Effect of Apple Pomace Addition During Fermentation on the Phenolic Content, Chemical Composition, and Sensory Properties of Cider
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apple Juice Extraction
2.3. Apple Pomace Addition
2.4. Basic Chemical Analysis
2.4.1. Titratable Acidity (TA) and pH
2.4.2. L-Malic and L-Lactic Acid Analysis
2.4.3. Phenolic Content
2.4.4. Antioxidant Activity of Cider
2.4.5. Alcohol Content and Brix Measurements
2.5. Volatile Compound Analysis
2.5.1. Volatile Solution Preparation
2.5.2. Gas Chromatography Flame Ionization Detection (GC-FID)
2.6. Sensory Analysis
2.6.1. Panel Training
2.6.2. Formal Evaluations
2.7. Statistical Analysis
3. Results and Discussion
3.1. Properties of Apple Juice and Cider Samples
3.2. Phenolic Analysis
3.3. Effect of Pomace Addition on Volatile Profile
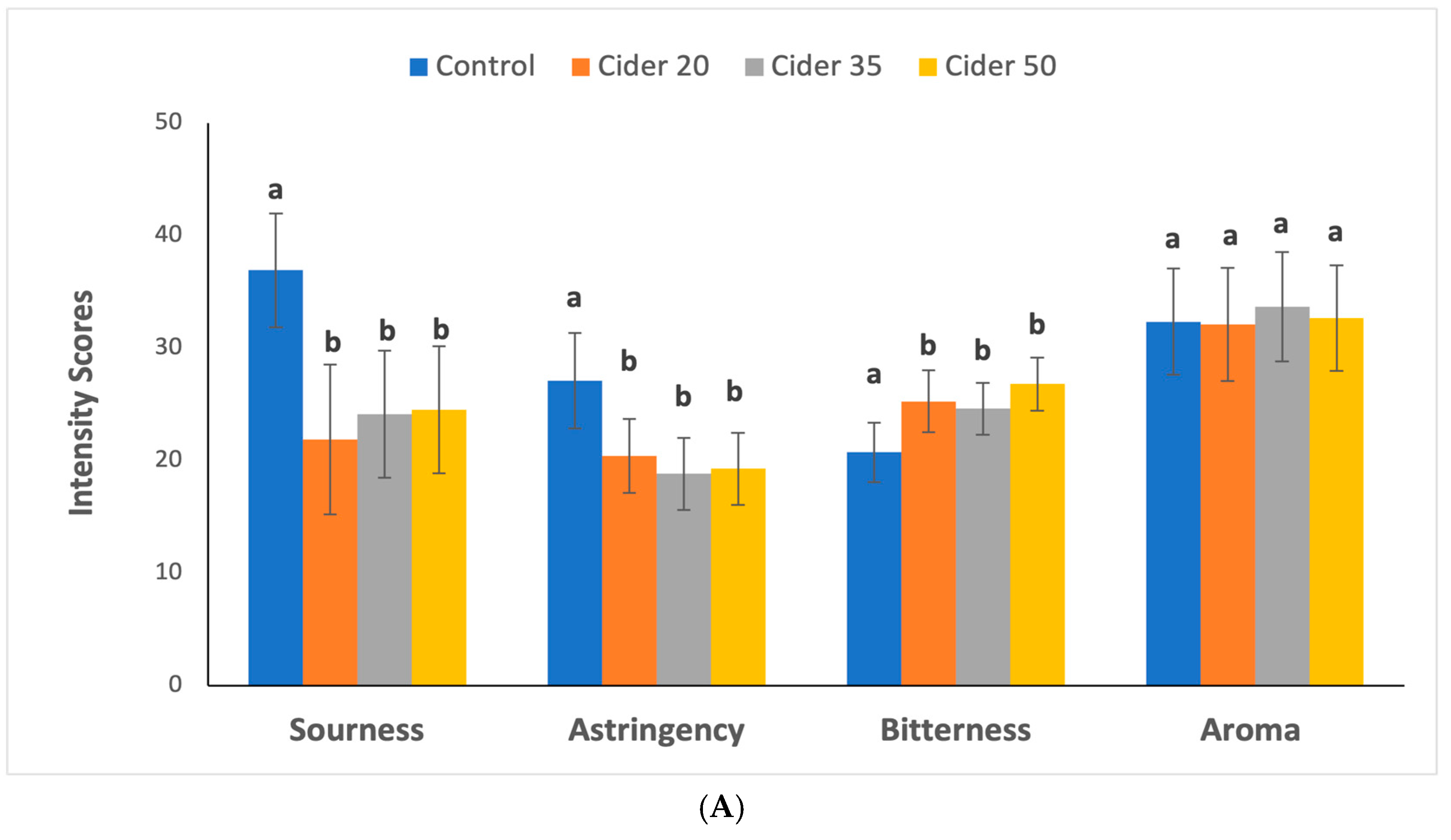
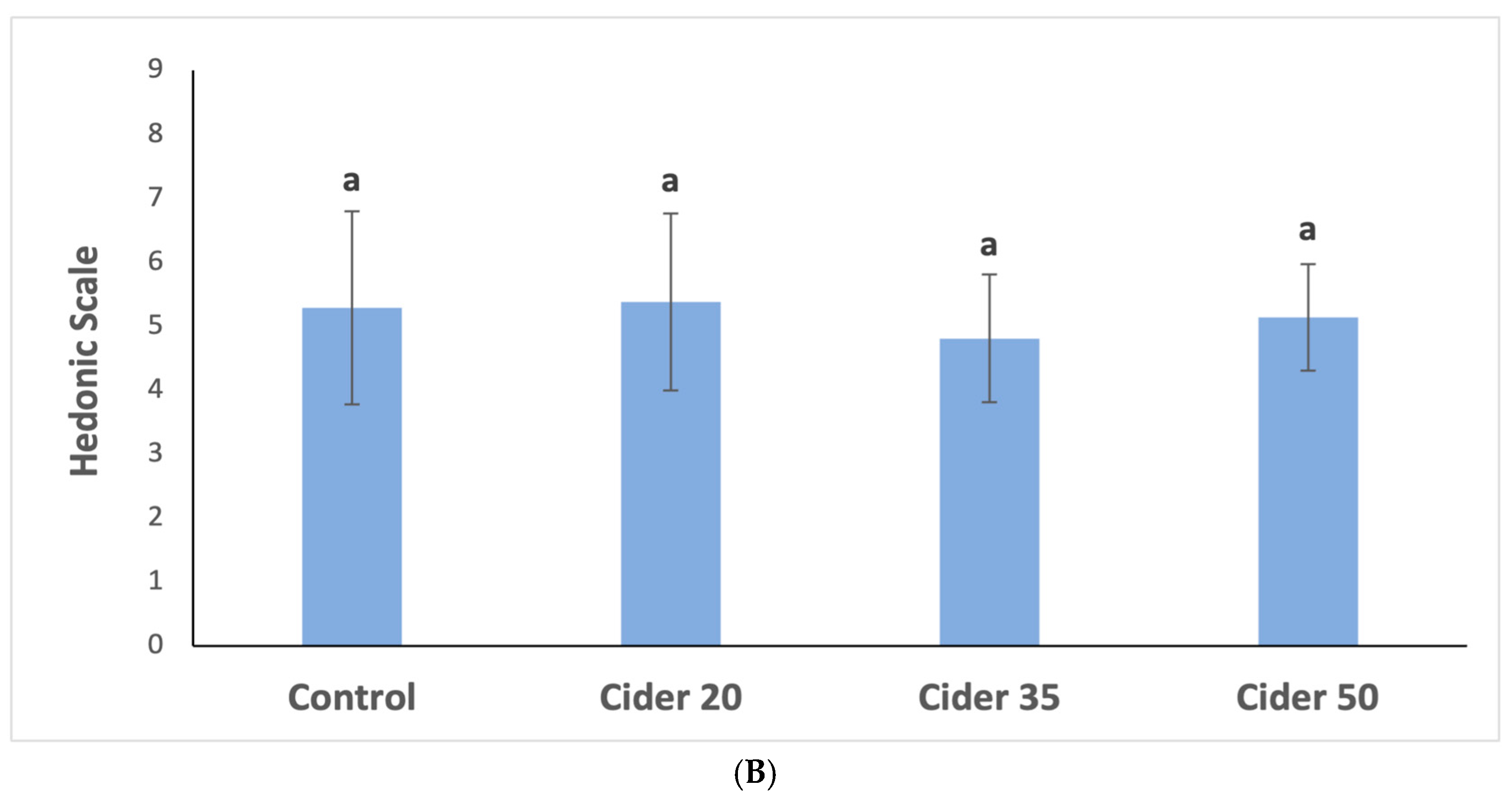
3.4. Sensory Analysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A new approach to the use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT-Food Sci. Technol. 2020, 126, 109316. [Google Scholar] [CrossRef]
- Beechum, D. A Cider Primer. In The Everything Hard Cider Book, 1st ed.; Laing, L., Guarco, A., Wissman, P., Palana-Shanahan, B., Eds.; Adams Media: Avon, MA, USA, 2013; Volume 1, pp. 11–19. [Google Scholar]
- Riekstina-Dolge, R.; Kruma, Z.; Dimins, F.; Straumite, E.; Karklina, D. Phenolic composition and sensory properties of ciders produced from Latvian apples. Rural. Sustain. Res. 2014, 31, 39–45. [Google Scholar] [CrossRef]
- Soomro, T.; Watts, S.; Migicovsky, Z.; Myles, S. Cider and dessert apples: What is the difference? Plants People Planet 2022, 4, 593–598. [Google Scholar] [CrossRef]
- Thompson-Witrick, K.A.; Goodrich, K.M.; Neilson, A.P.; Hurley, E.K.; Peck, G.M.; Stewart, A.C. Characterization of the polyphenol composition of 20 cultivars of cider, processing, and dessert apples (Malus × domestica Borkh.) grown in Virginia. J. Agric. Food Chem. 2014, 62, 10181–10191. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.; Guyot, S.; Marnet, N.; Lequéré, J.M.; Drilleau, J.F.; Wosiacki, G. Effect of Alcoholic Fermentation in the Content of Phenolic Compounds in Cider Processing. Braz. Arch. Biol. Technol. 2008, 51, 1025–1031. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods. 