Abstract
Cassava (Manihot esculenta) is a vital tropical staple crop with expanding relevance beyond food security, particularly in developing functional beverages and nutraceutical products. This review discusses the implications of selected chemicals in cassava roots for beverage production, notably cyanogenic glycosides and phenolic compounds. We further highlight the role of cassava as a substrate for beverage production, the nutritional significance of cassava-based beverages, and the health benefits and functional potential of cassava as a key ingredient in beverage production. We also discuss the probiotic and prebiotic properties and the antioxidant activity of chemicals in cassava-based beverages for health benefits. Additionally, we review the challenges, opportunities, and innovations regarding commercialization.
1. Introduction
Cassava (Manihot esculenta Crantz), native to South America (Brazil) and domesticated over 10,000 years ago, is a member of the Euphorbiaceae family that is highly valued for its starch content [1,2,3,4,5]. Cassava has emerged as a critical staple and commercial crop across tropical regions, particularly in Africa, Asia, and South America. Nigeria currently ranks as the world’s largest producer, with an annual output exceeding 60 million metric tons, followed by the Democratic Republic of the Congo, which contributes over 48.8 million metric tons to global production [1,6].
Moreover, the high starch content of cassava roots has particularly enhanced its significance in industrial applications in the pharmaceutical and beverage industries. Cassava starch provides sufficient substrate for the production of fermented products [7,8]. Cassava is emerging as a cheaper alternative to sorghum, wheat, and maize in beer brewing and the production of other beverages due to its chemical and nutritional diversity [9].
Cassava roots display significant chemical diversity. Besides starch, moisture, and fiber content, cassava harbors cyanogenic glycosides, low proteins, and sugars, such as glucose, fructose, and sucrose. Cassava also contains organic acids, such as lactic, oxalic, and citric acids, and several secondary metabolites, including phenolics and tannins [10,11,12,13]. These chemicals improve the cassava’s defense prowess and boost the nutritional values of the edible parts and its downstream products, such as beverages. Over the years, polyphenols have been demonstrated to enhance food’s nutritional qualities and shelf life. For example, (+)-gallocatechin, (+)-catechin, and (+)-catechin gallate have been reported to be present in cassava [12]. Consuming antioxidant-rich beverages can offer significant health benefits, such as improvement in cardiovascular health, antimicrobial activities, and antioxidant activities.
This review discusses the implications of selected chemicals in cassava roots for beverage production, notably cyanogenic glycosides and phenolic compounds, cassava as a substrate for beverage production, the nutritional significance of cassava-based beverages, and the health benefits and functional potential of cassava as the key ingredient in beverage production. We also highlighted its probiotic and prebiotic properties and reviewed the challenges, opportunities, and innovations regarding commercialization.
2. Chemical Composition and Diversity of Cassava
2.1. High Starch Content: Implications for Fermentation and Processing
Cassava has high starch content, which is crucial for its fermentation and processing, since it can serve as a substrate for microbial activities. This ultimately influences the qualitative and safety attributes of the resultant products, such as texture and taste. However, the starch contents of cassava vary among varieties, ranging from 20% to 40% [14], suggesting the level of fermentation also varies among cassava varieties. The fermentation process is critical for reducing harmful cyanogenic compounds, with studies indicating that extended fermentation times can significantly lower these compounds, thereby enhancing beverage safety [15]. Additionally, the starch granules in cassava undergo structural modifications during fermentation, leading to desirable qualities such as improved gel strength and enhanced pasting properties, which are vital for food applications. Consequently, understanding the implications of high starch content is essential for optimizing fermentation conditions and improving the overall quality of cassava-based food products [15,16].
A study investigated the nutritional composition of beer made from a blend of sorghum and hybrid yellow cassava. Proximate analysis was conducted on beer samples fermented over ten days at varying ratios of cassava to sorghum, revealing changes in pH and alcohol content, with pH ranging from 3.65 to 4.10 and alcohol content from 3.63% to 6.74%. Nutritional components such as fat, ash, moisture, and crude protein were measured, showing values between 0.30% to 0.59%, 0.42% to 0.61%, 76.10% to 80.97%, and 0.30% to 0.56%, respectively, alongside vitamin A and C contents ranging from 12.38 to 4.13 μg/100 mL and 12.21 to 17.39 mg/100 mL [15].
2.2. Secondary Metabolites
2.2.1. Cyanogenic Glycosides (Linamarin, Lotaustralin)—Implications for Processing Safety
Cassava, as a plant, contains high amounts of cyanogen compounds, which pose a severe threat to beverage production. The cyanogen compounds comprise three forms, namely, cyanogenic glucoside, composed of 95% linamarin and only 5% lotaustratin; cyanohydrins; and free cyanide [17] (Figure 1). Notably, linamarin and lotaustralin pose profound health implications for safety in the processing and consumption of cassava [18,19]. Ingested cyanogenic glycosides undergo enzymatic hydrolysis, resulting in the production of hydrogen cyanide (HCN), which is a highly toxic substance in humans, particularly interfering with cellular respiration through the inhibition of cytochrome c oxidase in human mitochondria, depriving cellular supply of oxygen [20]. HCN has also been reported to disrupt cardiovascular and respiratory architecture, notably causing cardiac arrest, low blood pressure, and irregular heartbeats [21,22,23]. Intriguingly, even a lower dosage, HCN can still have immeasurable health risks if consumed in large quantities, leading to vomiting, dizziness, and, in severe cases, respiratory failure and death [24]. Owing to these health implications of cyanogens in cassava, there is a need to reduce these toxins to tolerable levels before downstream beverage preparation. Although genetic engineering alternatives are being explored to mitigate cyanogen content in cassava, some schools of thought have suggested that shutting down cyanogen metabolism routes in cassava might compromise its defense machinery [13,25,26]. Similar observations in the genetic detoxification of gossypol in cotton [27] are a timely reminder for proper industrial cyanide detoxification in cassava before downstream beverage productions.
