Abstract
Tea has become one of the most popular drinks worldwide thanks to its pleasant sensory attributes and diverse health benefits. However, tannin-rich compositions have several negative effects and significantly impact the quality of tea beverages. Among various detannification methods, tannase treatment appears to be the most secure and environmentally friendly strategy. Although numerous microbial tannases have been identified and used in food processing, they are predominantly mesophilic with compromised heat tolerance, which limit their application in high-temperature tea extraction processing. Computer-assisted rational design and site-directed mutagenesis has emerged as a promising strategy in enzyme engineering to improve the thermostability of industrial enzymes. Nevertheless, relevant studies for tannase thermostability improvement remain lacking. In the present study, a novel thermophilic tannase called TanPL1 from marine fungus Penicillium longicatenatum strain SM102 was expressed in the food-grade host Yarrowia lipolytica. After purification and characterization, the thermostability of this enzyme was improved through site-directed mutagenesis guided by computer-aided rational design and molecular dynamics simulations. Then the thermostable mutant MuTanPL1 was applied in green tea processing for both polyphenol extraction and ester catechin hydrolysis. The tannase yield and specific activity values of 166.4 U/mL and 1059.3 U/mg, respectively, were achieved. The optimum pH and temperature of recombinant TanPL1 were determined to be 5.5 and 55 °C, respectively, and the enzyme exhibited high activity toward various gallic acid ester substrates. The site-directed mutagenesis method successfully generated a single-point mutant, MuTanPL1, with significantly enhanced thermostability and a higher optimum temperature of 60 °C. After 2 h of detannification by MuTanPL1, nearly all gallated catechins in green tea infusion were biotransformed. This resulted in a 202.4% and 12.1-fold increase in non-ester catechins and gallic acid levels, respectively. Meanwhile, the quality of the tea infusion was also markedly improved. Sensory evaluation and antioxidant activity assays revealed notable enhancements in these properties, while turbidity was reduced considerably. Additionally, the α-amylase inhibition activity of the tannase-treated tea infusion declined from 50.49% to 8.56%, revealing a significantly lower anti-nutritional effect. These findings suggest that the thermostable tannase MuTanPL1 holds strong application prospects in tea beverage processing.
1. Introduction
For centuries, tea has remained one of the world’s most popular beverages. Tea is valued not only for its sociocultural significance [1] but also for its pleasant sensory attributes [2] and diverse health benefits, including antioxidant, anticancer, and neuroprotective effects, which are largely attributed to tea polyphenols [3]. Compared to fermented teas such as black tea and oolong tea, green tea is especially rich in polyphenols, particularly catechins, which comprise 75–80% of its soluble compounds [4]. Green tea typically contains eight major catechins, with epigallocatechin gallate (EGCG) being the most abundant and accounting for over 50% of its total catechin content. The other main catechins in green tea are epigallocatechin (EGC), epicatechin gallate (ECG), and epicatechin (EC) [5]. Although EGCG is the most prominent catechin in green tea and possesses well-documented health benefits [6], it is classified as a hydrolyzable tannin along with other esterified catechins [7]. Tannins, which have molecular weights ranging from 300 to 20,000 Da, are the second most abundant group of plant polyphenols after lignin. They are widely distributed in higher plants, where they serve as defense molecules, protecting plant tissues against animal predation and microbial infection [8,9,10]. Tannins can easily bind with other food and feed components—such as proteins, polysaccharides, alkaloids, and metal ions—to form indigestible complexes that hinder the absorption of essential nutrients [11]. Additionally, tannins can inhibit digestive enzymes in the stomach, inducing anti-nutritional effects that can negatively impact both human and animal health [12]. Notably, tea gallotannins such as EGCG and ECG also cause other problems and are the primary components contributing to the astringent and bitter taste of green tea [13]. Additionally, when tea infusions cool down, the abundant tannins tend to interact with other molecules such as proteins and caffeine to form precipitates that lead to cloudiness and turbidity in the tea beverage (“tea cream”) during storage, making it less appealing to consumers [14]. Therefore, reducing tannin levels is critical for improving the quality of tea beverages. Conventional non-enzymatic detannification methods are usually unsuitable for the food processing sector. For example, tannins could be removed via alkaline ethanol precipitation [15]. Although achieving ~90% tannin removal, this method suffers from major drawbacks like high-content NaOH requirements and phenolic component loss. In contrast, enzymatic treatment strategy offers a more advisable alternative for tannin removal, suitable for large-scale applications due to its lower risk, reduced environmental impact, and minimal nutritional loss [11].
Tannases (EC 3.1.1.20), also known as tannin acyl hydrolases, are a family of serine esterases that specifically cleave the ester and depside bonds in hydrolyzable tannins (e.g., gallic acid [GA] esters), thereby releasing GA (Figure S1) [16]. These enzymes hold significant industrial value and are widely employed for detannification across various sectors, including food processing, animal feed production, pharmaceuticals, brewing, beverage manufacturing, cosmetics, leather tanning, and environmental remediation [17,18,19,20]. Given tannase’s significant industrial potential, the commercial adoptions for various fields have grown substantially in recent years, after Juelich Chiral Solutions GmbH (Münster, Germany) company pioneered tannase commercialization in extract form [17]. Based on the previous reports, tannases play a crucial role in the biotransformation of GA-esterified catechins in tea processing, such enzymatic modifications enhance the antioxidant capacity of tea [21], prevent tea cream formation [22], and improve the sensory attributes [14]. Ni et al. treated oolong tea infusion using the tannase from Aspergillus niger JMU-TS528, the commercial practice of hydrolyzed tea infusion demonstrated significant enhancement such as the increases in clarity and antioxidant activity [23]. In another study, the tannase from A. niger NRRL 3536 was heterologously expressed using Penicillium verruculosum fungus system, and the new recombinant tannase TAN2 was successfully applied in the black tea extracts, resulting in a substantial reduction of over 70% in tannin content [24]. Despite the growing number of studies on the applications of tannases in tea beverage processing, commercially available tannases are exclusively used in tea extract hydrolysis. Also, studies have demonstrated that tannases can be involved in disintegrating plant matrices, consequently facilitating the extraction of phenolic compounds [16]. However, during commercial tea beverage manufacturing, high-temperature extraction is typically used to maximize the recovery of soluble compounds [4]. Since all current commercial food-grade tannases are mesophilic and show low activity and stability at higher temperatures, they exhibit poor catalytic efficacy toward the extraction process. Hence, enzymatic treatment can only be carried out in tea infusions at lower temperatures after the water extraction stage, leading to quality deterioration and inefficiency [25]. The tannases suitable for standard tea manufacturing protocols that can efficiently participate into water extraction stage for integrated extraction–hydrolysis are still rather rare [7]. Consequently, the ones with thermophilic properties and improved thermal stability must be developed to enhance polyphenol extraction efficiency and overall processing performance in tea infusions at higher temperatures [4].
Although tannases are ubiquitously found in biological organisms, microbial enzymes have remained the focus of research due to their superior production efficiency and diverse biochemical properties [11,19]. While numerous microbial tannases have been identified, heterologously expressed, and purified, thermophilic and thermostable variants remain scarce [11]. Although a few robust tannases capable of maintaining activity at higher temperatures have been identified, many are not suitable for tea processing due to the lack of food-grade status [26,27]. To increase the probability of obtaining hyper-thermostable enzymes, tannases from extremophiles can be screened. However, this method is labor intensive and difficult to scale [28]. A more practical and feasible strategy is to enhance the thermal properties of existing tannases through protein engineering. Rational design has emerged as a promising strategy because it minimizes experimental efforts through computational analysis, enabling the enhancement of enzyme properties via targeted site mutation [29]. Widely adopted in enzyme engineering, this approach has successfully been applied to improve the thermostability of several hydrolases and is considered a paradigm in modern directed evolution strategies [29,30]. Nevertheless, studies applying this methodology specifically to improve tannase thermostability remain lacking.
In this study, we comprehensively characterized the biochemical properties of TanPL1, a novel thermophilic tannase, following its expression in the food-grade yeast Yarrowia lipolytica and subsequent purification. The thermal stability of TanPL1 was then enhanced through computer-assisted rational design and site-directed mutagenesis. Finally, we systematically evaluated the efficacy of the enhanced enzyme in ester catechin transformation during tea thermal extraction and its overall impact on the quality improvement of green tea infusions.
2. Materials and Methods
2.1. Materials, Strains, and Media
Methyl gallate (MG), propyl gallate (PG), tannic acid (TA), gallic acid (GA), (–)-epigallocatechin gallate (EGCG), (–)-epigallocatechin (EGC), (–)-epicatechin gallate (ECG), (–)-epicatechin (EC), (–)-gallocatechin gallate (GCG), (+)-gallocatechin (GC), (–)-catechin gallate (CG), and (+)-catechin (C) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). High-performance liquid chromatography (HPLC)-grade acetonitrile and trifluoroacetic acid (TFA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and rhodanine were obtained from Sigma Chemical Co., Ltd. (St. Louis, MO, USA). Other chemical reagents were of analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Autumn green tea was supplied by Qingdao Zhengli Tea Co., Ltd. (Qingdao, China). The pure water used in this study was purchased from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China).