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.; Fischer, T.; Zardo, D.; Nogueira, A.; Zielinski, A.; Alberti, A. Bioactive compounds recovered from apple pomace as ingredient in cider processing: Monitoring of compounds during fermentation. J. Food Sci. Technol. 2022, 59, 3349–3358. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.; Nogueira, A.; Zielinski, A.; Alberti, A. Effect of addition of phenolic compounds recovered from apple pomace on cider quality. LWT-Food Sci. Technol. 2019, 100, 348–354. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- CDR Cider Lab Determination of L-Malic Acid in Cider. Available online: https://www.cdrfoodlab.com/cdrciderlab/analyses/malic-acid-cider (accessed on 9 June 2025).
- CDR Cider Lab Determination of L-Lactic Acid in Cider. Available online: https://www.cdrfoodlab.com/cdrciderlab/analyses/lactic-acid-cider (accessed on 9 June 2025).
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH Assay for evaluating the antioxidant capacity of food additives—Inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y.; Han, Y. Quantification of volatile compounds in Chinese Ciders by Stir Bar Sorptive Extraction (SBSE) and Gas Chromatography-Mass Spectrometry (GC-MS). J. Inst. Brew. 2011, 117, 61–66. [Google Scholar] [CrossRef]
- American Society of Brewing Chemists. Beer–48. Headspace Gas Chromatography-Flame Ionization Detection Analysis of Beer Volatiles. In Methods of Analysis, 14th ed.; The Society: St. Paul, MN, USA, 2011. [Google Scholar]
- Mu, Y.; Zeng, C.; Qiu, R.; Yang, J.; Zhang, H.; Song, J.; Yan, J.; Sun, J.; Kang, S. Optimization of the Fermentation Conditions of Huaniu Apple Cider and Quantification of Volatile Compounds Using HS-SPME-GC/MS. Metabolites 2023, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; List, D.; Lu, Y.; Foo, L.Y.; Newman, R.H.; Sims, I.M.; Bain, P.J.S.; Hamilton, B.; Fenton, G. Apple Pomace and Products Derived from Apple Pomace: Uses, Composition and Analysis. In Analysis of Plant Waste Materials, 1st ed.; Linskens, H.F., Jackson, J.F., Eds.; Springer: Heidelberg, Germany, 1999; pp. 75–119. [Google Scholar]
- Jolicoeur, C. The Acids. In The New Cider Maker’s Handbook, 1st ed.; Watson, B., Walden, H., Eds.; Chelsea Green Publishing: White River Junction, VT, USA, 2013; pp. 177–186. [Google Scholar]
- Valles, B.S.; Bedriñana, R.P.; Tascón, N.F.; Simón, A.Q.; Madrera, R.R. Yeast species associated with the spontaneous fermentation of cider. Food Microbiol. 2007, 24, 25–31. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. App. Sci. 2021, 11, 7864. [Google Scholar] [CrossRef]