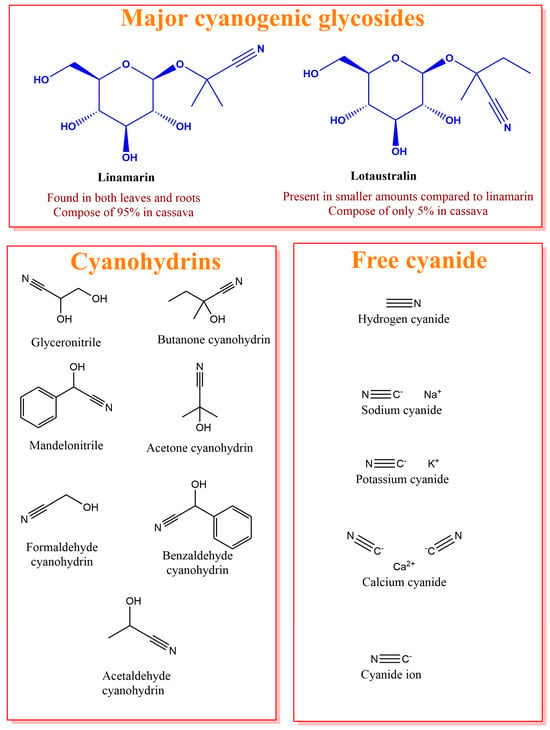
Figure 1.
Cyanogen compounds in cassava: cyanogenic glycoside, cyanohydrins, and free cyanide.
Early studies have outlined the processing techniques that can reduce cyanogen compounds in cassava to improve its ingestion. These include peeling, grating, drying, boiling, and fermentation [28]. Moreover, cyanogen detoxification processing has also been identified to improve the nutritional indicators of cassava and enhance the flavor of beverages. For example, fermentation enhances the bioavailability of certain minerals and improves the digestibility, shelf life, and sensory qualities of cassava [28,29]. More significantly, the fermentation of graded cassava reduced cyanogenic compounds than peeled ungraded cassava [28]. It has been suggested that the enzyme linamarase, which catalyzes the breakdown of cyanogenic glycosides, is inactivated or disrupted during grating and fermentation processes. Microbial fermentations, such as Lactobacillus plantarum, also enhance the leakage of bound cyanogenic glycosides, reducing the cyanide content of cassava. Similarly, Saccharomyces cerevisiae reduces HCN by 65.9% [28]. Detoxifying cassava during beverage production is a crucial step in healthy beverage production.
2.2.2. Relevance of Phenolic Compounds and Antioxidants in Cassava for Beverage Quality
The presence of phenolic compounds and antioxidants in cassava has received significant attention over the years owing to their crucial role in beverage quality improvement and health benefits [30,31]. These compounds also enhance the nutritional characteristics of cassava beverages and extend the shelf life of these products by acting as natural preservatives [30,31]. Cassava is rich in several phenolic compounds, such as hydroxycoumarins, flavonoids, and phenolic acids, which exhibit significant antioxidant characteristics. Phenolic compounds contribute to the bitterness and astringency characteristic of beverages. Under moderate amounts, they enhance the flavor of fermented cassava beverages and improve the health benefits of the end products [31] (Figure 2).
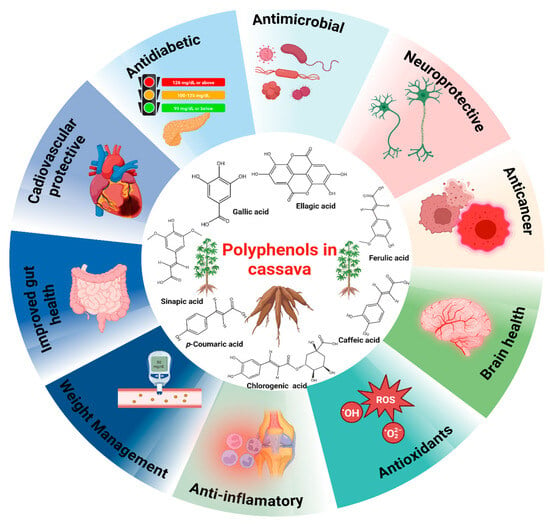
Figure 2.
Significance of polyphenols in cassava beverages.
Other antioxidants, including 1,1-diphenyl-2picrylhydrazyl, carotenoids, p-Coumaric acid, gallic acid, quercetin-3-Oglucosylrutinoside, and kaempferol-3-O-rutinoside are largely involved in the bitterness and astringency of most beverages [32,33,34]. Nevertheless, there are huge variations in the threshold of bitterness and astringency depending on these antioxidant capacities [33]. While kaempferol-3-O-glucoside (Kae-glu) and kaempferol-3-O-rutinoside (Kae-rut) contributed immensely to the astringency of green tea beverages, caffeine triggers the bitterness [33,35,36]. Caffeine is also known for its flavoring attributes [33]. The advent of new analytical tools and isolation techniques (macroporous resin separation column, solid phase extractor, and silica gel chromatography) could further help identify novel antioxidants involved in the quality improvement of beverages.
3. Cassava as a Substrate for Beverage Production
Cassava has emerged as a vital feedstock for global alcohol production, particularly in sub-Saharan Central Africa, where it has supplanted cereals as a primary source. Its high starch content and mild glycemic index make it a promising value addition to wine products [37,38]. However, previous research has highlighted challenges in utilizing cassava for alcoholic beverages, particularly due to inefficient starch saccharification, limiting ethanol conversion efficiency [39]. Building on earlier challenges in starch saccharification, Coelho, Ballesteros [38] employed biotechnological approaches to produce high-quality cassava spirits and beverages (Table 1). They generated a fermentable broth containing approximately 184 g L−1 of sugars by liquefying and saccharifying cassava flour with enzymatic cocktails. The by-product was fermented and distilled to produce spirits with 40% ethanol. The gas chromatography analysis revealed a profile dominated by yeast fermentation metabolites and wood extractives, resulting in spirits with desirable sensory traits and good acceptance by tasters. These findings on cassava spirits [38] highlight the importance of value addition in cassava products, an idea further explored by Malinao, Baniaga [40] in a study on cassava-based wine production. The latter study focused on the cassava-based alcoholic fermentation process and sensory attributes. The authors observed that using Saccharomyces cerevisiae as the primary yeast strain during the traditional fermentation process yielded wine with desirable sensory attributes. The cassava wine received favorable ratings for its aroma, appearance, and taste, with attributes like a light yellow-brown color and smooth texture contributing to its appeal [40]. However, fluctuations in fermentation parameters affected the wine’s taste and texture, resulting in some batches with a slightly sour taste [40]. As such, there is a need to optimize fermentation conditions to enhance the quality of cassava wine products. Indigenous people all over the world have explored various uses of cassava for diverse beverage production, and recently, some commercial products have emerged in the market. Table 1 indicates some of the known beverage productions in the literature, created using various parts of the cassava.

Table 1.
Some indigenous and commercial-based cassava beverages.
