volatile compounds in cold brew coffee using high-performance liquid chromatography and solid-phase microextraction—Gas chromatography-mass spectrometry. Foods 2020, 9, 1746. [Google Scholar] [CrossRef]
- Del Campo, G.; Berregi, I.; Caracena, R.; Zuriarrain, J. Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl) furfural in soluble coffees by 1H NMR spectrometry. Talanta 2010, 81, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Poerner Rodrigues, N.; Bragagnolo, N. Identification and quantification of bioactive compounds in coffee brews by HPLC–DAD–MSn. J. Food Compos. Anal. 2013, 32, 105–115. [Google Scholar] [CrossRef]
- Angelino, D.; Tassotti, M.; Brighenti, F.; Del Rio, D.; Mena, P. Niacin, alkaloids and (poly)phenolic compounds in the most widespread Italian capsule-brewed coffees. Sci. Rep. 2018, 8, 17874. [Google Scholar] [CrossRef]
- Midttun, Ø.; Ulvik, A.; Nygård, O.; Ueland, P.M. Performance of plasma trigonelline as a marker of coffee consumption in an epidemiologic setting. Am. J. Clin. Nutr. 2018, 107, 941–947. [Google Scholar] [CrossRef]
- Nugrahini, A.D.; Ishida, M.; Nakagawa, T.; Nishi, K.; Sugahara, T. Trigonelline: An alkaloid with anti-degranulation properties. Mol. Immunol. 2020, 118, 201–209. [Google Scholar] [CrossRef]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-carbolines in food: A review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef]
- Rommelspacher, H. β-Carbolines and Neuroprotection: Inhibition of Monoamine Oxidase. In Isoquinolines and Beta-Carbolines as Neurotoxins and Neuroprotectants: New Vistas in Parkinson’s Disease Therapy; Antkiewicz-Michaluk, L., Rommelspacher, H., Eds.; Springer: New York, NY, USA, 2012; pp. 115–124. [Google Scholar]
- Li, S.; Teng, L.; Liu, W.; Cheng, X.; Jiang, B.; Wang, Z.; Wang, C. Pharmacokinetic study of harmane and its metabolites in rat after intravenous and oral administration by UPLC-ESI-MS/MS. Pharm. Biol. 2016, 54, 1768–1781. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R.; Fedoruk-Wyszomirska, A.; Piechowska, P.; Mildner-Szkudlarz, S.; Bajerska, J.; Wojtowicz, E.; Przygoński, K.; Gurda, D.; Kubicka, W.; Wyszko, E. β-Carbolines in experiments on laboratory animals. Int. J. Mol. Sci. 2020, 21, 5245. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Casal, S.; Oliveira, B.M.P.P. Factors influencing the norharman and harman contents in espresso coffee. J. Agric. Food Chem. 2007, 55, 1832–1838. [Google Scholar] [CrossRef]
- Alves, R.C.; Mendes, E.; Oliveira, B.M.P.P.; Casal, S. Norharman and harman in instant coffee and coffee substitutes. Food Chem. 2010, 120, 1238–1241. [Google Scholar] [CrossRef]
- Herraiz, T. Identification and occurrence of the bioactive β-carbolines norharman and harman in coffee brews. Food Addit. Contam. 2002, 19, 748–754. [Google Scholar] [CrossRef]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.M.; Van Gent, T.; Buytenhek, R.; Princen, H.M.; Katan, M.B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; van der Weg, G.; Grdert, R.; van de Bovenkamp, P.; Charrier, A.; Katan, M.B. Levels of cafestol, kahweol and related diterpenoids in wild species of the coffee plant. J. Agric. Food Chem. 1997, 45, 3065–3069. [Google Scholar] [CrossRef]
- Dias, R.C.E.; de Faria-Machado, A.F.; Mercadante, A.Z.; Bragagnolo, N.; Benassi, M.T. Roasting process affects the profile of diterpenes in coffee. Eur. Food Res. Technol. 2014, 239, 961–970. [Google Scholar] [CrossRef]
- Gross, G.; Jaccaud, E.; Huggett, A.C. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem. Toxicol. 1997, 35, 547–554. [Google Scholar] [CrossRef]