- Reuss, R.M.; Stratton, J.E.; Smith, D.A.; Read, P.E.; Cuppett, S.L.; Parkhurst, A.M. Malolactic Fermentation as a Technique of the Deacidification of Hard Apple Cider. Food Chem. 2010, 75, C74–C78. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, F.; Dziugan, P.; Yao, Y.; Zhang, J.; LV, Z.; Zhang, B. Development of organic acids and volatile compounds in cider during malolactic fermentation. Czech J. Food Sci. 2014, 32, 69–76. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food. Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Way, M.; Jones, J.; Swarts, N.; Dambergs, R. Phenolic Content of Apple Juice for Cider Making as Influenced by Common Pre-Fermentation Processes using Two Analytical Methods. Beverages 2019, 5, 53. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffrey, D.W.; Grbin, P.R.; Muhlack, R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which Polyphenolic Compounds Contribute to the Total Antioxidant Activities of Apple? J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major Phenolics in Apple and Their Contribution to the Total Antioxidant Capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Wolniak, M.; Wojdylo, A.; Wawer, I. Comparative study of polyphenolic content and antiradical activity of cloudy and clear apple juices. J. Sci. Food Agric. 2007, 87, 573–579. [Google Scholar] [CrossRef]
- Pincinelli, L.A.; García, Y.D.; Sánchez, J.M.; Madrera, R.R.; Valles, B.S. Phenolic and antioxidant composition of cider. J. Food Compos. Anal. 2009, 22, 644–648. [Google Scholar] [CrossRef]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Exploring the chemical space of white wine antioxidant capacity: A combined DPPH, EPR and FT-ICR-MS study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimization of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Kliks, J.; Kawa-Rygielska, J.; Gasiński, A.; Rębas, J.; Szumny, A. Changes in the volatile composition of apple and apple/pear ciders affected by the different dilution rates in the continuous fermentation system. LWT 2021, 147, 111630. [Google Scholar] [CrossRef]
- Affonso, A.D. The Effects of Co-Fermentation of Cider and Apple Pomace on Cider Attributes. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2022. [Google Scholar]