4. Nutritional Significance of Cassava-Based Beverages
Extensive research has elucidated the nutritional benefits of cereal-based beverages, yet cassava-based drinks remain largely untapped. Plant-based beverages are excellent sources of carbohydrates and energy [57]. As a probiotic carrier, these beverages hold promises for women’s gut health [58], and, thus, the macronutrient composition of fermented wine products warrants further investigation (Figure 3). Fermentation enhances mineral bioavailability, particularly zinc and iron, by reducing anti-nutritional compounds such as tannins, phytates, and polyphenols prevalent in cassava (Figure 3). For instance, fermenting cassava decreased phytic acid by almost 90%, improving the nutritional bioavailability in the diet [59,60,61]. Anti-nutritional compounds in cassava-based diets interfere with mineral (calcium, molybdenum, magnesium, iron, zinc) absorption, halting nutrient bioavailability [62,63]. More evidence showed that tannins and polyphenols bind with proteins to prevent the digestion and absorption of mineral compounds.
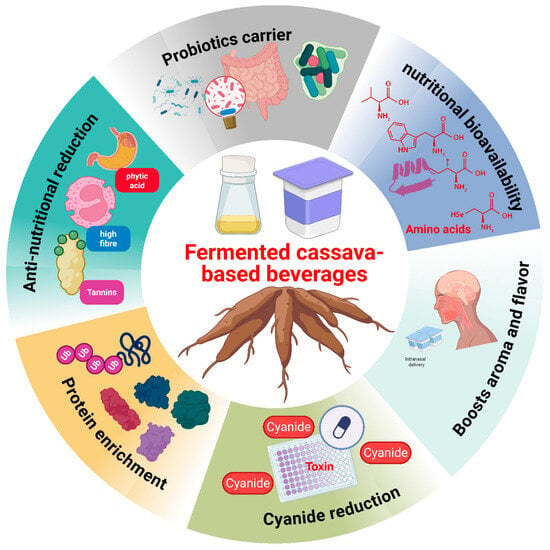
Figure 3.
Nutritional benefits of fermented cassava-based beverages.
Following this, protein precipitation occurs, and proteases are halted, resulting in amino acid deprivation (Figure 3). However, the fermentation of cassava or cereals effectively reduces the inhibitors, enhancing the digestibility of plant proteins. A previous study has shown a marked increase in the crude protein of cassava following a 48 h fermentation process [64]. Fermenting cassava chips with Saccharomyces cerevisiae heightened crude protein content from 2% to 32.4% [65]. Such increased protein content was attributed to microbial and enzymatic alterations [66]. These enzymes (lipase, amylase, phytase, and proteases) modify food products via the robust hydrolysis of lipids, polysaccharides, phytates, and proteins, respectively [67], to yield harmless and desirable by-products, with improved shelf life, taste, aroma, and nutritional value [67,68].
Fermentation produces a complex mix of volatile compounds (alkanes, organic acids, ketones, aldehyde, alcohol, terpenes, and nitrogen compounds), impacting food’s aroma and flavor profile [64] (Figure 3). Hasan, Sultan [69] opined that fermentation by Saccharomyces cerevisiae and lactic acid bacteria (LAB) enhances volatile compound production in fermented rice. Thus, the amount of volatile compounds produced varies depending on the microorganism present during fermentation [70]. Specifically, cereal-based fermented beverages are primarily fermented by lactic acid bacteria (LAB), including species of Streptococcus, Lactobacillus, Lactococcus, Pediococcus, with Saccharomyces and Candida yeasts also contributing [71]. LAB dominates fermentation, producing lactic acid and revolutionizing the beverages’ favorable properties [71]. This fermentation prolongs the shelf life of cereal beverages by boosting acidity while inhibiting harmful microorganisms and rapid spoilage [72,73]. Specifically, LAB and molds produce antibiotics and bacteriocins that further help prevent spoilage, improving the beverage’s shelf life and safety [74,75]. However, the traditional fermentation process often shortens the shelf life owing to suboptimal hygiene practices. Modern food processing techniques, such as drying and smart packaging techniques, were encouraged for cereals-based fermented beverages’ safety and shelf life extension [71]. Drying halts the growth of microorganisms that cause food spoilage and prevents toxin formation [76].
Ample evidence has shown that fermentation effectively reduces cyanide content in cassava [64] (Figure 3). It has also been revealed that a 24 h fermentation with L. plantarum drastically reduced cassava cyanide content by 97.92% [70]. Kobawila et al. [73] showed up to 70.67% reduction following a 48 h fermentation process, highlighting the importance of fermentation in cyanide reduction. While previous research centered on the benefits of cassava-based fermented beverages, future studies should explore emerging areas such as microbial metagenomics, rheology, shelf life extension, starter culture development, and kinetic modeling to facilitate innovation.
5. Health Benefits and Functional Potential
5.1. Probiotic and Prebiotic Properties
Cassava-based formulations have gained increasing attention in functional food research, especially in relation to symbiotic systems that combine probiotics and prebiotics. Symbiotic formulations using cassava starch are emerging due to cassava’s high carbohydrate content and fermentability in the gut. Studies have shown that modified cassava starch supports the growth of numerous probiotic strains, such as Lactobacillus acidophilus and Bifidobacterium bifidum, enhancing gut health and improving host immunity [77,78,79,80]. The cassava roots are mainly a source of carbohydrates, especially starch. Cassava leaves are a good source of proteins, vitamins (A, C, B1, B2), and minerals. While the cassava peels are not as nutritious as the roots or leaves, they do contain fiber and some minerals. The valorization of cassava has demonstrated its prebiotic potential through converting it to a functional food and recovering the compounds found in its pulp and peels [81,82,83,84,85].
Fibers and resistant starch allow cassava to exhibit strong prebiotic properties. Resistant starch, particularly type II and retrograded type III from cassava, has been shown to resist digestion and undergo fermentation in the colon, supporting beneficial microbiota. Malfado et al. showed in their research that cassava varieties induce positive changes in the composition and metabolic activity of the human intestinal microbiota of celiac disease patients during colonic fermentation [86]. Moreover, cassava-based dietary fiber improves short-chain fatty acid (SCFA) production, which contributes to colonic health and systemic anti-inflammatory effects [87].