- Sridevi, V.; Giridhar, P.; Ravishankar, G.A. Evaluation of roasting and brewing effect on antinutritional diterpenes-cafestol and kahweol in coffee. Glob. J. Med. Res. 2017, 11, 1–7. [Google Scholar]
- Araujo, J.M.A.; Sandi, D. Extraction of coffee diterpenes and coffee oil using supercritical carbon dioxide. Food Chem. 2006, 101, 1087–1094. [Google Scholar] [CrossRef]
- Oigman, S.S.; de Souza, R.; dos Santos Júnior, H.M.; Hovell, A.M.C.; Hamerski, L.; Rezende, C.M. Microwave-assisted methanolysis of green coffee oil. Food Chem. 2012, 134, 999–1004. [Google Scholar] [CrossRef]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. Annu. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef]
- Moreira Novaes, F.J.; Calvente Bayan, F.; Radler de Aquino Neto, F.; Moraes Rezende, C. The occurrence of cafestol and kahweol diterpenes in different coffee brews. Coffee Sci. 2019, 14, 265–280. [Google Scholar] [CrossRef]
- De Souza Gois Barbosa, M.; dos Santos Scholz, M.B.; Sorane Good Kitzberger, C.; de Toledo Benassi, M. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Leitão, A.L. Occurrence of ochratoxin A in coffee: Threads and solutions—A mini-review. Beverages 2019, 5, 36. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Abdi, L.; Sengling Cebin Coppa, C.F.; Tuanny Franco, L.; Fernandes de Oliveira, C.A. The concentration and prevalence of ochratoxin A in coffee and coffee-based products: A global systematic review, meta-analysis and meta-regression. Fungal Biol. 2019, 123, 611–617. [Google Scholar] [CrossRef]
- Heintz, M.M.; Doepker, C.L.; Wikoff, D.S.; Hawks, S.E. Assessing the food safety risk of ochratoxin A in coffee: A toxicology-based approach to food safety planning. J. Food Sci. 2021, 86, 4799–4810. [Google Scholar] [CrossRef] [PubMed]
- La Pera, L.; Avellone, G.; Lo Turco, V.; Di Bella, G.; Agozzino, P.; Dugo, G. Influence of roasting and different brewing processes on Ochratoxin A content in coffee determined by HPLC-FLD. Food Addit. Contam. 2008, 25, 1257–1263. [Google Scholar] [CrossRef]
- Pakshir, K.; Dehghani, A.; Nouraei, H.; Zareshahrabadi, Z.; Zomorodian, K. Evaluation of fungal contamination and ochratoxin A detection in different types of coffee by HPLC-based method. J. Clin. Lab. Anal. 2021, 35, e24001. [Google Scholar] [CrossRef] [PubMed]
- Gopinandhan, T.N.; Kannan, G.S. Effect of roasting and brewing on ochratoxin A in coffee. J. Food Sci. Technol. 2009, 46, 459–462. [Google Scholar]
- Casal, S.; Mendes, E.; Rui Alves, M.; Alves, R.C.; Beatriz, M.; Oliveira, P.P.; Ferreira, M.A. Free and conjugated biogenic amines in green and roasted coffee beans. J. Agric. Food Chem. 2004, 52, 6188–6192. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Wagner, M.; Mayer, H.K. Thermogenic formation of biogenic amines during commercial coffee roasting processes. LWT-Food Sci. Technol. 2022, 154, 112664. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2015, 175, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Özdestan, Ö. Evaluation of bioactive amine and mineral levels in Turkish coffee. Food Res. Int. 2014, 61, 167–175. [Google Scholar] [CrossRef]
- Tfouni, S.A.V.; Serrate, C.S.; Leme, F.M.; Camargo, M.C.R.; Teles, C.R.A.; Cipolli, K.M.V.A.B.; Furlani, R.P.Z. Polycyclic aromatic hydrocarbons in coffee brew: Influence of roasting and brewing procedures in two Coffea cultivars. LWT-Food Sci. Technol. 2013, 50, 526–530. [Google Scholar] [CrossRef]
- Houessou, J.K.; Maloug, S.; Leveque, A.S.; Delteil, C.; Heyd, B.; Camel, V. Effect of roasting conditions on the polycyclic aromatic hydrocarbon content in ground Arabica coffee and coffee brew. J. Agric. Food Chem. 2007, 55, 9719–9726. [Google Scholar] [CrossRef]