The Escherichia coli DH5α strain, used for expression vector preservation after construction, was cultured at 37 °C in Luria–Bertani (LB) medium containing 50 μg/mL kanamycin if required. The pINA1312 vector and the uracil mutant strain Y. lipolytica URA– were used for tanPL1 gene expression. The Y. lipolytica transformants were cultivated on YNB plates (10.0 g/L glucose, 5.0 g/L (NH4)2SO4, 1.7 g/L yeast nitrogen base, and 20.0 g/L agar). Meanwhile, GPPB medium (30.0 g/L glucose, 2.0 g/L yeast extract, 1.0 g/L (NH4)2SO4, 3.0 g/L K2HPO4, 2.0 g/L KH2PO4, 0.1 g/L MgSO4·7H2O, pH 6.8) was applied for recombinant TanPL1 expression [31].
2.2. Bioinformatics Analysis of the TanPL1 Sequence
Online server SignalP 6.0 (https://services.healthtech.dtu.dk/services/SignalP-6.0/, accessed on 12 October 2024) was employed to predict the N-terminal signal peptide sequence of TanPL1. The online InterProScan tool (http://www.ebi.ac.uk/interpro/search/sequence/, accessed on 10 November 2024) was utilized for functional domain analysis. Theoretical isoelectric point (pI) and molecule weight (Mw) calculations were performed using the online pI/Mw Tool (https://web.expasy.org/compute_pi/, accessed on 10 November 2024). DNAMAN 6.0 (Lynnon Biosoft, Foster City, CA, USA) was employed for multiple amino acid sequence alignment analysis of TanPL1 and other tannases from the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 10 November 2024).
2.3. Expression and Purification of Recombinant Tannase TanPL1
The codon-optimized tanPL1 gene was synthesized by Genscript Biotech Co., Ltd. (Nanjing, China) and ligated into the expression vector pINA1312. Specifically, the XPR2 signal peptide sequence and a hexahistidine tag were appended to the 5′ and 3′ terminals of the tanPL1 gene. Then, the pINA1312 vector containing the tanPL1 gene was transformed into the E. coli DH5α strain for preservation. In the next step, the expression vector was extracted from the E. coli DH5α cells and linearized before it was transformed into Y. lipolytica URA– using the LiAc method [32]. After 84 h of culture in GPPB plate at 28 °C, the positive Y. lipolytica transformant with the best extracellular tannase activity was further cultured for 72 h of liquid fermentation. After incubation, the cultured supernatant (containing the crude enzyme) was harvested by centrifugation at 4 °C and 10,000× g and filtered using a 0.22 µm syringe filter. It was then loaded onto a His60 Ni Superflow column (Clontech Laboratories, Dalian, China) that was previously equilibrated with a buffer composed of 50 mM Tris-HCl (pH 7.0) and 300 mM NaCl. The column was initially washed with an equilibration buffer containing 20 mM imidazole. Subsequently, elution was performed using a linear imidazole gradient (50–400 mM) in the same buffer. Tannase-active fractions were combined and concentrated via ultrafiltration using a 3 kDa molecular weight cutoff (MWCO) centrifugal filter (Amicon Ultra, Millipore, Burlington, MA, USA). Finally, the Mw of recombinant TanPL1 was determined using 12% (w/v) SDS-PAGE.
2.4. Quantification of Tannase Activity
Tannase activity was determined based on the method reported by Liu et al. [31]. Briefly, 0.5 mL of appropriately diluted enzyme was mixed with 4.5 mL of 0.5% (w/v) PG (pH 5.5) and incubated at 55 °C for 10 min. GA production was quantified based on the formation of a violet chromogen following a chemical reaction with rhodanine in methanol (0.667%, w/v). Color development was initiated through 0.5 M KOH addition, and the absorbance was recorded at 520 nm by an UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). Tannase activity (U) was calculated in terms of 1 μmol GA generated per minute. The total protein content was measured using the Bradford assay, with BSA as the standard [33].
2.5. Effects of Temperature and pH on TanPL1 Activity and Stability
Enzymatic activity measurements between 20 °C and 70 °C revealed the temperature optimum of TanPL1, and the data were normalized to its peak activity (100%). The thermal stability profile of TanPL1 was generated by quantifying residual activity after 2 h of incubation across the same temperature range, with reference to untreated samples (100%). The optimum pH of TanPL1 was assessed within the range of pH 2.0–9.0 using PG substrate in 100 mM buffer, with maximal activity set to 100%. pH stability was tested after 2 h of incubation at 20 °C across this pH range, with residual activity measured relative to untreated controls (100%).
2.6. Tannase-Mediated Degradation of Gallic Acid Esters
The substrate specificity of recombinant TanPL1 was evaluated by comparing its enzymatic activity toward various 0.5% (w/v) GA esters, including MG, PG, and TA, and the tea catechin derivatives EGCG, ECG, GCG, and CG. To examine the capacity of TanPL1 for tea catechin degradation and product formation, reaction mixtures containing 0.5% (w/v) of each substrate were incubated with excess enzyme (2.5 U/mL) at 55 °C under constant agitation. Following 2 h of incubation, the samples were immediately filtered through Millipore 3 kDa MWCO centrifugal filters (Burlington, MA, USA) for enzyme removal prior to analysis on the Waters 2695 HPLC platform equipped with the 2489 Dual Wavelength Absorbance Detector (Waters, Milford, MA, USA). The Symmetry C18 column (4.6 × 250 mm, particle size 5 μm) was used, with the temperature set at 35 °C and flow velocity set at 0.8 mL/min. The mobile phase was composed of 5% acetonitrile in 0.035% TFA (A) and 80% acetonitrile in 0.025% TFA (B). The gradient elution protocol was as follows: 0% B for 5 min, increased to 10% B for 5–10 min, to 30% B for 10–35 min, back to 0% B for 5 min, and maintained there for another 5 min before the next round of injection. The injection volume and detection wavelength were set at 10 μL and 280 nm, respectively.
2.7. Rational Design and Site-Directed Mutagenesis of TanPL1
The homology modeling of TanPL1 was performed using the Alphafold3 tool (https://alphafold.com, accessed on 26 November 2024). The molecular dynamics (MD) simulations were performed with GROMACS Version 2020 [34]. The root mean square fluctuation (RMSF) values at 20 °C and 60 °C were calculated to predict conformational fluctuations in each amino acid and identify those with potential negative effects on the thermal stability of recombinant TanPL1. Then, the Pythia algorithm (https://pythia.wulab.xyz, accessed on 29 November 2024) was applied to conduct virtual saturation mutagenesis at each candidate site, systematically assessing the folding free energy changes (ΔΔG) values of replacing the wild-type residue with each of the other 19 canonical amino acids, to identify the optimal substitutions that may improve the thermostability of TanPL1 [35]. Site-directed mutagenesis was performed to introduce selected stabilizing mutations into TanPL1, with candidate sites prioritized on the basis of higher Pythia-derived ΔΔG values predicting more possibly enhanced structural stability. The mutant genes were synthesized by Genscript Biotech Co., Ltd. (Nanjing, China), and the TanPL1 mutants were expressed and purified using the same method described in Section 2.3. The biochemical properties of all the mutants were characterized and compared to those of wild-type TanPL1.
2.8. Extraction and Tannase Treatment of Green Tea
Green tea infusions were prepared using three methods. Method 1 was carried out as follows. Autumn green tea leaves were pulverized in a mortar, and 4 g of ground tea powder was then infused in 200 mL pure water (1:50, w/v). The infusion was prepared in a 500 mL beaker placed on a magnetic stirrer at 90 °C for 30 min. This was followed by centrifugation at 12,000× g for 10 min and supernatant filtration through a 500-mesh screen. Afterward, the tannase TanPL1 and its mutant MuTanPL1 were separately added to the green tea infusion at a concentration of 2.5 U/mL. After agitated hydrolysis at 65 °C (MuTanPL1) or 55 °C (TanPL1) for 120 min, the samples were removed, inactivated by boiling for 10 min, and filtered through a 500-mesh screen. Method 2 was similar to Method 1; however, in Method 2, tannase treatment was performed without discarding the tea leaf powder. Instead, the system temperature was directly lowered to 65 °C or 55 °C. Both centrifugation and filtration were performed after 2 h of enzymatic treatment. In Method 3 tannase was added to the tea and water mixture (1:50, w/v) before the extraction stage. Both enzymatic extraction and hydrolysis were performed simultaneously at 65 °C or 55 °C for 2 h. Samples from the system lacking tannase were taken as controls.
2.9. Determination of the Total Polyphenol, Catechin, and GA Content
The total polyphenol contents of tannase-treated and untreated green tea infusions were determined using the Folin–Ciocalteu method [36], with GA serving as the calibration standard. The results were expressed as GA equivalents (GAE). The concentrations of catechins (EGCG, EGC, ECG, EC, GCG, GC, CG, C) and GA in tea infusions were examined using an HPLC system, as described above in Section 2.6.
2.10. Measurements of pH and Turbidity
The pH values were measured using a Mettler Toledo SG2 pH meter (Shanghai, China). Meanwhile, the absorbance at 672 nm was recorded to examine sample turbidity using Shimadzu UV-1800 spectrophotometer (Kyoto, Japan).
2.11. Sensory Evaluation of Green Tea Infusions
Ten professional tea tasters from the Northern Tea Research Institute (Laoshan District, Qingdao, China) conducted preference tests for the green tea infusions to evaluate four key attributes—bitterness intensity, astringency level, sweet aftertaste, and overall acceptability—using a standardized 10-point hedonic scale [37]. The scale ranged from 0 to 10, with intermediate ratings signifying the following: 0–2 (very weak), 2–4 (weak), 4–6 (neutral), 6–8 (strong), and 8–10 (very strong) [37]. Following the standardized tasting protocol established by Cao et al. [13], each panelist independently scored the sensory attributes of the green tea infusions. Subsequent statistical analysis was performed to identify the significant differences among mean values for different treatment groups.