Cassava-resistant starch has also contributed to the stability and sustained delivery of biomolecules. Zhu et al. showed that cassava starch could prevent the oxidation of Epigallocatechin gallate (EGCG) during delivery, leading to the bioavailability of EGCG [88,89]. Similarly, incorporating cassava starch nanoparticles promoted a sustained drug delivery system, supporting a targeted drug delivery system [90] and contributing to pharmaceuticals and medicine. Additionally, another study conducted using the pulsed electric field esterification of cassava demonstrates an increase in the cassava-resistant starch content products, promoting the desired digestibility of the formulation [91]. In a modified cassava starch formulation, the quality characteristics of a fermented soybean beverage are improved by improving sensory activities based on turning pH, acidity, soluble solids, syneresis, and microbiological quality [78,92].
5.2. Antioxidant Activity
Cassava peels and roots are rich in polyphenolic compounds, which contribute significantly to antioxidant activity. Investigations have identified key phenolics, such as catechins, ferulic acid, and rutin, especially in the peels [93,94,95,96]. These compounds exhibit radical scavenging capacity and inhibit lipid peroxidation, making cassava by-products viable for functional ingredients in antioxidant-rich formulations. These compounds have also been characterized using LC-MS for antioxidant properties in different food-related varieties [97,98]. In assessing the total phenolic content, the peels have a higher amount than the roots, with the concentration of 681.5 mg GAE/100 g, with an antioxidant activity of 19% [99]. Hence, despite the chelating disadvantage of polyphenols of some micronutrients, they have more relevance in antioxidant activity in cassava.
Furthermore, the antioxidant mechanisms play a critical role in reducing oxidative stress, thereby potentially mitigating chronic diseases such as cardiovascular conditions and certain cancers. Fermented cassava products have demonstrated enhanced antioxidant capacity and flavor, likely due to the biotransformation of the phenolic compounds during fermentation [100,101]. Cassava–acerola juice showed a marked increase in antioxidant activity, using 1,1-diphenyl-2picrylhydrazyl (DPPH) and 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity within 24 h of fermentation. It was found that β-galactosidase liberates free phenolic compounds to boost the DPPH and ABTS antioxidant capacity [100]. Nevertheless, the ascorbic acid activity weakened during the 24 h fermentation, suggesting the impact of fermentation on antioxidant capacity [100].
5.3. Detoxification and Gut Health
Cassava fermentation significantly improves its functional attributes by supporting beneficial gut microbiota and aiding in detoxification [102]. Fermented cassava increases microbial diversity in the gut, especially in promoting short-chain fatty acids (SCFA)-producing species and beneficial Bacteroides [103,104]. These microbial shifts improve intestinal barrier function and support immune modulation. Traditional cassava-based drinks, such as “fermented gari water” and “chicha”, have long been utilized in indigenous practices for their detoxifying and digestive benefits. Ethnobotanical studies confirm the role of these beverages in alleviating gastrointestinal discomfort and improving metabolic balance [105].
6. Challenges and Opportunities
6.1. Safety Concerns
One of the primary safety concerns with cassava is its cyanogenic glucoside content, which can produce toxic hydrogen cyanide upon improper processing. Efforts to manage cyanide toxicity include optimized fermentation, drying, and thermal treatment protocols [106]. However, variability in traditional processing methods leads to inconsistent detoxification, necessitating standardized protocols. The standardization of fermentation processes is crucial for the safe commercialization of cassava-based functional products. Recent biotechnological advancements, including the use of defined microbial starters, have improved consistency in detoxification and functional benefits [107]. The way forward for cassava safety will require intensive research on the safe use of cassava.
6.2. Innovation and Commercialization
Bioprocessing innovations are unlocking new avenues for the development of cassava-derived functional beverages. Enzyme-assisted extraction and controlled fermentation have produced prebiotic-rich and probiotic-fortified drinks with stable shelf life and sensory appeal [108]. Market trends in health and wellness underscore significant commercial potential for cassava-based functional products, particularly in regions with high cassava cultivation, mostly in tropical regions such as Africa, Asia, and parts of America. Functional beverages incorporating cassava-derived ingredients align with consumer demand for natural, gut health-promoting, and plant-based options [109]. The commercial value of the cassava plant is underutilized despite its resilience, cheapness, and productivity.
Agricultural innovation remains a crucial phenomenon capable of revolutionizing cassava production by enhancing high yields through disease resistance measures, biotechnology, and tissue culture. The application of precision farming techniques, including Geographic Information System, remote sensing, and drones for yield prediction and soil analysis, will further drive innovation in cassava production and promote its use as a commercial beverage product. Agricultural mechanization will also help reduce labor demands and minimize post-harvest losses.
The industrial processing of cassava for beverages and value-added products represents another innovation that can enhance its commercial value by extracting relevant phytochemicals for industries such as brewing, pharmaceuticals, and adhesives. In commercializing cassava, business models for small-scale farmers should be integrated with exporters and processors. Public–private partnerships should be developed to provide the necessary infrastructure for cassava processing. Additionally, branding cassava-based beverage products will be essential to strengthening their market presence.
7. Conclusions
Cassava offers a highly affordable source of phytochemicals for the beverage industry, providing potential antioxidant and anti-inflammatory compounds that serve as a nutritional feedstock with health benefits in beverage products. Bioprocessing innovations such as controlled fermentation, enzyme-assisted extraction, and biotechnological advancements have mitigated cassava’s toxicity, making it safer for bio-nutritional applications. With its numerous bioactive compounds, cassava provides health benefits including antioxidant, anticancer, antidiabetic, and antimicrobial activities. To fully harness cassava’s potential, future research must focus on enhancing its health-promoting properties while ensuring environmental sustainability and socio-economic accessibility, especially for the communities that depend on it the most.
Author Contributions
V.N., J.Z. and O.O.A. conceived the idea and drafted the manuscript. V.N. drew the figures, and J.Z. and O.O.A. formatted the manuscript. N.K., S.C., F.D.D., M.S.U., L.A.A., A.K.A.-N. and J.A. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by: Constructor University Bremen Germany (School of Science), C. K. T. University of Technology and Applied Sciences Ghana (School of Chemical and Biochemical Sciences), the Project of National Key Laboratory for Tropical Crop Breeding (NO. NKLTCBYWF202405) and the Central Public-interest Scientific Institution Basal Research Fund (NO. 1630032022007) in China.