- Yeretzian, C.; Jordan, A.; Badoud, R.; Lindinger, W. From the green bean to the cup of coffee: Investigating coffee roasting by on-line monitoring of volatiles. Eur. Food Res. Technol. 2002, 214, 92–104. [Google Scholar] [CrossRef]
- Houessou, J.K.; Goujot, D.; Heyd, B.; Camel, V. Modeling the formation of some polycyclic aromatic hydrocarbons during the roasting of Arabica coffee samples. J. Agric. Food Chem. 2008, 56, 3648–3656. [Google Scholar] [CrossRef]
- Orecchio, S.; Paradiso Ciotti, V.; Culotta, L. Polycyclic aromatic hydrocarbons (PAHs) in coffee brew samples: Analytical method by GC–MS, profile, levels and sources. Food Chem. Toxicol. 2009, 47, 819–826. [Google Scholar] [CrossRef]
- Viegas, O.; Pinho, O.; Ferreira, I.M.P.L.V.O. Polycyclic aromatic hydrocarbons. In Coffee: Production, Quality and Chemistry; Farah, A., Farah, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 705–725. [Google Scholar]
- Navarro, R.P.; Ishikawa, H.; Morimoto, K.; Tatsumi, K. Enhancing the release and plant uptake of PAHs with a water-soluble purine alkaloid. Chemosphere 2009, 76, 1109–1113. [Google Scholar] [CrossRef]
- Aresta, A.M.; Zambonin, C. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Coffee Samples by DI-SPME-GC/MS. Food Anal. Methods 2023, 16, 1009–1016. [Google Scholar] [CrossRef]
- Hamrah, R.P.; Rahimi, A.; Yarahmadi, M.; Talebi-Ghane, E.; Mehri, F. Health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in coffee-based products: A meta-analysis study and systematic review. J. Agric. Food Res. 2024, 18, 101508. [Google Scholar] [CrossRef]
- Rattanarat, P.; Chindapan, N.; Devahastin, S. Comparative evaluation of acrylamide and polycyclic aromatic hydrocarbons contents in Robusta coffee beans roasted by hot air and superheated steam. Food Chem. 2021, 341 Pt 1, 128266. [Google Scholar] [CrossRef]
- IARC. Some Industrial Chemicals; IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; International Agency for Research on Cancer: Lyon, France, 1994; Volume 60, pp. 389–441. [Google Scholar]
- Guenther, H.; Anklam, E.; Wenzl, T.; Stadler, R.H. Acrylamide in coffee: Review of progress in analysis, formation and level reduction. Food Addit. Contam. 2007, 24, 60–70. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Delatour, T.; Périsset, A.; Goldmann, T.; Riediker, S.; Stadler, R.H. Improved sample preparation to determine acrylamide in difficult matrixes such as chocolate powder, cocoa, and coffee by liquid chromatography tandem mass spectroscopy. J. Agric. Food Chem. 2004, 52, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Fagt, S. Analysis of acrylamide in coffee and dietary exposure to acrylamide from coffee. Anal. Chim. Acta 2004, 520, 177–182. [Google Scholar] [CrossRef]
- Alves, R.C.; Soares, C.; Casal, S.; Fernandes, J.O.; Beatriz, M.; Oliveira, B.M.P.P. Acrylamide in espresso coffee: Influence of species, roast degree and brew length. Food Chem. 2010, 119, 929–934. [Google Scholar] [CrossRef]
- Mesias, M.; Morales, F.J. Acrylamide in coffee: Estimation of exposure from vending machines. J. Food Comp. Anal. 2016, 48, 8–12. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Angeloni, S.; Bartolucci, D.; Fioretti, L.; Sagratini, G.; Vittori, S.; Caprioli, G. Effect of brewing methods on acrylamide content and antioxidant activity: Studying eight different filter coffee preparations. Antioxidants 2023, 12, 1888. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Romani, S. Acrylamide in coffee: Formation and possible mitigation strategies—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3807–3821. [Google Scholar] [CrossRef]