2.12. Assessment of Antioxidant Capacity
The antioxidant capacities of tea infusions were assessed using the DPPH radical scavenging assay [23]. Briefly, the stock solution of DPPH (197 mg DPPH in 250 mL methanol) was diluted 10-fold with methanol to prepare a working solution. Properly diluted tea infusion samples were transferred to the DPPH working solution at a 1:1 (v/v) ratio. The mixtures were incubated for 30 min at room temperature (25 °C) in the dark. The absorbance was measured at 517 nm by a Shimadzu UV-1800 spectrophotometer (Kyoto, Japan) against a blank mixture in which the tea infusion was replaced with methanol. The antioxidant capacity was calculated as follows: DPPH scavenging activity (%) = (A0 − A1)/A0 × 100%, where A0 and A1 represent the absorbance values of the control and test samples, respectively.
2.13. Determination of α-Amylase Inhibitory Activity
The α-amylase inhibitory activities of green tea infusions (with and without tannase treatment) were evaluated using a modified enzymatic assay. Briefly, 200 μL of each diluted tea infusion sample was pre-incubated with 100 μL of α-amylase solution (12.5 U/mL in 50 mM sodium phosphate buffer, pH 6.9) for 10 min. The reaction was initiated by adding 700 μL of 1% (w/v) starch solution, followed by 10 min of incubation at 37 °C. The reducing sugar content was quantified using the DNS method [38], with glucose as the calibration standard. A negative control (tea infusion replaced with ultrapure water) was tested, and inhibition (%) was calculated as follows: Inhibition activity (%) = (C0 − C1)/C0 × 100%, where C0 and C1 represent the reducing sugar concentration produced in the control and test reactions, respectively.
2.14. Statistical Analysis
The data are presented as the mean ± standard deviation (SD) of triplicate measurements. Differences among samples were assessed for statistical significance (p < 0.05) through analysis of variance (ANOVA) and Duncan’s multiple range test using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Sequence Analysis of TanPL1
The marine Penicillium longicatenatum SM102 is an endophytic fungal strain that was isolated from mangroves root tissue growing on the coast of the South China Sea. The in silico genomic studies of this fungus led to the identification of a tannase-encoding gene named tanPL1 (Accession no. PV590102; length 1149 bp). The putative protein encoded by this gene is tannase TanPL1, which is 382 amino acids in length and has a theoretical pI and Mw of 4.88 and 42.4 kDa, respectively. Additionally, it lacks an N-terminal signal peptide, implying that it may be an intracellular enzyme. In this study, sequence retrieval using the InterProScan online server showed that TanPL1 belongs to the tannase and ferulic acid esterase family, with conserved domains associated with the α/β-hydrolase superfamily. Sequence alignment between TanPL1 and other putative tannases from annotated fungal genomes, including the tannases from Penicillium malachiteum (Accession no. XM_057095642), Penicillium cataractarum (Accession no. XM_056704852), Penicillium verhagenii (Accession no. XM_057160424), Penicillium daleae (Accession no. XM_056916065), Aspergillus eucalypticola CBS 122712 (Accession no. XM_025537034), Aspergillus saccharolyticus JOP 1030-1 (Accession no. XM_025579098.1), Penicillium pulvis (Accession no. XM_057071264), Penicilliopsis zonata CBS 506.65 (Accession no. XM_022722132), Penicillium canariense (Accession no. XM_056692087), and P. daleae (Accession no. XM_056904532), and the characterized tannase Antan1 from A. niger FJ0118 (Accession no. XM_001401772) [4], is shown in Figure 1. The results revealed a pentapeptide active site motif G/A-C-S-X-G (boxed in red in Figure 1) that appears to be relatively conserved across serine hydrolases. Here, Ser20 unites two other residues, Asp247 and His293 (marked by red circles in Figure 1), to form the “CS-D-HC” catalytic triad [31]. Additionally, the two other amino acids in this motif (Cys19 and Cys294, highlighted with blue circles in Figure 1) are directly linked, forming a disulfide bond that stabilizes the active center [4].
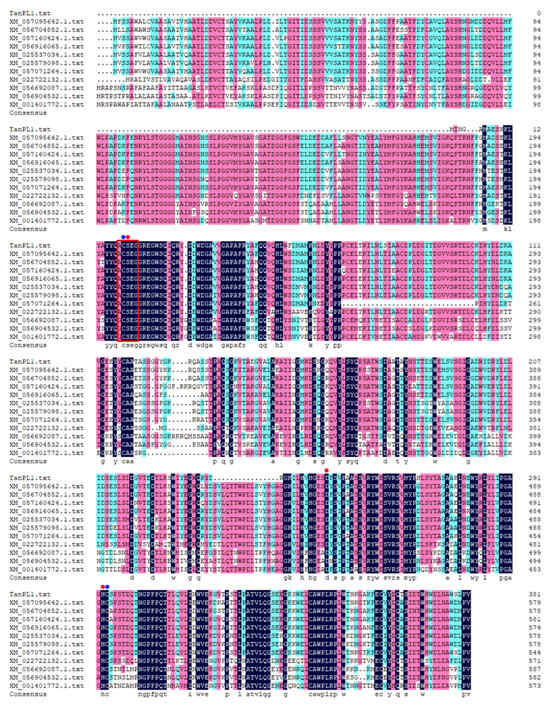
Figure 1.
Multiple sequence alignment of TanPL1 with other putative tannases derived from the annotated genomic sequences of Penicillium spp. and Aspergillus spp. The conserved domain G/A-C-S-X-G is boxed in red, the catalytic sites are marked with red circles, and the cysteine residues forming disulfide bonds that link the active sites are highlighted by blue circles.
3.2. Secretory Expression and Purification of Recombinant TanPL1
Although the demand for tannases is increasing continuously, native tannases cannot fulfill these needs owing to their complicated production processes and low yields. Thus, profitable, large-scale tannase production must be achieved using an efficient and safe expression system. In this study, the food-grade chassis strain Y. lipolytica URA–, which shows promising post-translational processing and extracellular secretion capacity and does not require any antibiotics or inducers during its enzyme-producing fermentation process, was adopted for the heterologous expression of the recombinant tannase TanPL1 [39]. Furthermore, the yeast Y. lipolytica has been identified by FDA as GRAS (generally regarded as safe), establishing this system recognized as a favorable choice for heterologous protein expression [39]. After 72 h of shake-flask cultivation, the highest extracellular tannase activity was found to be 166.4 U/mL, representing a significantly higher yield than most previously reported values, i.e., 76.63 U/mL from A. niger [40], 98.6 U/mL from Pestalotiopsis guepinii URM 7114 [41], and 34.7 U/mL from Penicillium atramentosum KM [42]. After Ni-IDA agarose affinity chromatography (Table 1), purified TanPL1 was obtained with a specific activity of 1059.3 U/mg, similar to that of the tannases derived from Kluyveromyces marxianus NRRL Y-8281 (1026.12 U/mg) [16] and Enterococcus faecalis (1096 U/mg) [43], but lower than that of the tannase derived from Bacillus subtilis PAB2 (2868.75 U/mg) [27]. However, the specific activity of purified TanPL1 was still much higher than that of most previously identified tannases [11].

Table 1.
Summary of the TanPL1 purification.
Different multistep purification steps can lead to a loss of enzyme activity [24]. Gonçalves et al. adopted DEAE-cellulose and Sephacryl S-200 to obtain a purified tannase with a low yield of 29.95% [26]. In another study, three chromatography steps based on the ion exchange and steric exclusion methods were utilized to purify the tannase TanLpl, which showed a specific activity of 84.34 U/mg but a poor recovery of 4.8% [44]. However, 1026.12 U/mg tannase activity and 64.6% yield were achieved through one chromatography step using Sephadex G-200 by Mahmoud et al. [16]. Similarly, the use of only the Ni-affinity step in the current study led to a yield of 73.1%, and this recovery rate was much higher than that reported previously (Table 1).
The analysis of purified tannase TanPL1 using SDS-PAGE revealed a single dominant protein band with an apparent molecular mass of approximately 43 kDa (Figure 2), consistent with the predicted size. The molecular masses of fungal tannases generally range from 45 to 320 kDa owing to the potential presence of multiple subunits or glycosylation [45]. Even bacterial tannases that typically contain one subunit and do not have any modifications have Mw values ranging from 31 to 90 kDa [11]. This indicated that TanPL1 has a very low Mw among all the microbial tannases, which could explain its high specific activity. In addition, proteins with low Mw are usually expressed and secreted more efficiently by microbial expression systems, enabling greater tannase production.