Data Availability Statement
This article generated no data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olsen, K.M.; Schaal, B.A. Evidence on the origin of cassava: Phylogeography of Manihot esculenta. Proc. Natl. Acad. Sci. USA 1999, 96, 5586–5591. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.; Nolorbe-Payahua, C. Ethnobotanical Diversity of Cassava (Manihot esculenta Crantz) in the Peruvian Amazon. Diversity 2022, 14, 252. [Google Scholar] [CrossRef]
- Alves-Pereira, A.; Zucchi, M.I.; Clement, C.R.; Viana, J.P.G.; Pinheiro, J.B.; Veasey, E.A.; de Souza, A.P. Selective signatures and high genome-wide diversity in traditional Brazilian manioc (Manihot esculenta Crantz) varieties. Sci. Rep. 2022, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Thuy, C.T.L.; Lopez-Lavalle, L.A.B.; Vu, N.A.; Hy, N.H.; Nhan, P.T.; Ceballos, H.; Newby, J.; Tung, N.B.; Hien, N.T.; Tuan, L.N.; et al. Identifying New Resistance to Cassava Mosaic Disease and Validating Markers for the CMD2 Locus. Agriculture 2021, 11, 829. [Google Scholar] [CrossRef]
- Parmar, A.; Sturm, B.; Hensel, O. Crops that feed the world: Production and improvement of cassava for food, feed, and industrial uses. Food Secur. 2017, 9, 907–927. [Google Scholar] [CrossRef]
- Otekunrin, O.A. Cassava (Manihot esculenta Crantz): A global scientific footprint—Production, trade, and bibliometric insights. Discov. Agric. 2024, 2, 94. [Google Scholar] [CrossRef]
- Kaur, K.; Ahluwalia, P. Cassava as potential crop for the food and fermentation industry: A review. Int. J. Food Ferment. Technol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Falade, K.O.; Akingbala, J.O. Utilization of cassava for food. Food Rev. Int. 2010, 27, 51–83. [Google Scholar] [CrossRef]
- Qi, M.; Jiang, L.; Song, J.; Li, L.; Xu, M.; Li, Y.; Ma, C.; Chen, S.; Li, H. Enhancing cassava beer quality: Extrusion-induced modification of cassava starch structure boosts fermentable sugar content in wort. Int. J. Biol. Macromol. 2024, 278, 134895. [Google Scholar] [CrossRef]
- Drapal, M.; Barros de Carvalho, E.; Ovalle Rivera, T.M.; Becerra Lopez-Lavalle, L.A.; Fraser, P.D. Capturing Biochemical Diversity in Cassava (Manihot esculenta Crantz) through the Application of Metabolite Profiling. J. Agric. Food Chem. 2019, 67, 986–993. [Google Scholar] [CrossRef]
- Manano, J.; Ogwok, P.; Byarugaba-Bazirake, G.W. Chemical composition of major cassava varieties in Uganda, targeted for industrialisation. J. Food Res. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Blagbrough, I.S.; Bayoumi, S.A.; Rowan, M.G.; Beeching, J.R. Cassava: An appraisal of its phytochemistry and its biotechnological prospects. Phytochemistry 2010, 71, 1940–1951. [Google Scholar] [CrossRef]
- Gazola, D.; Zucareli, C.; Ringenberg, R.; de Oliveira, M.C.N.; da Graça, J.P.; de Oliveira Nunes, E.; Hoffmann-Campo, C.B. Secondary metabolite contents in different parts of cassava plants infested by Phenacoccus manihoti Matile-Ferrero (Hemiptera: Pseudococcidae). Arthropod-Plant Interact. 2019, 13, 359–366. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Adeloye, A.A.; Olaomo, O.O.; Kayitesi, E. Effect of fermentation time on physicochemical properties of starch extracted from cassava root. Food Biosci. 2020, 33, 100485. [Google Scholar] [CrossRef]
- Akpoghelie, P.O.; Edo, G.I. Proximate and nutritional composition of beer produced from malted sorghum blended with yellow cassava. Biocatal. Agric. Biotechnol. 2022, 45, 102535. [Google Scholar] [CrossRef]
- Daly, L. “The Spirits Drink Cassava Beer”: The More-Than-Human Politics of Self-Help in Amazonian Guyana. Med. Anthropol. 2025, 44, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Processing Techniques to Reduce Toxicity and Antinutrients of Cassava for Use as a Staple Food. Compr. Rev. Food Sci. Food Saf. 2009, 8, 17–27. [Google Scholar] [CrossRef]
- Cereda, M.P.; de Vasconcellos, S.P. Cassava cyanogenic glycosides: Importance, toxicity, and dosage methods. In Varieties and Landraces: Cultural Practices and Traditional Uses; Elsevier: Amsterdam, The Netherlands, 2023; pp. 179–209. [Google Scholar]
- Nyamekye, C.A. Health Issues Related to the Production and Consumption of Cassava as a Staple Food. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2021. [Google Scholar]
- Cressey, P.; Saunders, D.; Goodman, J. Cyanogenic glycosides in plant-based foods available in New Zealand. Food Addit. Contam. Part A 2013, 30, 1946–1953. [Google Scholar] [CrossRef]
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 931–951. [Google Scholar] [CrossRef]
- Wahl-Schott, C.; Biel, M. HCN channels: Structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 2008, 66, 470. [Google Scholar] [CrossRef]
- Roubille, F.; Tardif, J.-C. New therapeutic targets in cardiology: Heart failure and arrhythmia: HCN channels. Circulation 2013, 127, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Alitubeera, P.H. Outbreak of cyanide poisoning caused by consumption of cassava flour—Kasese District, Uganda, September 2017. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Siritunga, D.; Sayre, R. Transgenic approaches for cyanogen reduction in cassava. J. AOAC Int. 2007, 90, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Siritunga, D.; Sayre, R.T. Generation of cyanogen-free transgenic cassava. Planta 2003, 217, 367–373. [Google Scholar] [CrossRef]
- Ninkuu, V.; Liu, Z.; Zhou, Y.; Sun, X. The nutritional and industrial significance of cottonseeds and genetic techniques in gossypol detoxification. Plants People Planet 2024, 6, 271–286. [Google Scholar] [CrossRef]
- Panghal, A.; Claudia, M.; Paras, S.; Chhikara, N. Cassava toxicity, detoxification and its food applications: A review. Toxin Rev. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products–A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- de Oliveira, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New frontiers in the exploration of phenolic compounds and other bioactives as natural preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Liao, C.; Chen, L.; Wang, J.; Zeng, L. Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: A study using response surface methodology. Food Chem. 2018, 269, 24–34. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Aluko, O.O.; Mo, Z.; Mao, J.; Zhang, H.; Liu, X.; Ma, M.; Wang, Q.; Liu, H. Bitter and astringent substances in green tea: Composition, human perception mechanisms, evaluation methods and factors influencing their formation. Food Res. Int. 2022, 157, 111262. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Aluko, O.O.; Jianpei, Y.; Chen, S.; Zeng, H.; Dakora, F.D. Phenylpropanoids metabolism: Recent insight into stress tolerance and plant development cues. Front. Plant Sci. 2025, 16, 1571825. [Google Scholar] [CrossRef]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted Analysis Using Ultraperformance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Zhang, L.; Qing-Qing, C.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Andrés–Meza, P.; Aguilar–Rivera, N.; Meneses–Márquez, I.; Del Rosario–Arellano, J.L.; Bolio–López, G.I.; Leyva–Ovalle, O.R. Cassava cultivation; current and potential use of agroindustrial co–products. AIMS Environ. Sci. 2024, 11, 248–278. [Google Scholar] [CrossRef]