- Banchero, M.; Pellegrino, G.; Manna, L. Supercritical fluid extraction as a potential mitigation strategy for the reduction of acrylamide level in coffee. J. Food Eng. 2013, 115, 292–297. [Google Scholar] [CrossRef]
- Cha, M. Enzymatic control of the acrylamide level in coffee. Eur. Food Res. Technol. 2013, 236, 567–571. [Google Scholar] [CrossRef]
- Anese, M. Chapter 9—Acrylamide in Coffee and Coffee Substitutes. In Acrylamide in Food Analysis, Content and Potential Health Effects, 1st ed.; Gokmen, V., Ed.; Academic Press: London, UK, 2016; pp. 181–195. [Google Scholar]
- Bedade, D.K.; Sutar, Y.B.; Singhal, R.S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chem. 2019, 275, 95–104. [Google Scholar] [CrossRef]
- Akillioglu, H.G.; Gökmen, V. Mitigation of acrylamide and hydroxymethyl furfural in instant coffee by yeast fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hu, G.; Hong, D.; Guo, T.; Li, J.; Li, Z.; Qiu, M. Review on factors affecting coffee volatiles: From seed to cup. J. Sci. Food Agric. 2022, 102, 1341–1352. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, S.J.; Yu, J.S.; Lee, D.Y. Interactive effect of post-harvest processing method, roasting degree, and brewing method on coffee metabolite profiles. Food Chem. 2022, 397, 133749. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joët, T. Climatic factors directly impact the volatile organic compound fingerprint in green Arabica coffee bean as well as coffee beverage quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Girma, B.; Sualeh, A. A review of coffee processing methods and their influence on aroma. Int. J. Food Eng. Technol. 2022, 6, 7–16. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Dulsat-Serra, N.; Quintanilla-Casas, B.; Vichi, S. Volatile thiols in coffee: A review on their formation, degradation, assessment and influence on coffee sensory quality. Food Res. Int. 2016, 89, 982–988. [Google Scholar] [CrossRef]
- Gill, S.; Kavanagh, M.; Cherry, W.; Barker, M.; Weld, M.; Cooke, G.M. A 28-day gavage toxicity study in male Fischer 344 Rats with 2-methylfuran. Toxicol. Pathol. 2014, 42, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Ruosi, M.R.; Cagliero, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Quantitative analysis of volatiles from solid matrices of vegetable origin by high concentration capacity headspace techniques: Determination of furan in roasted coffee. J. Chromatogr. A 2011, 1218, 753–762. [Google Scholar] [CrossRef]
- Becalski, A.; Halldorson, T.; Hayward, S.; Roscoe, V. Furan, 2-methylfuran and 3-methylfuran in coffee on the Canadian market. J. Food Comp. Anal. 2016, 47, 113–119. [Google Scholar] [CrossRef]
- Altaki, M.S.; Santos, F.J.; Galceran, M.T. Occurrence of furan in coffee from Spanish market: Contribution of brewing and roasting. Food Chem. 2011, 126, 1527–1532. [Google Scholar] [CrossRef]
- Pavesi Arisseto, A.; Vicente, E.; Soares Ueno, M.; Verdiani Tfouni, S.A.; De Figueiredo Toledo, M.C. Furan levels in coffee as influenced by species, roast degree, and brewing procedures. J. Agric. Food Chem. 2011, 59, 3118–3124. [Google Scholar] [CrossRef]
- Zoller, O.; Sager, F.; Reinhard, H. Furan in food: Headspace method and product survey. Food Addit. Contam. 2007, 24 (Suppl. 1), 91–107. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for public health related to the presence of furan and methylfurans in food. EFSA J. 2017, 15, e05005. [Google Scholar]
- Rahn, A.; Yeretzian, C. Impact of consumer behavior on furan and furan-derivative exposure during coffee consumption. A comparison between brewing methods and drinking preferences. Food Chem. 2019, 272, 514–522. [Google Scholar] [CrossRef]