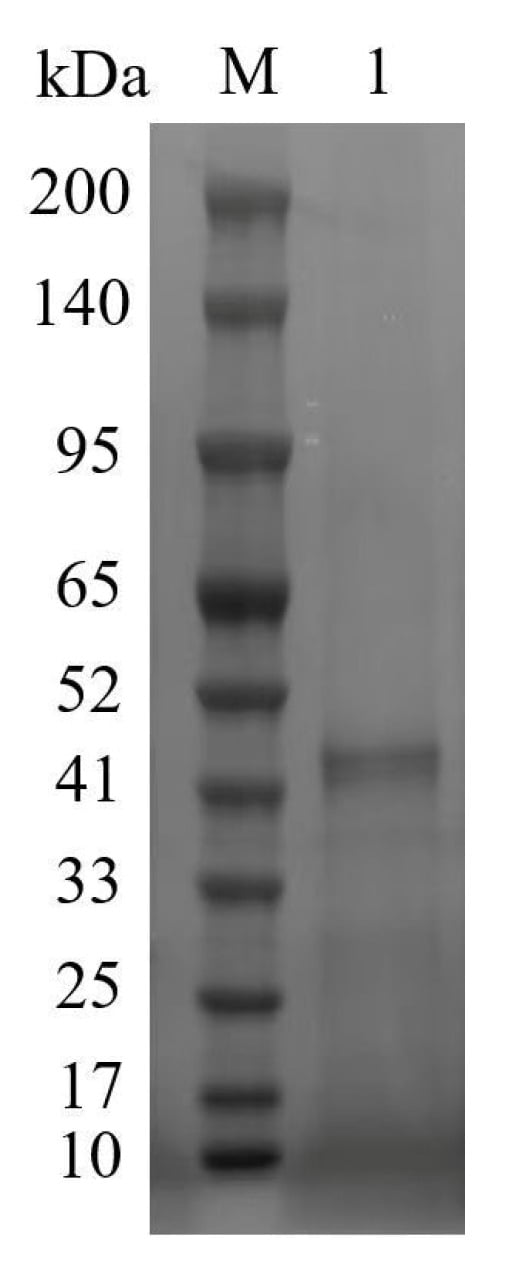
Figure 2.
SDS-PAGE analysis of purified TanPL1. Lane M: pre-stained protein marker; Lane 1: purified TanPL1.
3.3. Effects of Temperature and pH on TanPL1 Activity and Stability
As shown in Figure 3a, recombinant TanPL1 exhibited the highest activity at 55 °C, and over 80% relative activity was retained at 40–60 °C, demonstrating its thermophilic properties. The activity of TanPL1 reduced at temperatures outside this range (Figure 3a). However, even at lower temperatures of 20–25 °C, over 50% of its activity was retained (Figure 3a). These interesting findings demonstrated that thermophilic TanPL1 can also adapt to lower temperatures and could thus be applied in processes where excessive heating is contraindicated. However, the thermophilic properties of TanPL1 were more valuable for diverse industrial food applications since higher temperatures are used during food production and processing [26]. In general, microbial tannases show optimum temperatures between 30 °C and 60 °C [11]; however, thermophilic tannases with an optimum temperature above 50 °C are quite rare (Table S1) [16,24,26,27,31,44,46,47,48,49,50,51,52,53]. In this study, TanPL1 showed relative activities of 82.3% and 46.2% even at 60 °C and 65 °C, respectively, which could be very beneficial for tea beverage processing at elevated temperatures [4].
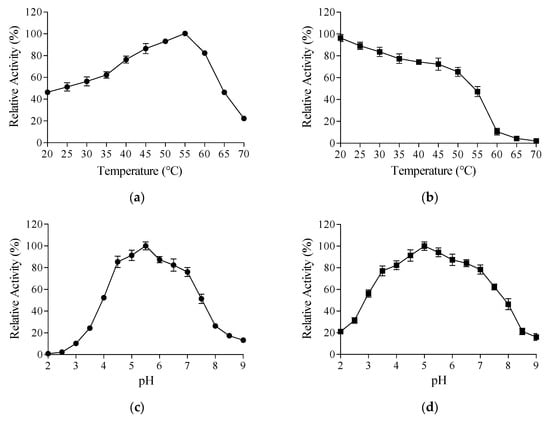
Figure 3.
Effects of temperature and pH on TanPL1 activity and stability. (a) Optimum temperature. Enzyme activities were assessed across a temperature range of 20–70 °C, and relative activities were calculated through normalization based on the maximum observed activity (100%). (b) Thermal stability. Residual activities after 2 h of incubation at 20–70 °C (initial activity = 100%). (c) Optimum pH. Enzyme activities in different buffers (pH 2.0–9.0), normalized to peak activity (100%). (d) pH stability. Residual activities following 2 h of incubation at 20 °C across pH values of 2.0–9.0, measured under standard assay conditions (initial activity = 100%).
In addition to enzyme activity, enzyme thermostability is also a key property since it determines how long a tannase can sustain its catalytic ability at higher temperatures. Despite showing extremely high activity, a tannase that undergoes denaturation at higher temperatures, causing catalytic inactivation before complete substrate degradation, would fail to effectively meet industrial requirements [54]. For tea beverage manufacturing, at least 1–2 h of treatment is required to improve the quality of a tea infusion [37]. Therefore, the thermal stability of TanPL1 over a 2 h period was examined in this study (Figure 3b). The activity of TanPL1 remained robust for 2 h at 20–55 °C, demonstrating its superior thermostability. Moreover, >50% activity was preserved after 2 h at 55 °C. However, at temperatures above 60 °C, <10% activity was retained after 2 h, which was undesirable for tea infusion treatment. Tannases whose thermostable range extends beyond 60 °C are very rare. For instance, the tannase from Aspergillus phoenicis retains merely 10% of its original activity following 60 min of incubation at 60 °C, demonstrating poor thermal stability under these conditions [55]. Although some thermostable tannases that show heat tolerance at 60 °C exist, they are not of food grade and are thus unsafe for food and beverage applications [26]. In this context, protein engineering methods appear suitable for improving the thermostability of current tannases.
In pH experiments, recombinant TanPL1 exhibited maximum activity at pH 5.5, while retaining over 50% relative activity across the pH range of 4.0–7.5 (Figure 3c). TanPL1 also showed stability within the wide pH range of 3–8 (Figure 3d). Similarly, most fungal tannases show maximal catalytic activity under acidic pH conditions while sustaining functional stability across a broad pH spectrum [23]. The tannase from Aureobasidium melanogenum T9 exhibits an optimum pH of 6.0 and stability within the pH range of 3.5–7.5 [31]. Meanwhile, the tannase derived from A. niger JMU-TS528 displays peak activity at pH 5.5 and remains active and stable at pH values of 3.0–7.0 [23]. Unlike fungal tannases, bacterial tannases typically show an optimum pH range of 7–9 [11]. However, most tea beverages are acidic, with a pH value of 5–6 [13]. Therefore, fungal tannases such as TanPL1 are more suitable for tea infusion treatment (Table S1).
3.4. Substrate Specificity and Degradation Product Analysis
Considering the complexity and diversity of tannin structures, the substrate preference of tannases is a crucial consideration for their application [10]. The hydrolyzable tannins present in green tea are gallated catechins, mainly EGCG and ECG. Thus, the substrate specificity of TanPL1 was assessed to determine its application prospects for green tea infusion treatment. The catalytic activities of TanPL1 toward MG, PG, TA, EGCG, ECG, GCG, and CG were determined to be 1059.3, 856.8, 987.4, 1035.5, 1096.2, 917.3, and 955.6 U/mg under optimum conditions. This indicated that TanPL1 is a multifunctional tannase with the ability to degrade both ester and depside bonds and can thus act on a multitude of substrates, including natural tannins (EGCG, ECG, GCG, CG, and TA) and synthetic ones (PG and MG). After the catalytic decomposition of natural tea ester catechins by TanPL1 (2 h at 55 °C), no residual gallotannins could be detected in the reaction mixture (Figure S2). However, the transformed products detected included EGC, EC, GC, C, and GA, as shown in Figure S2. The tannase from K. marxianus NRRL Y-8281 was shown to exhibit substrate preference in the following order: TA (100% relative activity) > MG (74.3%) > PG (64.5%) >> EGCG (10.5%) [16]. Meanwhile, the tannase Ss-Tan from Streptomyces sviceus ATCC 29,083 showed a preference for degrading depside bonds and exhibited stronger activity against TA and digallic acid [10]. By contrast, TanPL1 showed high catalytic activity toward tea catechins (EGCG, ECG, GCG, CG), implying that it could successfully achieve detannification in green tea infusions. Owing to the complex chemical nature of tannins, the use of single enzymes with obvious substrate preference is often insufficient for substrate hydrolysis, necessitating the use of tannase with different substrate specificity combinations for more efficient detannification [10]. However, tannase TanPL1—with its ability to degrade several types of substrates—showed the potential to be used independently in practical scenarios for achieving the complete digestion of hydrolyzable tannins.
3.5. Rational Design and Site-Directed Mutagenesis of TanPL1
To further improve the thermostability of TanPL1 at temperatures over 60 °C and facilitate its application in the detannification of green tea infusions, a computer-assisted rational design strategy was adopted to assess the feasibility of site-directed mutagenesis. Enzyme thermostability is affected by several factors, and not all mutation strategies yield positive outcomes [30]. Therefore, we first performed MD simulations, which have often been applied for guiding mutant selection in order to improve enzyme thermostability [30]. The RMSF values of all amino acids in TanPL1 at temperatures of 20 °C and 60 °C were calculated (Figure 4a). The results indicated that three medial regions (marked by green line) of the TanPL1 protein may negatively affect its thermal stability. Thus, amino acids showing the highest conformational fluctuations were selected as possible sites for mutation. It is recommended that mutation sites be distant from the central catalytic site so as to ensure that enzyme activity remains unaffected [56]. Moreover, sites in conserved domains should also be excluded as these regions play critical roles in maintaining the structural stability of enzymes [29]. These rules were followed in the present study. The 3D structure of TanPL1 was generated and used to select candidate mutation sites (Figure 4b). Finally, seven sites that were spatially distant from the active center (Ser123, Tyr127, Arg130, Asp199, Lys224, Tyr228, and Ser334) were chosen for further screening (Figure 4b).