- Coelho, E.; Ballesteros, L.F.; Domingues, L.; Vilanova, M.; Teixeira, J.A. Production of a Distilled Spirit Using Cassava Flour as Raw Material: Chemical Characterization and Sensory Profile. Molecules 2020, 25, 3228. [Google Scholar] [CrossRef]
- Kubo, R.; Funakawa, S.; Araki, S.; Kitabatake, N. Production of indigenous alcoholic beverages in a rural village of Cameroon. J. Inst. Brew. 2014, 120, 133–141. [Google Scholar] [CrossRef]
- Malinao, C.W.M.; Baniaga, G.E.; Cay, J.J.G. Local Production Techniques and Sensory Evaluation of Cassava Wine. J. Interdiscip. Perspect. 2025, 3, 214–220. [Google Scholar]
- Chacón, G.; Arias, G.; José Sandoval-Cañas, G.; Ordoñez Araque, R. Ancestral fermented indigenous beverages from South America made from cassava (Manihot esculenta). Ciência E Tecnol. De Aliment. 2020; ahead of print. [Google Scholar] [CrossRef]
- Mgm, E.; Krishnadath, I.; van Eer, E.; Ym, S.; Mans, D.; Sgs, V. Metabolic syndrome in Indigenous Amerindian women in Suriname; less on waist and more on weight? J. Obes. Overweig. 2017, 3, 201. [Google Scholar]
- Mayorga, G.A.C.; Palma, G.B.A.; Sandoval-Cañas, G.J.; Ordoñez-Araque, R.H. Ancestral fermented indigenous beverages from South America made from cassava (Manihot esculenta). Food Sci. Technol. 2021, 41. [Google Scholar] [CrossRef]
- Ngozi Joan, A.; Peter, A.S. Mutation Breeding: A Tool in Nutritional Improvement of Cassava. In Cassava—Recent Updates on Food, Feed, and Industry; Frediansyah, A., Ed.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Lima, T.T.M.; Hosken, B.d.O.; Venturim, B.C.; Lopes, I.L.; Martin, J.G.P. Traditional Brazilian fermented foods: Cultural and technological aspects. J. Ethn. Foods 2022, 9, 35. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; de Almeida, E.G.; Duarte, W.F.; Schwan, R.F. Study of the physicochemical parameters and spontaneous fermentation during the traditional production of yakupa, an indigenous beverage produced by Brazilian Amerindians. World J. Microbiol. Biotechnol. 2014, 30, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Motlhanka, K.; Zhou, N.; Lebani, K. Microbial and chemical diversity of traditional non-cereal based alcoholic beverages of Sub-Saharan Africa. Beverages 2018, 4, 36. [Google Scholar] [CrossRef]
- Andrade, J.S.; Pantoja, L.; Maeda, R.N. Improvement on beverage volume yield and on process of alcoholic beverage production from pejibaye (Bactris gasipaes Kunth). Food Sci. Technol. 2003, 23, 34–38. [Google Scholar]
- Harsono, G. Analisa Dan Perancangan Sistem Manajemen Gudang Pada Perusahaan Jasa Maklon/E-Contract Manufacturing (Studi Kasus: CV. Sakura Satrya Jaya). JUSIBI J. Sist. Inf. Dan E-Bisnis 2020, 2, 374–390. [Google Scholar]
- Cereda, M.P.; dos Santo Brito, V.H. Fermented Foods and Beverages from Cassava (Manihot esculenta Crantz) in South America. In Fermented Foods of Latin America; CRC Press: Boca Raton, FL, USA, 2017; pp. 202–223. [Google Scholar]
- Wireko-Manu, F.D. Development and quality assessment of cassava-sweetpotato non-alcoholic beverage. MOJ Food Process. Technol. 2016, 2, 1–5. [Google Scholar]
- Palacio, J. The Garifuna, A Nation Across Borders: Essays in Social Anthropology; Cubola Prod: Benque Viejo Del Carmen, Belize, 2005. [Google Scholar]
- Gico, E.T.; Ybarzabal, E.R. Indigenous rice wine making in Central Panay, Philippines. Patubas 2015, 10, 49–73. [Google Scholar]
- Gregorio, C.G.C. Philippine Traditional Alcoholic Beverages: A Germinal Study. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Mérillon, J.-M., Riviere, C., Lefèvre, G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–30. [Google Scholar] [CrossRef]
- Dobermann, D.; Field, L.M.; Michaelson, L.V. Using Hermetia illucens to process Ugandan waragi waste. J. Clean. Prod. 2019, 211, 303–308. [Google Scholar] [CrossRef]
- Cereda, M.P.; de Almeida Lima, U. Etanol, spirits and beer produced from underground starchy raw materials. In Traditional Starch Food Products; Elsevier: Amsterdam, The Netherlands, 2025; pp. 205–236. [Google Scholar]
- Scaria, S.; Balasubramanian, B.; Arun, M.; Jaison, J.; Gangwar, J.; Kurian, J.; Pappuswamy, M.; Pusparaj, K.; Park, S.; Joseph, K.S. Cassava (Manihot esculenta Crantz)—A potential source of phytochemicals, food, and nutrition—An updated review. eFood 2024, 5, e127. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Osungbaro, T. Physical and nutritive properties of fermented cereal foods. Afr. J. Food Sci. 2008, 3, 23–27. [Google Scholar]
- Marfo, E.K.; Simpson, B.K.; Idowu, J.S.; Oke, O.L. Effect of local food processing on phytate levels in cassava, cocoyam, yam, maize, sorghum, rice, cowpea, and soybean. J. Agric. Food Chem. 1990, 38, 1580–1585. [Google Scholar] [CrossRef]
- Adinsi, L.; Mestres, C.; Akissoé, N.; Vieira-Dalodé, G.; Anihouvi, V.; Noel, D.; Hounhouigan, D. Comprehensive quality and potential hazards of gowe, a malted and fermented cereal beverage from West Africa. A diagnostic for a future re-engineering. Food Control 2017, 82, 18–25. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Krebs, N.F. Dietary reference intakes for zinc may require adjustment for phytate intake based upon model predictions. J. Nutr. 2008, 138, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.; Ameha, K.; Biruhtesfa, A. Cassava based foods: Microbial fermentation by single starter culture towards cyanide reduction, protein enhancement and palatability. Int. Food Res. J. 2014, 21, 1751–1756. [Google Scholar]
- Manivanh, N.; Preston, T.R.; Le Van, A.; Tran, H. Improving nutritive value of cassava root (Manihot esculenta crantz) by fermentation with yeast (saccharomyces cerevisiae), urea and di-ammonium phosphate. Livest. Res. Rural Dev. 2018, 30. Available online: http://www.lrrd.org/lrrd30/5/noup30094.html (accessed on 23 June 2025).