- Genovese, A.; De Vivo, A.; Aprea, A.; Tricarico, M.C.; Sacchi, R.; Sarghini, F. Particle size and variety of coffee used as variables in mitigation of furan and 2-methylfuran content in espresso coffee. Food Chem. 2021, 361, 130037. [Google Scholar] [CrossRef]
- Dorfner, R.; Ferge, T.; Kettrup, A.; Zimmermann, R.; Yeretzian, C. Real-time monitoring of 4-vinylguaiacol, guaiacol, and phenol during coffee roasting by resonant laser ionization time-of-flight mass spectrometry. J. Agric. Food Chem. 2003, 51, 5768–5773. [Google Scholar] [CrossRef]
- Semmelroch, P.; Grosch, W. Analysis of roasted coffee powders and brews by gas chromatography-olfactometry of headspace samples. LWT-Food Sci. Technol. 1995, 28, 310–313. [Google Scholar] [CrossRef]
- Semmelroch, P.; Grosch, W. Studies on character impact odorants of coffee brews. J. Agric. Food Chem. 1996, 44, 537–543. [Google Scholar] [CrossRef]
- Czerny, M.; Grosch, W. Potent odorants of raw Arabica coffee. Their changes during roasting. J. Agric. Food Chem. 2000, 48, 868–872. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on coffee aroma: II. Roasted coffee. J. Food Comp. Anal. 2007, 20, 297–307. [Google Scholar] [CrossRef]
- Schenker, S.; Heinemann, C.; Huber, M.; Pompizzi, R.; Perren, R.; Escher, R. Impact of roasting conditions on the formation of aroma compounds in coffee beans. J. Food Sci. 2002, 67, 60–66. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef]
- Petisca, C.; Pérez-Palacios, T.; Farah, A.; Pinho, O.; Ferreira, I.M. Furans and other volatile compounds in ground roasted and espresso coffee using headspace solid-phase microextraction: Effect of roasting speed. Food Bioprod. Process. 2013, 91, 233–241. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Hirano, Y.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Characterization of headspace aroma compounds of freshly brewed Arabica coffees and studies on a characteristic aroma compound of Ethiopian coffee. J. Food Sci. 2008, 73, C335–C346. [Google Scholar] [CrossRef]
- Toledo, P.R.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the different aspects related to coffee quality and their volatile compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef]
- De Vivo, A.; Genovese, A.; Tricarico, M.C.; Aprea, A.; Sacchi, R.; Sarghini, F. Volatile compounds in espresso resulting from a refined selection of particle size of coffee powder. J. Food Comp. Anal. 2022, 114, 104779. [Google Scholar] [CrossRef]
- Mayer, F.; Czerny, M.; Grosch, W. Sensory study of the character impact aroma compounds of a coffee beverage. Eur. Food Res. Technol. 2000, 211, 272–276. [Google Scholar] [CrossRef]
- Acree, T.E. Peer Reviewed: GC/Olfactometry GC with a sense of smell. Anal. Chem. 1997, 69, 170A–175A. [Google Scholar] [CrossRef]
- Zellner, B.D.A.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavor analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Toci, A.T.; Boldrin, M.V.Z. Coffee beverages and their aroma compounds. In Natural and Artificial Flavoring Agents and Food Dyes; Academic Press: Cambridge, MA, USA, 2018; pp. 397–425. [Google Scholar]
- Laukaleja, I.; Koppel, K. Aroma active compound perception in differently roasted and brewed coffees by gas chromatography–olfactometry. J. Sens. Stud. 2021, 36, e12708. [Google Scholar] [CrossRef]
- Mahmud, M.C.; Keast, R.; Mohebbi, M.; Shellie, R.A. Identifying aroma-active compounds in coffee-flavored dairy beverages. J. Food Sci. 2022, 87, 982–997. [Google Scholar] [CrossRef]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.M.; Blekas, G.; Paraskevopoulou, A. Single origin coffee aroma: From optimized flavor protocols and coffee customization to instrumental volatile characterization and chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Huang, D.; Chen, S.; Zhu, S. Characterization and discrimination of volatile compounds in roasted Arabica coffee beans from different origins by combining GC-TOFMS, GC-IMS, and GC-E-Nose. Food Chem. 2025, 481, 144079. [Google Scholar] [CrossRef]