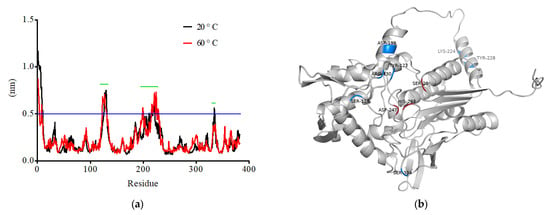
Figure 4.
(a) RMSF values for all the amino acids present in TanPL1 were calculated under temperature conditions of 20 °C and 60 °C. The three regions possibly exerting negative effects on the thermostability of TanPL1 are highlighted by green lines. (b) A 3D model of tannase TanPL1. The catalytic active and candidate mutation sites are labeled in red and blue, respectively.
The TanPL1 protein structural stability was computationally analyzed using the Pythia algorithm, and the computational evaluation encompassed all possible amino acid substitutions at each candidate mutation site, systematically assessing the ΔΔG values of replacing the wild-type residue with each of the other 19 canonical amino acids. Single-point variants exhibiting the largest ΔΔG absolute values (indicating more enhanced structural stability) were selected for further experimental validation (Table S2). Finally, among the seven candidate substitutions, the S123A, Y127A, R130N, and Y228E mutants with higher theoretical ΔΔG absolute values were chosen for subsequent expression and purification, and their thermostabilities and activities were compared to the wild-type TanPL1 enzyme. The thermostabilities of all four single-point mutants increased to different degrees at higher temperatures, with mutants Y228E and S123A showing greater stability at temperatures above 50 °C than the other two mutants (Figure 5a). The relative activities of the Y228E mutant after 2 h of incubation at 55 °C, 60 °C, and 65 °C were found to be 76.35%, 68.43%, and 51.24%, respectively. The corresponding values for the S123A mutant were 68.83%, 59.61%, and 39.69%, respectively. Thus, both mutants showed relative activities after prolonged incubation at these temperatures than the wild-type tannase (42.75%, 20.58%, and 13.25%) (Figure 5a). These results confirmed the accuracy of the computer-assisted virtual saturation mutagenesis strategy, and the Y228E mutant with the higher calculated ΔΔG value (−5.7502 kcal/mol) was found to show stronger heat resistance. The findings also showed that generating a substitution at only one site could be very useful in enhancing the thermostability of the whole protein [57]. However, the initial activities of all the mutants were also compared because amino acid substitution can induce both positive and negative effects on enzyme activity [34]. Figure 5b presents the relative activities of wild-type TanPL1 and its mutants at 20–70 °C. Notably, the activity of the mutant Y228E reduced dramatically. At 60 °C, its activity was equivalent to only two-thirds of that of the S123A mutant, whereas its thermostability was just a little higher than that of S123A. Some studies have shown that mutations causing the improvement of heat tolerance may induce a shift in optimum enzyme temperature [28]. Interestingly, in this study, the optimum temperature of the mutants S123A and Y228E increased by 5 °C and 10 °C, reaching 60 °C and 65 °C, respectively (Figure 5b). However, even at 65 °C, the catalytic efficiency of Y228E appeared to be lower than that of the S123A mutant (Figure 5a,b). By comprehensively considering both enzyme activity and stability, we selected the mutant S123A for subsequent use in green tea infusion treatment at 65 °C and named it “MuTanPL1”. In addition, we confirmed that this mutation caused no other remarkable differences between MuTanPL1 and wild-type TanPL1 with respect to other properties, including optimum pH and stability as well as substrate specificity. The catalytic activity of MuTanPL1 toward EGCG, ECG, GCG, and CG was determined as 1050.4, 995.6, 923.4.3, and 873.1 U/mg, respectively, with no pronounced difference when compared to wild-type TanPL1.
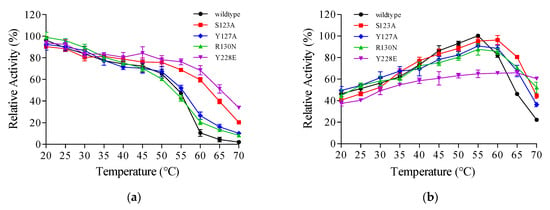
Figure 5.
Optimal temperatures and thermal stabilities of wild-type TanPL1 and its mutants. (a) Thermal stability. The activities were measured after 2 h of incubation at 20–70 °C, with the respective initial activities being considered 100%. (b) Optimum temperature. The activities were determined at 20–70 °C, with the highest activity of the wild-type tannase set at 100%.
In recent years, researchers have increasingly used computer-assisted rational design analysis and MD simulations to enhance the heat resistance of enzymes [29,35]. However, there are few similar precedents for the application of this strategy in tannases. Given the increasing use of tannases in food processing and other industrial fields, rational design and site-directed mutagenesis strategies hold considerable potential for improving the properties of these enzymes.
3.6. Effects of Tannase MuTanPL1 Application in Green Tea Infusion Extraction/Hydrolysis
Tannase treatment can enhance the quality and nutritional value of tea beverages. For instance, it can reduce their turbidity and bitter and astringent taste while enhancing their antioxidant capacity through the biotransformation of hydrolyzable tannins, mainly gallate-esterified catechins [11]. To explore these effects, three approaches (Method 1, Method 2, and Method 3) for easter-catechin extraction/hydrolysis in green tea infusions were adopted and compared in the present study, the changes in catechin composition, turbidity, sensory quality, antioxidant activity, and anti-nutritional effects after enzymatic hydrolysis were surveyed across all groups.
3.6.1. Effects of Tannase-Assisted Extraction/Hydrolysis on the Total Phenolic Content and Catechin Composition
First, the effects of enzyme-mediated extraction using tannase TanPL1 (55 °C) and MuTanPL1 (65 °C) were compared based on the total phenolic content. As shown in Table 2, enzymatic extraction remarkably improved the total phenolic content in infusions prepared using both Method 2 and Method 3, consistent with a previous study [4]. However, the total phenolic content was higher in the Method 2 group because pre-extraction at 90 °C aided substance recovery. By hydrolyzing the tannin–plant matrices complex, tannases induce structural modifications in bound polyphenols, increasing their solubility and extraction efficiency [58]. Enzymatic treatment with Penicillium commune tannase was found to increase the extractable phenolic content of Hibiscus tea by 8.6%, as quantified using Folin–Ciocalteu assay [59]. Additionally, higher temperatures have been shown to be important for enzymatic extraction [4]. MuTanPL1, with its optimum temperature of 60 °C and prolonged stability at 65 °C, showed superiority over wild-type tannase TanPL1, which could only be used at temperatures below 55 °C due to its limited thermostability. In Method 2 or Method 3, despite the similar enzymatic activities toward ester catechins, extraction using MuTanPL1 at 65 °C led to an obviously higher total phenolic content than that using TanPL1 at 55 °C (Table 2). Therefore, only MuTanPL1 was examined at 65 °C in subsequent tests. Furthermore, when using MuTanPL1, the pre-extraction step at 90 °C appeared to be unnecessary, as it only provided a slight increase in the total phenolic content (5.10 to 5.32 mg/mL) while adding to both energy and time costs. Finally, polyphenol recovery was enhanced by 27.5% with MuTanPL1 treatment using Method 3.

Table 2.
Changes in the total phenolic contents of tea infusions after 2 h of hydrolysis using wild-type tannase TanPL1 and the mutant MuTanPL1.
After tannase-mediated hydrolysis using the three different methods, the changes in catechins and GA contents in the tea infusions were examined using HPLC (Figure S3); the concentrations of the compounds are listed in Table 3. There were no significant differences in total catechin contents among the three extraction methods for the control groups without tannase treatment. The total catechin concentration was 3.99 mg/mL after extraction at 65 °C for 2 h (Method 3), 4.01 mg/mL after extraction at 90 °C for 30 min (Method 1), and 4.23 mg/mL after extraction at 90 °C for 30 min combined with 65 °C for 2 h (Method 2). However, once MuTanPL1 was added, some discrepancies appeared. After 2 h of hydrolysis, the total catechin and GA contents obtained were 3.52 and 1.21 mg/mL with Method 3 and 3.57 and 1.29 mg/mL with Method 2. All these values were much higher than the yields obtained with Method 1 (3.16 and 0.76 mg/mL, respectively), in which the tannase only induced catechin biotransformation without aiding extraction. However, there were no significant differences in the total catechin and GA contents between Method 2 and Method 3 (Table 3), further indicating that the 90 °C pre-extraction stage was unnecessary. Thus, in subsequent experiments, tea infusions were only treated with MuTanPL1 using Method 3. Shao et al. used enzymatic extraction to obtain a higher total concentration of catechins and GA, achieving a higher total phenolic content than water-based extraction without tannase addition [4].

Table 3.
The changes in tea catechins and gallic acid concentrations (mg/mL), and pH values of tea infusions after tannase hydrolysis through three methods.