- Herrero, M.; García, L.A.; Díaz, M. The effect of SO2 on the production of ethanol, acetaldehyde, organic acids, and flavor volatiles during industrial cider fermentation. J. Agric. Food Chem. 2003, 51, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.P.; DeOrduña, R.M.; Pilone, G.J.; Liu, S. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef] [PubMed]
- De la Roza, C.; Laca, A.; García, L.A.; Díaz, M. Ethanol and ethyl acetate production during the cider fermentation from laboratory to industrial scale. Proc. Biochem. 2003, 38, 1451–1456. [Google Scholar] [CrossRef]
- Cavazza, A.; Poznanski, E.; Guzzon, R. Must treatments and wild yeast growth before and during alcoholic fermentation. Ann. Micribiol. 2011, 61, 41–48. [Google Scholar] [CrossRef]
- Kauser, S.; Murtaza, M.A.; Hussain, A.; Imran, M.; Kabir, K.; Najam, A.; An, Q.U.; Akram, S.; Fatima, H.; Batool, S.A.; et al. Apple pomace, a bioresource of functional and nutritional components with potential of utilization in different food formulations: A review. Food Chem. Adv. 2024, 4, 100598. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepeen, K.J. Physiology, ecology, and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J. Synthesis of higher alcohols during cider processing. Food Chem. 1999, 67, 287–294. [Google Scholar] [CrossRef]
- Castro, L.F.; Ross, C.F. Correlation Between Sensory Descriptive Analysis and Volatile Composition of Beer Using Multivariate Analysis: The Effect of the NonVolatile Matrix on the Sensory Perception and Volatile Fraction Behavior. J. Am. Soc. Brew. Chem. 2018, 76, 86–95. [Google Scholar] [CrossRef]
- CoSeteng, M.Y.; McLellan, M.R.; Downing, D.L. Influence of Titratable Acidity and pH of Intensity of Sourness of Citric, Malic, Tartaric, Lactic and Acetic Acids Solutions and on the Overall Acceptability of Imitation Apple Juice. Can. Inst. Food Sci. Technol. J. 1989, 22, 46–51. [Google Scholar] [CrossRef]
- Batali, M.E.; Cotter, A.R.; Frost, S.C.; Ristenpart, W.D.; Guinard, J.X. Titratable Acidity, Perceived Sourness, and Liking of Acidity in Drip Brewed Coffee. ACS Food Sci. Technol. 2021, 1, 559–569. [Google Scholar] [CrossRef]
- Bergentall, M.K.; Niimi, J.; Persson, I.; Calmet, E.; As, D.; Plovie, A.; Malafronte, L.; Melin, P. Malolactic fermentation in lingonberry juice and its use as a preservative. Food Microbiol. 2024, 121, 104500. [Google Scholar] [CrossRef] [PubMed]