- Bala, J.D.; Ijah, U.; Abioye, P.; Emele, L.C. Protein enrichment of Cassava with yeasts for Garri production. Biotechnol. Indian J. 2012, 6, 120–126. [Google Scholar]
- Chelule, P.K.; Mbongwa, H.P.; Carries, S.; Gqaleni, N. Lactic acid fermentation improves the quality of amahewu, a traditional South African maize-based porridge. Food Chem. 2010, 122, 656–661. [Google Scholar] [CrossRef]
- Halake, N.; Chinthapalli, B. Fermentation of Traditional African Cassava Based Foods: Microorganisms Role in Nutritional and Safety Value. J. Exp. Agric. Int. 2020, 42, 56–65. [Google Scholar] [CrossRef]
- Hasan, M.; Sultan, Z.; Mar-E-Um, M. Significance of Fermented Food in Nutrition and Food Science. J. Sci. Res. 2014, 6, 16530. [Google Scholar] [CrossRef]
- Halake, N.; Chinthapalli, B.; Chitra, D. Role of Selected Fermentative Microorganisms on Cyanide Reduction, Protein Enhancement and Palatability of Cassava Based Food. Int. J. Res. Agric. For. 2019, 6, 1–12. [Google Scholar]
- Shumye Gebre, T.; Admassu Emire, S.; Okomo Aloo, S.; Chelliah, R.; Vijayalakshmi, S.; Hwan Oh, D. Unveiling the potential of African fermented cereal-based beverages: Probiotics, functional drinks, health benefits and bioactive components. Food Res. Int. 2024, 191, 114656. [Google Scholar] [CrossRef]
- Ignat, M.V.; Salanță, L.C.; Pop, O.L.; Pop, C.R.; Tofană, M.; Mudura, E.; Coldea, T.E.; Borșa, A.; Pasqualone, A. Current functionality and potential improvements of non-alcoholic fermented cereal beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef]
- Phiri, S.; Schoustra, S.E.; van den Heuvel, J.; Smid, E.J.; Shindano, J.; Linnemann, A. Fermented cereal-based Munkoyo beverage: Processing practices, microbial diversity and aroma compounds. PLoS ONE 2019, 14, e0223501. [Google Scholar] [CrossRef]
- Gemechu, T. Review on lactic acid bacteria function in milk fermentation and preservation. Afr. J. Food Sci. 2015, 9, 170–175. [Google Scholar]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules 2023, 28, 458. [Google Scholar] [CrossRef]
- Keawyok, K.; Waree, W.; Jodnak, S. Prebiotic properties of isomaltooligosaccharides from cassava as a potential ingredient in high-protein drinks for athletes. Bioact. Compd. Health Dis. Online 2023, 6, 38–55. [Google Scholar] [CrossRef]
- Souza, C.M.; Bastos, T.S.; Kaelle, G.C.; Bortolo, M.; de Oliveira, S.G.; Félix, A.P. Fine cassava fibre utilization as a dietary fibre source for dogs: Effects on kibble characteristics, diet digestibility and palatability, faecal metabolites and microbiota. J. Anim. Physiol. Anim. Nutr. 2023, 107, 18–29. [Google Scholar] [CrossRef]
- de Souza, C.B.; Roeselers, G.; Troost, F.; Jonkers, D.; Koenen, M.; Venema, K. Prebiotic effects of cassava bagasse in TNO’s in vitro model of the colon in lean versus obese microbiota. J. Funct. Foods 2014, 11, 210–220. [Google Scholar] [CrossRef]
- Hayati, S.R.; Pattarapanawan, M.; Phuengjayaem, S.; Akrimajirachoote, N.; Laohakunjit, N.; Kovitvadhi, A.; Kotatha, D. Preparation, characterization, and prebiotic potential of resistant maltodextrin from the remaining starch in cassava pulp. Int. J. Biol. Macromol. 2025, 297, 139894. [Google Scholar] [CrossRef]
- Rogoski, W.; Pereira, G.N.; Cesca, K.; da Silva, M.A.; Zanella, E.; Stambuk, B.U.; Ávila, P.F.; Goldbeck, R.; de Oliveira, D.; de Andrade, C.J. Production of cassava peel-based xylooligosaccharides using endo-1, 4-β-xylanase from Trichoderma longibrachiatum: The effect of alkaline pretreatment. Biomass Convers. Biorefinery 2024, 14, 11351–11363. [Google Scholar] [CrossRef]
- Poletto, P.; Pereira, G.N.; Monteiro, C.R.; Pereira, M.A.F.; Bordignon, S.E.; de Oliveira, D. Xylooligosaccharides: Transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem. 2020, 91, 352–363. [Google Scholar] [CrossRef]
- Ratnadewi, A.A.I.; Rahma, M.T.; Nurhayati, N.; Santoso, A.B.; Senjarini, K.; Labes, A.; Reza, M. Production of xylooligosaccharide from cassava pulp’s waste by endo-β-1, 4-D-xylanase and characterization of its prebiotic effect by fermentation of Lactobacillus acidophilus. Fermentation 2022, 8, 488. [Google Scholar] [CrossRef]
- Osundahunsi, O.F.; Williams, A.O.; Oluwalana, I.B. Prebiotic effects of cassava fibre as an ingredient in cracker-like products. Food Funct. 2012, 3, 159–163. [Google Scholar] [CrossRef]
- Mafaldo, Í.M.; Araújo, L.M.; Cabral, L.; Barão, C.E.; Noronha, M.F.; Fink, J.R.; de Albuquerque, T.M.R.; dos Santos Lima, M.; Vidal, H.; Pimentel, T.C. Cassava (Manihot esculenta) Brazilian cultivars have different chemical compositions, present prebiotic potential, and beneficial effects on the colonic microbiota of celiac individuals. Food Res. Int. 2024, 195, 114909. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. WJG 2007, 13, 2826. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, B.; Wang, F.; Huang, D.; Zhong, F.; Li, Y. Characterization and in vitro digestion properties of cassava starch and epigallocatechin-3-gallate (EGCG) blend. LWT 2021, 137, 110398. [Google Scholar] [CrossRef]