Autumn green tea usually contains higher amounts of catechins, especially gallated ones such as EGCG and ECG [13]. EGCG typically accounts for nearly 70% of all green tea catechins, followed by EGC (nearly 20%), ECG (5% more or less), and EC (2–5%). Hence, gallate-ester catechins comprise over 70% of the total catechins in green tea [5]. Shao et al. found that the concentration of total catechins in the water extract of green tea was 4.158 mg/mL (207.9 mg/g tea leaves), with EGCG being the most abundant (2.687 mg/mL, 64.62% of weight percentage) and more than 88% of catechins being gallate-esterified [4]. A total catechin content of 3.88 mg/mL (194 mg/g green tea leaves) was obtained at 50 °C after 3 h of extraction by Cao et al., with the gallated ones and EGCG accounting for 69.33% and 55.93%, respectively [13]. In this study, based on the data in Table 3, similar total catechin (4.23 mg/mL and 211.5 mg/g tea leaves) levels were obtained through water-based extraction. However, a 57.2% proportion of ester catechins and 42.8% proportion of EGCG were detected, much lower than previous reports. These differences were attributed to variations in tea cultivars, geographical origins, harvest times, and processing conditions. The autumn green tea used in this study was grown on the Laoshan Mountain, a famous tea-growing area in Northern China, the green tea obtained here always has a better taste and unique aroma when compared to the green tea from other places [60].
As shown in Table 3, after tannase-mediated extraction and hydrolysis using Method 3, the contents of gallated catechins (EGCG, ECG, and GCG) in the green tea infusions decreased dramatically. This was accompanied by an increase in ungallated catechins. The EGC, EC, GC, and GA contents increased relatively to 205.1%, 247.4%, 171.4%, and 12.1-fold compared to the controls, reaching 2.40, 0.47, 0.48, and 1.21 mg/mL, respectively. Lu et al. studied the influence of tannases on catechin conversion, revealing that the concentration of the degallated catechins EGC and EC can increase by 1.5- and 5.0-fold after tannase treatment, respectively [14]. Interestingly, Cao et al. discovered that the catalytic efficiency of tannase was dramatically lower when it was added to a tea leaf and infusion mixture, with only 5% of gallated catechins being successfully degraded [13]. The tea leaves in the infusion likely adsorbed the enzyme, influencing its activity and the hydrolysis of gallated catechins [61]. However, such a phenomenon was not detected in the present study. Similarly, Shao et al. also used tannase in a green tea leaf and infusion mixture and observed no noticeable reduction in enzyme activity. Almost all the ester catechins were degraded, and the content of non-ester ones was enhanced by 4.76 times [4]. In addition, in our study, the pH decreased to 4.86 after 2 h of hydrolysis due to GA accumulation (Table 3). Fortunately, the pH variations (6.1 to 4.8) of the tea infusion were perfectly aligned with the optimum pH and stability range of MuTanPL1. Thus, the pH changes had little effect on catechin biotransformation (Figure 3c,d).
3.6.2. Improvement of Tea Infusion Quality Through Tannase-Assisted Extraction/Hydrolysis
Sensory attributes are a key quality consideration for green tea. Tea catechins act as major contributors to the bitter, astringent, and sweet tastes of tea beverages [37]. The astringency and bitterness of tea arise from the loss of oral lubricity due to the strong binding activity of tannins with salivary proteins [62]. Generally, non-esterified catechins such as EGC and EC taste bitter and astringent and have a sweet aftertaste. Meanwhile, gallate-esterified catechins (especially EGCG and ECG) taste much more bitter and astringent, without providing any sweet aftertaste [37]. In untreated green infusions, the highly abundant gallate-ester catechins mask the sweetness of less abundant non-ester catechins [63]. Additionally, green tea has a high content of caffeine (Figure S3), which adds strong bitterness and has been shown to act synergistically with EGCG, with both compounds enhancing each other’s bitterness [37]. Notably, the enzymatic degradation of gallated catechins releases free GA, which is a key contributor to the characteristic sweet aftertaste in green tea infusions [13]. Thus, treating green tea infusions with tannases is important to improve their mouthfeel. In the present study, sensory evaluation (Table 4) revealed that untreated green tea infusions exhibited pronounced bitterness and astringency, with minimal sweet aftertaste. However, following 2 h of MuTanPL1 treatment, a significant reduction in bitterness and astringency was observed, along with a marked enhancement of sweet aftertaste and overall acceptability scores. These results are consistent with previous reports [13,37].

Table 4.
Effects of tannase-mediated hydrolysis on the sensory scores of green tea infusions.
During storage, the tannins in tea infusions, such as EGCG and ECG, readily bind with other molecules such as proteins and caffeine to form undesirable tea cream and pronounced turbidity, causing the appearance of the tea beverage to deteriorate [11]. Centrifugation and filtration methods used to remove such cloudy precipitates may lead to the loss of bioactive compounds and negatively affect the flavor of tea infusions [64]. Therefore, tannase treatment has emerged as a better approach to prevent tea cream formation. In this study, the effect of tannase treatment on tea cream formation was evaluated after one week of storage at 4 °C. The OD672nm value of tannase-treated tea infusions was found to be 0.064, much lower than that of control infusions (0.498), indicating better storage stability at low temperatures (Table 5). Lu et al. reported that although the initial turbidity value of tannase-treated green tea (16.1 NTU) was not significantly different from that of the untreated sample (17.1 NTU), after 4 weeks of storage, the turbidity increased to 19.1 NTU and 50.0 NTU, respectively [14]. In another study, the reduction in tannin content after tannase treatment caused the absorbance at 660 nm to decrease by 0.629, from 0.874 to 0.245 [12]. Consistent with our findings, Ni et al. reported a 2.4-fold enhancement in tea infusion clarity (from 33.4% to 81.1% transmittance at 640 nm) following tannase treatment, demonstrating the enzyme’s efficacy in reducing turbidity [23].

Table 5.
Effects of tannase-mediated hydrolysis on the turbidity, antioxidant activity, and α-amylase inhibitory activity of green tea infusions.
In this study, the DPPH assay was utilized to evaluate the free radical scavenging activity of MuTanPL1-treated green tea infusions. A summary of the DPPH inhibition results is presented in Table 5. After tannase treatment, the scavenging capacity of the infusions increased by 16.76 percentage points (p < 0.05), from 69.51% to 86.27%, representing a 24.1% relative enhancement of antioxidant activity. Meanwhile, corresponding measurements were also obtained under the other two extraction/hydrolysis methods. Following tannase treatment, DPPH inhibition rates were 87.33% (treated) vs. 72.62% (control) for Method 2, and 75.22% (treated) vs. 71.53% (control) for Method 1. The increased antioxidant activity across all the tea infusions after enzymatic treatment might be attributed to the GA releasing during tannin degradation—a potent antioxidant [12]. Notably, the antioxidant activity levels of tea infusions obtained through all the three preparation methods exactly match their total phenolic content data (Table 2), suggesting that polyphenols (e.g., catechins and GA) are primary contributors to antioxidant efficacy. Similarly, Aharwar and Parihar reported that the DPPH inhibition capacity of two green tea infusions increased from 52.57% to 81.6% and 48.79% to 70.6%, respectively, following tannase treatment [12]. Another comparative analysis revealed significantly enhanced free radical scavenging activity in two types of tannase-treated tea infusions (57.8% and 69.5%) compared to their untreated controls (35.6% and 44.8%), further demonstrating the potential of tannase to boost the antioxidant activity of tea infusions [65].
The tannins in tea infusions can bind to digestive enzymes, forming complexes in the digestive tract that exert anti-nutritional effects and pose health concerns in both humans and animals [11,12]. Therefore, the enzymatic removal of tannins in tea infusions is integral to reduce their impact on nutrient absorption following tea consumption. In this study, an α-amylase inhibition assay was used to assess the anti-nutritional effects of green tea infusions after tannase treatment. The untreated infusion inhibited 50.49% of α-amylase activity, whereas the tannase-treated sample inhibited only 8.56%, revealing a significantly lower anti-nutritional effect (Table 5). In another study, the α-amylase inhibition capacity of Hibiscus tea was found to decrease to 49.32% after tannase treatment, in contrast to 68.48% in the untreated sample [59].
4. Conclusions
In this study, a novel thermophilic tannase, TanPL1, was heterologously expressed in the food-grade chassis Y. lipolytica. A tannase yield of 166.4 U/mL was achieved after 72 h of shake-flask fermentation, and purification through Ni-IDA affinity chromatography provided a specific activity of 1059.3 U/mg. TanPL1 was found to be a multifunctional enzyme, with high activity toward diverse GA-ester substrates and an optimum temperature and pH of 55 °C and 5.5, respectively. The thermal stability of TanPL1 was enhanced through computer-assisted rational design and site-directed mutagenesis, leading to the generation of a single-point mutant MuTanPL1, which had an optimum temperature of 60 °C and showed significantly enhanced thermostability. The thermostable tannase MuTanPL1 was applied for green tea infusion treatment, and its effects on both polyphenol recovery and gallated catechin biotransformation were tested. After 2 h of detannification, almost all the ester catechins were degraded by MuTanPL1, and the EGC, EC, GC, and GA contents of the green tea infusion increased to 205.1%, 247.4%, 171.4%, and 12.1-fold compared to the control groups. Meanwhile, the sensory quality and antioxidant activity of the tea infusions also showed obvious improvements after MuTanPL1 treatment, and the turbidity and digestive enzyme inhibitory activity were greatly reduced. The findings of the present study demonstrate that the enhanced tannase, MuTanPL1, holds immense promise as a superior candidate for tea beverage processing. In addition, rational design and site-directed mutagenesis strategies have considerable application prospects for improving the quality of tannases.