- Gantner, M.; Kostyra, E.; Górska-Horczyczak, E.; Piotrowska, A. Effect of Temperature and Storage on Coffee’s Volatile Compound Profile and Sensory Characteristics. Foods 2024, 13, 3995. [Google Scholar] [CrossRef]
- Clarke, R.; Vitzthum, O.G. Coffee: Recent Developments; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Miyazato, H.; Nakamura, M.; Hashimoto, S.; Hayashi, S. Identification of the odor-active cyclic diketone cis-2,6-dimethyl-1,4-cyclohexanedione in roasted Arabica coffee brew. Food Chem. 2013, 138, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Scheidig, C.; Czerny, M.; Schieberle, P. Changes in key odorants of raw coffee beans during storage under defined conditions. J. Agric. Food Chem. 2007, 55, 5768–5775. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Cristalli, G.; Maggi, F.; Odello, L.; Ricciutelli, M.; Sagratini, G.; Sirocchi, V.; Tomassoni, G.; Vittori, S. Optimization of espresso machine parameters through the analysis of coffee odorants by HS-SPME–GC/MS. Food Chem. 2012, 135, 1127–1133. [Google Scholar] [CrossRef]
- Mestdagh, F.; Davidek, T.; Chaumonteuil, M.; Folmer, B.; Blank, I. The kinetics of coffee aroma extraction. Food Res. Int. 2014, 63, 271–274. [Google Scholar] [CrossRef]
- Maeztu, L.; Sanz, C.; Andueza, S.; Paz de Pena, M.; Bello, J.; Cid, C. Characterization of espresso coffee aroma by static headspace GC–MS and sensory flavor profile. J. Agric. Food Chem. 2001, 49, 5437–5444. [Google Scholar] [CrossRef]
- Rocha, S.; Maeztu, L.; Barros, A.; Cid, C.; Coimbra, M.A. Screening and distinction of coffee brews based on headspace solid phase microextraction/gas chromatography/principal component analysis. J. Sci. Food Agric. 2004, 84, 43–51. [Google Scholar] [CrossRef]
- Michishita, T.; Akiyama, M.; Hirano, Y.; Ikeda, M.; Sagara, Y.; Araki, T. Gas chromatography/olfactometry and electronic nose analyses of retronasal aroma of espresso and correlation with sensory evaluation by an artificial neural network. J. Food Sci. 2010, 75, S477–S489. [Google Scholar] [CrossRef]
- López-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006, 54, 8560–8566. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.; Romano, A.; Yener, S.; Barnabà, M.; Navarini, L.; Märk, T.D.; Biasoli, F.; Gasperi, F. Understanding flavor perception of espresso coffee by the combination of a dynamic sensory method and in-vivo nose space analysis. Food Res. Int. 2015, 69, 9–20. [Google Scholar] [CrossRef]
- Lolli, V.; Acharjee, A.; Angelino, D.; Tassotti, M.; Del Rio, D.; Mena, P.; Caligiani, A. Chemical Characterization of Capsule-Brewed Espresso Coffee Aroma from the Most Widespread Italian Brands by HS-SPME/GC-MS. Molecules 2020, 25, 1166. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Pineau, N.; Matthey-Doret, W.; Ali, S.; Sudre, J.; Germain, J.C.; Kolodziejczyk, E.; Pollien, P.; Labbe, D.; Jarisch, C.; et al. Impact of crema on the aroma release and the in-mouth sensory perception of espresso coffee. Food Funct. 2012, 3, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Dold, S.; Lindinger, C.; Kolodziejczyk, E.; Pollien, P.; Ali, S.; Germain, J.C.; Perin, S.G.; Pineau, N.; Folmer, B.; Engel, K.-H.; et al. Influence of foam structure on the release kinetics of volatiles from espresso coffee prior to consumption. J. Agric. Food Chem. 2011, 59, 11196–11203. [Google Scholar] [CrossRef]