- Oguntoye, M.A.; Ezekiel, O.O.; Oridupa, O.A. Viability of Lactobacillus rhamnosus GG in provitamin A cassava hydrolysate during fermentation, storage, in vitro and in vivo gastrointestinal conditions. Food Biosci. 2021, 40, 100845. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Zhu, Y.; Lan, Y. Preparation and drug-loading properties of amphoteric cassava starch nanoparticles. Nanomaterials 2022, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-R.; Xiao, Y.; Ali, M.; Xu, F.-Y.; Li, J.; Wang, R.; Zeng, X.-A.; Teng, Y.-X. Improving resistant starch content of cassava starch by pulsed electric field-assisted esterification. Int. J. Biol. Macromol. 2024, 276, 133272. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, J.D.; Rodriguez-Sandoval, E.; Hernandez, M.S.; Melo-Brito, N.B.; Mejía-Villota, A. Physicochemical and microbiological quality of a fermented soybean beverage: Effect of modified cassava starches. Food Sci. Technol. 2024, 44. [Google Scholar] [CrossRef]
- Ekeledo, E.; Latif, S.; Abass, A.; Müller, J. Antioxidant potential of extracts from peels and stems of yellow-fleshed and white cassava varieties. Int. J. Food Sci. Technol. 2021, 56, 1333–1342. [Google Scholar] [CrossRef]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef]
- Laya, A.; Koubala, B.B. Polyphenols in cassava leaves (Manihot esculenta Crantz) and their stability in antioxidant potential after in vitro gastrointestinal digestion. Heliyon 2020, 6, e03567. [Google Scholar] [CrossRef]
- Lehmane, H.; Kohonou, A.N.; Tchogou, A.P.; Ba, R.; Dah-Nouvlessounon, D.; Didagbé, O.; Sina, H.; Senou, M.; Adjanohoun, A.; Baba-Moussa, L. Antioxidant, anti-inflammatory, and anti-cancer properties of amygdalin extracted from three cassava varieties cultivated in Benin. Molecules 2023, 28, 4548. [Google Scholar] [CrossRef]
- Ziemah, J.; Ullrich, M.S.; Kuhnert, N. Antibacterial activity potential of industrial food production waste extracts against pathogenic bacteria: Comparative analysis and characterization. Foods 2024, 13, 1902. [Google Scholar] [CrossRef]
- Elshamy, S.; Kuhnert, N.; El-Shazly, M.; Ziemah, J.; Handoussa, H. Comparative metabolomic study of twelve Acacia species by UHPLC-q-tof-ESI-MS coupled with chemometrics in correlation with antibacterial activity. Fitoterapia 2025, 181, 106378. [Google Scholar] [CrossRef]
- Linn, K.Z.; Myint, P.P. Estimation of nutritive value, total phenolic content and in vitro antioxidant activity of Manihot esculenta Crantz.(Cassava) leaf. J. Med. Plants 2018, 6, 73–78. [Google Scholar]
- Lu, T.; Song, B.; Yang, J.; Tan, H.; Qiao, H.; Zhi, W.; Chen, R.; Sheng, Z. Lactobacillus HNC7-YLC92 Improves the Fermentation Quality of Cassava–Acerola Cherry Beverage. Fermentation 2024, 10, 90. [Google Scholar] [CrossRef]
- Ojo, I.; Apiamu, A.; Egbune, E.O.; Tonukari, N.J. Biochemical characterization of solid-state fermented cassava stem (Manihot esculenta Crantz-MEC) and its application in poultry feed formulation. Appl. Biochem. Biotechnol. 2022, 194, 2620–2631. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zi, X.; Lv, R.; Zhang, L.; Ou, W.; Chen, S.; Hou, G.; Zhou, H. Cassava foliage effects on antioxidant capacity, growth, immunity, and ruminal microbial metabolism in Hainan black goats. Microorganisms 2023, 11, 2320. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, T.; Zi, X.; Lv, R.; Gu, L. Effects of Feeding Fermented Cassava Leaves on Intestinal Morphology, Cecal Microbiota, and Metabolome in Hybrid Geese. Microorganisms 2025, 13, 660. [Google Scholar] [CrossRef]
- Okrathok, S.; Sirisopapong, M.; Mermillod, P.; Khempaka, S. Modified dietary fiber from cassava pulp affects the cecal microbial population, short-chain fatty acid, and ammonia production in broiler chickens. Poult. Sci. 2023, 102, 102265. [Google Scholar] [CrossRef]
- Khota, W.; Kaewpila, C.; Suwannasing, R.; Srikacha, N.; Maensathit, J.; Ampaporn, K.; Patarapreecha, P.; Thip-Uten, S.; Sawnongbue, P.; Subepang, S. Ensiling cyanide residue and In vitro rumen fermentation of cassava root silage treated with cyanide-utilizing bacteria and cellulase. Fermentation 2023, 9, 151. [Google Scholar] [CrossRef]
- Posridee, K.; Oonsivilai, A.; Oonsivilai, R. Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer. Foods 2024, 13, 766. [Google Scholar] [CrossRef]
- Adinsi, L.; Akissoé, N.; Escobar, A.; Prin, L.; Kougblenou, N.; Dufour, D.; Hounhouigan, D.J.; Fliedel, G. Sensory and physicochemical profiling of traditional and enriched gari in Benin. Food Sci. Nutr. 2019, 7, 3338–3348. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Chu-ky, S.; Luong, H.N.; Nguyen, H.V. Effect of pretreatment methods on enzymatic kinetics of ungelatinized cassava flour hydrolysis. Catalysts 2020, 10, 760. [Google Scholar] [CrossRef]
- Kumari, K.; Nagar, S.; Goyal, S.; Maan, S.; Chugh, V.; Kumar, V.; Kharor, N. Xylooligosaccharide Production From Lignocellulosic Biomass and Their Health Benefits as Prebiotics. Biochem. Res. Int. 2024, 2024, 6179375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).