However, this study does not involve the mechanism by which site-directed mutagenesis improved the thermal stability of tannase TanPL1. A follow-up article will focus on elucidating the mechanism, particularly in terms of changes in molecular interactions and the solvent accessibility of amino acid residues. Additionally, the synergistic effects of tannase and other food-grade enzymes in enhancing the quality of various teas, such as black tea, white tea, etc., warrant further investigation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/beverages11040099/s1. Table S1: Comparison of the properties of TanPL1 and other microbial tannases; Table S2: Single-point mutations selected for tannase TanPL1 based on ΔΔG values; Figure S1: General chemical structures of some tannase substrates and products; Figure S2: Chromatograms demonstrating catechin biotransformation by tannase TanPL1; Figure S3: Chromatograms demonstrating the changes in catechins and gallic acid in tea infusions after tannase MuTanPL1 hydrolysis.
Author Contributions
Conceptualization, H.-X.Z. and J.-C.Z.; methodology, S.-N.C. and C.-S.Z.; software, H.-X.Z.; validation, L.-F.Z. and J.C. (Jing Chen 2); formal analysis, J.-C.Z.; investigation, J.C. (Jing Chen 1) and Q.-B.M.; resources, H.-X.Z.; data curation, Y.-Y.T.; writing—original draft preparation, H.-X.Z.; writing—review and editing, S.-N.C., Y.-Y.T. and J.S.; visualization, M.W.; supervision, J.S. and J.-C.Z.; project administration, C.-S.Z. and M.W.; funding acquisition, H.-X.Z., S.-N.C., and J.-C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Qingdao People’s Livelihood Science and Technology Plan Project, grant number 24-1-8-xdny-16-nsh; Innovation Project of Agricultural Science and Technology, Shandong Academy of Agricultural Sciences, grant number CXGC2025F19, CXGC2025G13; the Open Project of Key Laboratory of Biology and Genetic Improvement of Oil Crops, Ministry of Agriculture and Rural Affairs, P. R. China, grant number KF2023009.
Institutional Review Board Statement
The sensory evaluation study protocol was approved by the Institutional Ethics Committee at Medical College of Qingdao University (code: QDU-HEC-2025467; approved on 15 March 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the data of this study are available within the paper and its Supplementary Materials file.
Acknowledgments
The authors would like to thank all the reviewers and editors who participated in the review.
Conflicts of Interest
Author Jing Chen was employed by Qingdao Zepha Agricultural Science and Technology Development Co., Ltd. She participated in HPLC detection in the study. The role of the company was supplement of the HPLC platform. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TFA | Trifluoroacetic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| MG | Methyl gallate |
| PG | Propyl gallate |
| TA | Tannic acid |
| EGCG | Epigallocatechin gallate |
| EGC | Epigallocatechin |
| ECG | Epicatechin gallate |
| EC | Epicatechin |
| GCG | Gallocatechin gallate |
| GC | Gallocatechin |
| CG | Catechin gallate |
| C | Catechin |
| GA | Gallic acid |
| HPLC | High-performance liquid chromatography |
| RMSF | Root mean square fluctuation |
| MD | Molecular dynamics |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| Mw | Molecule weight |
| pI | Isoelectric point |
| NCBI | National Center of Biotechnology Information |
References
- Yu, S.D.; Zhang, J.H. Reinventing a tradition: East Asian tea cultures in the contemporary world. Asian J. Soc. Sci. 2022, 50, 167–170. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Jiang, H.; Wang, W.; Huang, J.; Ye, S.; Chen, Y.; Liu, S.; Liu, J. Theaflavins are improved by the oxidation of catechins in tannase treatment during black tea fermentation. Molecules 2025, 30, 452. [Google Scholar] [CrossRef]
- Chen, Z.M.; Lin, Z. Tea and human health: Biomedical functions of tea active components and current issues. J. Zhejiang Univ. B 2015, 16, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhang, Y.H.; Zhang, F.; Yang, Q.M.; Weng, H.F.; Xiao, Q.; Xiao, A.F. Thermostable tannase from Aspergillus niger and its application in the enzymatic extraction of green tea. Molecules 2020, 25, 952. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, D.Y.; Javaid, H.M.A.; Sahar, N.E.; Lee, H.N.; Hong, S.J.; Huh, J.Y.; Kim, Y.M. Bio-transformation of green tea infusion with tannase and its improvement on adipocyte metabolism. Enzym. Microb. Technol. 2020, 135, 109496. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef]
- Kumar, M.; Mehra, R.; Yogi, R.; Singh, N.; Salar, R.K.; Saxena, G.; Rustagi, S. A novel tannase from Klebsiella pneumoniae KP715242 reduces haze and improves the quality of fruit juice and beverages through detannification. Front. Sustain. Food Syst. 2023, 7, 1173611. [Google Scholar] [CrossRef]
- Garcia, D.E.; Glasser, W.G.; Pizzi, A.; Paczkowski, S.P.; Laborie, M.P. Modification of condensed tannins: From polyphenol chemistry to materials engineering. New J. Chem. 2016, 40, 36–49. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Revathi, S.; Rameshkumar, N.; Krishnan, M.; Kayalvizhi, N. Microbial tannase: Current perspectives and biotechnological advances. Biocatal. Agric. Biotechnol. 2016, 6, 168–175. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; McKinstry, W.J.; Ren, B. Characterization of a tannin acyl hydrolase from Streptomyces sviceus with substrate preference for digalloyl ester bonds. Appl. Microbiol. Biotechnol. 2015, 99, 2663–2672. [Google Scholar] [CrossRef]
- Tang, Z.; Shi, L.; Liang, S.; Yin, J.; Dong, W.; Zou, C.; Xu, Y. Recent advances of tannase: Production, characterization, purification, and application in the tea industry. Foods 2025, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Aharwar, A.; Parihar, D.K. Talaromyces verruculosus tannase immobilization, characterization, and application in tea infusion treatment. Biomass Conv. Bioref. 2023, 13, 261–272. [Google Scholar] [CrossRef]
- Cao, Q.; Zou, C.; Zhang, Y.; Du, Q.; Yin, J.; Shi, J.; Xue, S.; Xu, Y. Improving the taste of autumn green tea with tannase. Food Chem. 2019, 277, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chu, S.; Yan, L.; Chen, C. Effect of tannase treatment on protein-tannin aggregation and sensory attributes of green tea infusion. Lwt Food Sci. Technol. 2009, 42, 338–342. [Google Scholar] [CrossRef]
- Gong, X.; Li, Y.; Qu, H. Removing tannins from medicinal plant extracts using an alkaline ethanol precipitation process: A case study of danshen injection. Molecules 2014, 19, 18705–18720. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Fathy, S.A.; Rashad, M.M.; Ezz, M.K.; Mohammed, A.T. Purification and characterization of a novel tannase produced by Kluyveromyces marxianus using olive pomace as solid support, and its promising role in gallic acid production. Int. J. Biol. Macromol. 2018, 107, 2342–2350. [Google Scholar] [CrossRef]
- Aguilar, C.N.; Gutierrez-Sanchez, G. Review: Sources, properties, applications and potential uses of tannin acyl hydrolase. Food Sci. Technol. Int. 2001, 7, 373–382. [Google Scholar] [CrossRef]
- Fathy, S.A.; Mahmoud, A.E.; Rashad, M.M.; Ezz, M.K.; Mohammed, A.T. Improving the nutritive value of olive pomace by solid state fermentation of Kluyveromyces marxianus with simultaneous production of gallic acid. Int. J. Recycl. Org. Waste Agric. 2018, 7, 135–141. [Google Scholar] [CrossRef]
- Jana, A.; Halder, S.K.; Banerjee, A.; Paul, T.; Pati, B.R.; Mondal, K.C.; Mohapatra, P.K.D. Biosynthesis, structural architecture and biotechnological potential of bacterial tannase: A molecular advancement. Bioresour. Technol. 2014, 157, 327–340. [Google Scholar] [CrossRef]
- Lekha, P.K.; Lonsane, B.K. Production and application of tannin acyl hydrolase: State of the art. Adv. Appl. Microbiol. 1997, 44, 215–260. [Google Scholar]
- Lu, M.J.; Chen, C. Enzymatic modification by tannase increases the antioxidant activity of green tea. Food Res. Int. 2008, 41, 130–137. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hu, X.F.; Du, Q.Z.; Zeng, L.; Wang, S.Y.; Yin, J.F.; Xu, Y.Q. Effect of tannase on sediment formation in green tea infusion. J. Food Meas. Charact. 2020, 14, 1957–1965. [Google Scholar] [CrossRef]
- Ni, H.; Chen, F.; Jiang, Z.D.; Cai, M.Y.; Yang, Y.F.; Xiao, A.F.; Cai, H.N. Biotransformation of tea catechins using Aspergillus niger tannase prepared by solid state fermentation on tea byproduct. Lwt Food Sci. Technol. 2015, 60, 1206–1213. [Google Scholar] [CrossRef]