- Bicchi, C.P.; Panero, O.M.; Pellegrino, G.M.; Vanni, A.C. Characterization of roasted coffee and coffee beverages by solid phase microextraction–gas chromatography and principal component analysis. J. Agric. Food Chem. 1997, 45, 4680–4686. [Google Scholar] [CrossRef]
- Kıvançlı, J.; Elmacı, Y. Characterization of Turkish-style boiled coffee aroma by gas chromatography and mass spectrometry and descriptive analysis techniques. Int. J. Food Prop. 2016, 19, 1671–1686. [Google Scholar] [CrossRef]
- Amanpour, A.; Selli, S. Differentiation of volatile profiles and odor activity values of Turkish coffee and French press coffee. J. Food Process. Preserv. 2016, 40, 1116–1124. [Google Scholar] [CrossRef]
- Ayseli, M.T.; Kelebek, H.; Selli, S. Elucidation of aroma-active compounds and chlorogenic acids of Turkish coffee brewed from medium and dark roasted Coffea arabica beans. Food Chem. 2021, 338, 127821. [Google Scholar] [CrossRef] [PubMed]
- Azizah, D.R.; Sunarharum, W.B.; Mahatmanto, T.; Kartika, A.A.; Hakim, L. Exploring the impact of various manual brewing techniques on the physicochemical and sensory characteristics of brewed coffee. IOP Conf. Ser. Earth Environ. Sci. 2024, 1299, 012011. [Google Scholar] [CrossRef]
- López-Galilea, I.; De Pena, M.P.; Cid, C. Correlation of selected constituents with the total antioxidant capacity of coffee beverages: Influence of the brewing procedure. J. Agric. Food Chem. 2007, 55, 6110–6117. [Google Scholar] [CrossRef]
- Sanz, C.; Maeztu, L.; Zapelena, M.J.; Bello, J.; Cid, C. Profiles of volatile compounds and sensory analysis of three blends of coffee: Influence of different proportions of Arabica and Robusta and influence of roasting coffee with sugar. J. Sci. Food Agric. 2002, 82, 840–847. [Google Scholar] [CrossRef]
- Vezzulli, F.; Rocchetti, G.; Lambri, M.; Lucini, L. Metabolomics combined with sensory analysis reveals the impact of different extraction methods on coffee beverages from Coffea arabica and Coffea canephora var. robusta. Foods 2022, 11, 807. [Google Scholar] [CrossRef]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavor characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Zhou, Q.; Cheng, C.; Xing, Z.; Zhou, Y.; Liu, X.; Hua, S.; Wei, W.; Tan, J.; et al. Characterization of key aroma compounds in cold brew coffee prepared by negative-pressure extraction technology and its changes during storage. LWT-Food Sci. Technol. 2024, 197, 115919. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Z.; Pan, X.; Gao, M.; Wu, M.; Wu, J.; Lao, F. Comparative profiling of hot and cold brew coffee flavor using chromatographic and sensory approaches. Foods 2022, 11, 2968. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef]
- Maksimowski, D.; Pachura, N.; Oziembłowski, M.; Nawirska-Olszańska, A.; Szumny, A. Coffee roasting and extraction as a factor in cold brew coffee quality. Appl. Sci. 2022, 12, 2582. [Google Scholar] [CrossRef]
- Yu, J.M.; Chu, M.; Park, H.; Park, J.; Lee, K.G. Analysis of volatile compounds in coffee prepared by various brewing and roasting methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef]
- Lapčíková, B.; Lapčík, L.; Barták, P.; Valenta, T.; Dokládalová, K. Effect of extraction methods on aroma profile, antioxidant activity and sensory acceptability of specialty coffee brews. Foods 2023, 12, 4125. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H.; Sagara, Y. Analysis of freshly brewed espresso using a retronasal aroma simulator and influence of milk addition. Food Sci. Technol. Res. 2009, 15, 233–244. [Google Scholar] [CrossRef]
- Roberts, D.D.; Acree, T.E. Simulation of retronasal aroma using a modified headspace technique: Investigating the effects of saliva, temperature, shearing, and oil on flavor release. J. Agric. Food Chem. 1995, 43, 2179–2186. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Civitella, A.; Sacchi, R. Effect of human saliva and sip volume of coffee brews on the release of key volatile compounds by a retronasal aroma simulator. Food Res. Int. 2014, 61, 100–111. [Google Scholar] [CrossRef]
- Crnjar, R.; Solari, P.; Sollai, G. The Human nose as a chemical sensor in the perception of coffee aroma: Individual variability. Chemosensors 2023, 11, 248. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, Y.C. Impact of brand and testing method on coffee taste perception between genders: A comparative study of two leading coffee brands in Taiwan. SAGE Open 2025, 15, 21582440251316087. [Google Scholar] [CrossRef]
- Spence, C.; Carvalho, F.M. The coffee drinking experience: Product extrinsic (atmospheric) influences on taste and choice. Food Qual. Prefer. 2020, 80, 103802. [Google Scholar] [CrossRef]
- Chapko, M.J.; Seo, H.S. Characterizing product temperature-dependent sensory perception of brewed coffee beverages: Descriptive sensory analysis. Food Res. Int. 2019, 121, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, J.; Chambers, E., IV; Koppel, K. Impact of consumption temperature on sensory properties of hot brewed coffee. Food Res. Int. 2019, 115, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Steen, I.; Waehrens, S.S.; Petersen, M.A.; Münchow, M.; Bredie, W.L. Influence of serving temperature on flavor perception and release of Bourbon Caturra coffee. Food Chem. 2017, 219, 61–68. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, Y.; Qu, F.; Cui, H.; Tao, G.; Zhang, X.; Xu, S. Influence of aroma binding factors of coffee matrix on characteristic aroma in a model system. LWT-Food Sci. Technol. 2024, 211, 116930. [Google Scholar] [CrossRef]
- Spence, C.; Van Doorn, G. Visual communication via the design of food and beverage packaging. Cogn. Res. Princ. Implic. 2022, 7, 42. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Spence, C. The shape of the cup influences aroma, taste, and hedonic judgements of specialty coffee. Food Qual. Pref. 2018, 68, 315–321. [Google Scholar] [CrossRef]
- Labbe, D.; Rytz, A.; Strube, A.; Leloup, V. Impact of mug shape and beverage volume on instant coffee perception. Food Qual. Pref. 2021, 89, 104150. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Spence, C. Do metallic-coated cups affect the perception of specialty coffees? An exploratory study. Int. J. Gastron. Food Sci. 2021, 23, 100285. [Google Scholar] [CrossRef]




| Water | Ground Coffee | Extraction | Cup |
|---|---|---|---|
| Temperature | Amount | Pressure | Cup size (volume) |
| Volume | Particle size distribution | Flow rate | Ratio of water/ground coffee |
| Quality (hardness) | Compaction | Flow time | |
| Shape of the coffee bed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, A.; Caporaso, N.; Baiano, A. The Impact of Brewing Methods on the Quality of a Cup of Coffee. Beverages 2025, 11, 125. https://doi.org/10.3390/beverages11050125
Genovese A, Caporaso N, Baiano A. The Impact of Brewing Methods on the Quality of a Cup of Coffee. Beverages. 2025; 11(5):125. https://doi.org/10.3390/beverages11050125
Chicago/Turabian StyleGenovese, Alessandro, Nicola Caporaso, and Antonietta Baiano. 2025. "The Impact of Brewing Methods on the Quality of a Cup of Coffee" Beverages 11, no. 5: 125. https://doi.org/10.3390/beverages11050125
APA StyleGenovese, A., Caporaso, N., & Baiano, A. (2025). The Impact of Brewing Methods on the Quality of a Cup of Coffee. Beverages, 11(5), 125. https://doi.org/10.3390/beverages11050125