- Osipov, D.; Matys, V.Y.; Nemashkalov, V.; Rozhkova, A.; Shashkov, I.; Satrutdinov, A.; Kondratyeva, E.; Sinitsyn, A. Cloning, isolation, and properties of a new recombinant tannase from the Aspergillus niger fungus. Appl. Biochem. Microbiol. 2022, 58, 958–965. [Google Scholar] [CrossRef]
- Hong, Y.H.; Jung, E.Y.; Park, Y.; Shin, K.S.; Kim, T.Y.; Yu, K.W.; Chang, U.J.; Suh, H.J. Enzymatic improvement in the polyphenol extractability and antioxidant activity of green tea extracts. Biosci. Biotechnol. Biochem. 2013, 77, 22–29. [Google Scholar] [CrossRef]
- Gonçalves, H.B.; Riul, A.J.; Terenzi, H.F.; Jorge, J.A.; Guimarães, L.H.S. Extracellular tannase from Emericella nidulans showing hypertolerance to temperature and organic solvents. J. Mol. Catal. B Enzym. 2011, 71, 29–35. [Google Scholar] [CrossRef]
- Jana, A.; Maity, C.; Halder, S.K.; Das, A.; Pati, B.R.; Mondal, K.C.; Das Mohapatra, P.K. Structural characterization of thermostable, solvent tolerant, cytosafe tannase from Bacillus subtilis PAB2. Biochem. Eng. J. 2013, 77, 161–170. [Google Scholar] [CrossRef]
- Guo, J.; Rao, Z.; Yang, T.; Man, Z.; Xu, M.; Zhang, X.; Yang, S.T. Enhancement of the thermostability of Streptomyces kathirae SC-1 tyrosinase by rational design and empirical mutation. Enzym. Microb. Technol. 2015, 77, 54–60. [Google Scholar] [CrossRef]
- Xu, K.; Fu, H.; Chen, Q.; Sun, R.; Li, R.; Zhao, X.; Zhou, J.; Wang, X. Engineering thermostability of industrial enzymes for enhanced application performance. Int. J. Biol. Macromol. 2025, 291, 139067. [Google Scholar] [CrossRef]
- Zong, Z.; Gao, L.; Cai, W.; Yu, L.; Cui, C.; Chen, S.; Zhang, D. Computer-assisted rational modifications to improve the thermostability of β-glucosidase from Penicillium piceum H16. Bioenerg. Res. 2015, 8, 1384–1390. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Zhou, X.F.; Li, Z.; Zhou, H.X.; Song, W.Q. Characterization and secretory expression of a thermostable tannase from Aureobasidium melanogenum T9: Potential candidate for food and agricultural industries. Front. Bioeng. Biotechnol. 2022, 9, 769816. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–34. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–4254. [Google Scholar] [CrossRef]
- Teng, Z.; Pan, X.; Liu, Y.; You, J.; Zhang, H.; Zhao, Z.; Qiao, Z.; Rao, Z. Engineering serine hydroxymethyltransferases for efficient synthesis of L-serine in Escherichia coli. Bioresour. Technol. 2024, 393, 130153. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, T.; Cui, Y.; Wu, B. Structure-based self-supervised learning enables ultrafast protein stability prediction upon mutation. Innovation 2025, 6, 100750. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.–The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yin, J.F.; Chen, J.X.; Wang, F.; Du, Q.Z.; Jiang, Y.W.; Xu, Y.Q. Improving the sweet aftertaste of green tea infusion with tannase. Food Chem. 2016, 192, 470–476. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica: Recent achievements in heterologous protein expression and pathway engineering. Appl. Microbiol. Biotechnol. 2015, 99, 4559–4577. [Google Scholar] [CrossRef]
- Afolayan, T.V.; Adah, M.E.; Ibidapo, O.I.; Orotope, M.O.; Orji, F.A.; Famotemi, A.C.; Ehiwuogu-Onyibe, J.; Lawal, A.K. Screening and optimization of culture conditions for local production of tannase from fungal species. Trop. J. Nat. Prod. Res. 2021, 4, 1178–1181. [Google Scholar]
- Amanda, R.D.S.; Gouveia, M.J.; Tonny, C.C.L.; Aparecida Moreira, K.; Sandra, A.D.A. Production, Characterization and application of a thermostable tannase from Pestalotiopsis guepinii URM 7114. Food Technol. Biotechnol. 2014, 52, 459–467. [Google Scholar]
- Selwal, M.K.; Selwal, K.K. High-level tannase production by Penicillium atramentosum KM using agro residues under submerged fermentation. Ann. Microbiol. 2012, 62, 139–148. [Google Scholar] [CrossRef]
- Goel, G.; Kumar, A.; Beniwal, V.; Raghav, M.; Puniya, A.K.; Singh, K. Degradation of tannic acid and purification and characterization of tannase from Enterococcus faecalis. Int. Biodeterior. Biodegrad. 2011, 65, 1061–1065. [Google Scholar] [CrossRef]
- Iwamoto, K.; Tsuruta, H.; Nishitaini, Y.; Osawa, R. Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917(T). Syst. Appl. Microbiol. 2008, 31, 269–277. [Google Scholar] [CrossRef]
- Aharwar, A.; Parihar, D.K. Tannases: Production, properties, applications. Biocatal. Agric. Biotechnol. 2018, 15, 322–334. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, L.; Saxena, R.K. Purification, immobilization and characterization of tannase from Penicillium variable. Bioresour. Technol. 2008, 99, 2544–2551. [Google Scholar] [CrossRef]
- Chhokar, V.; Sangwan, M.; Beniwal, V.; Nehra, K.; Nehra, K.S. Effect of additives on the activity of tannase from Aspergillus awamori MTCC9299. Appl. Biochem. Biotechnol. 2010, 160, 2256–2264. [Google Scholar] [CrossRef]
- Farag, A.M.; Hassan, S.W.; El-Says, A.M.; Ghanem, K.M. Purification, characterization and application of tannase enzyme isolated from marine Aspergillus nomius GWA5. J. Pure Appl. Microbiol. 2018, 12, 1939–1949. [Google Scholar] [CrossRef]
- Pan, J.; Wang, N.N.; Yin, X.J.; Liang, X.L.; Wang, Z.P. Characterization of a robust and pH-Stable tannase from mangrove-derived yeast Rhodosporidium diobovatum Q95. Mar. Drugs 2020, 18, 546. [Google Scholar] [CrossRef]
- Ong, C.B.; Ibrahim, D.; Mohd Kassim, M.J.N. The tannase from red yeast Rhodotorula glutinis: Purification and characterization. Biocatal. Biotransform. 2024, 42, 110–117. [Google Scholar] [CrossRef]
- Beniwal, V.; Kumar, A.; Goel, G.; Chhokar, V. A novel low molecular weight acido-thermophilic tannase from Enterobacter cloacae MTCC 9125. Biocatal. Agric. Biotechnol. 2013, 2, 132–137. [Google Scholar] [CrossRef]
- Guan, L.; Wang, K.; Gao, Y.; Li, J.; Yan, S.; Ji, N.; Ren, C.; Wang, J.; Zhou, Y.; Li, B. Biochemical and structural characterization of a novel bacterial tannase from Lachnospiraceae bacterium in ruminant gastrointestinal tract. Front. Bioeng. Biotechnol. 2021, 9, 806788. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Unban, K.; Nguyen, T.H.; Haltrich, D.; Khanongnuch, C. Expression and biochemical characterization of a new alkaline tannase from Lactobacillus pentosus. Protein Expr. Purif. 2019, 157, 36–41. [Google Scholar] [CrossRef]
- Gayen, S.; Ghosh, U. Purification and characterization of tannin acyl hydrolase produced by mixed solid state fermentation of wheat bran and marigold flower by Penicillium notatum NCIM 923. BioMed Res. Int. 2013, 2013, 596380. [Google Scholar] [CrossRef]
- Riul, A.J.; Gonçalves, H.B.; Jorge, J.A.; Guimarães, L.H.S. Characterization of a glucose- and solvent-tolerant extracellular tannase from Aspergillus phoenicis. J. Mol. Catal. B Enzym. 2013, 85, 126–133. [Google Scholar] [CrossRef]
- Yuan, S.; Yan, R.; Lin, B.; Li, R.; Ye, X. Improving thermostability of Bacillus amyloliquefaciens alpha-amylase by multipoint mutations. Biochem. Biophys. Res. Commun. 2023, 653, 69–75. [Google Scholar] [CrossRef]
- Li, T.; Cui, N.; Fan, J.; Liu, X.; Guan, J.; Zhang, W.; Li, Y.; Gao, Q.; Liu, Y.; Xu, Y.; et al. Engineering β-agarase Aga0917 from Pseudoalteromonas fuliginea YTW1-15-1 to improve the thermostability and enzyme activity. ACS Sustain. Chem. Eng. 2022, 10, 15437–15449. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Østergaard, P.; Kauppinen, S.; Williamson, G. Hydrolysis of diethyl diferulates by a tannase from Aspergillus oryzae. Carbohyd. Polym. 2001, 44, 319–324. [Google Scholar] [CrossRef]
- Mostafa, H.S. Production of low-tannin Hibiscus sabdariffa tea through D-optimal design optimization of the preparation conditions and the catalytic action of new tannase. Food Chem. X 2023, 17, 100562. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Ye, J.H.; Jin, J.; Liang, H.L.; Lu, J.L.; Du, Y.Y.; Zheng, X.Q.; Liang, Y.R. Using tea stalk lignocelluloses as an adsorbent for separating decaffeinated tea catechins. Bioresour. Technol. 2009, 100, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, D.; Bongaerts, J.H.H.; Wantling, E.; Stokes, J.R.; Williamson, A.M. Astringency of tea catechins: More than an oral lubrication tactile percept. Food Hydrocoll. 2009, 23, 1984–1992. [Google Scholar] [CrossRef]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Boadi, D.; Neufeld, R. Encapsulation of tannase for the hydrolysis of tea tannins. Enzym. Microb. Technol. 2001, 28, 590–595. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Khanongnuch, C.; Mathivanan, K.; Shyu, D.J.H.; Sharma, K.P.; Mandal, S.D. In-vitro biotransformation of tea using tannase produced by Enterobacter cloacae 41. J. Food Sci. Technol. 2021, 58, 3235–3242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).