- Symoneaux, R.; Chollet, S.; Bauduin, R.; Quéré, J.M.L.; Baron, A. Impact of apple procyanidins on sensory perception in model cider (part 2): Degree of polymerization and interactions with the matrix components. LWT-Food Sci. Technol. 2014, 57, 28–34. [Google Scholar] [CrossRef]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Ilak Peršurić, A.S.; Jeromel, A.; Radeka, S. Comparison of different maceration and non-maceration treatments for enhancement of phenolic composition, colour intensity, and taste attributes of Malvazija istarska (Vitis vinifera L.) white wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Karl, A.D.; Zakalik, D.L.; Cook, B.S.; Kumar, S.K.; Peck, G.M. The biochemical and physiological basis for hard cider apple fruit quality. Plants People Planet 2022, 5, 178–189. [Google Scholar] [CrossRef]
- Sommer, S.; Anderson, A.F.; Cohen, S.D. Analytical Methods to Assess Polyphenols, Tannin Concentration, and Astringency in Hard Apple Cider. Appl. Sci. 2022, 12, 9409. [Google Scholar] [CrossRef]
| Sample | pH | TA (g/L) | Malic Acid (mg/L) | Lactic Acid (mg/L) | Degrees Brix (°Bx) | ABV (%v/v) |
|---|---|---|---|---|---|---|
| Apple Juice | 3.65 ± 0.01 a | 3.72 ± 0.01 a | 3.01 ± 0.12 a | 0.07 ± 0.77 a | 14.68 ± 0.77 a | - |
| Control | 3.59 ± 0.02 a | 4.98 ± 0.77 b | 3.39 ± 0.17 a | 0.11 ± 0.10 a | 2.25 ± 0.09 b | 7.78 ± 0.10 a |
| Cider 20 | 3.92 ± 0.46 b | 2.90 ± 0.07 c | 0.27 ± 0.24 b | 2.34 ± 0.08 b | 1.81 ± 0.02 c | 7.91 ± 0.03 b |
| Cider 35 | 3.82 ± 0.113 b | 3.55 ± 0.64 c | 1.28 ± 1.10 b | 1.93 ± 0.62 b | 1.95 ± 0.10 c, d | 7.92 ± 0.01 b |
| Cider 50 | 3.83 ± 0.04 b | 3.27 ± 0.20 c | 0.55 ± 0.26 b | 2.42 ± 0.23 b | 2.03± 0.02 d | 7.90 ± 0.02 b |
| Sample | TPC (mg Gallic Acid/L) | DPPH (TEAC) |
|---|---|---|
| Apple Juice | 720 ± 29 a | - |
| Control | 400 ± 49 b | 6.02 ± 0.48 a |
| Cider 20 | 524 ± 74 b,c | 5.90 ± 0.18 a |
| Cider 35 | 647 ± 85 c | 6.29 ± 0.43 a |
| Cider 50 | 597 ± 25 c | 6.25 ± 0.05 a |
| Sample | Acetaldehyde | Ethyl Acetate | Isoamyl Alcohol | Phenylethyl Alcohol |
|---|---|---|---|---|
| Control | 18.94 ± 1.04 a | 6.95 ± 0.84 a | 142.52 ± 1.78 a | 88.34 ± 0.74 a |
| Cider 20 | 11.76 ± 1.34 b | 14.80 ± 0.85 b | 153.61 ± 0.50 b | 85.75 ± 1.96 a |
| Cider 35 | 9.58 ± 1.15 b | 14.02 ± 0.40 b | 153.20 ± 2.34 b | 97.37 ± 1.77 b |
| Cider 50 | 10.13 ± 0.75 b | 19.72 ± 0.90 c | 166.36 ± 5.34 b | 120.20 ± 2.04 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, L.F.; Affonso, A.D.; Perry, K.P. Effect of Apple Pomace Addition During Fermentation on the Phenolic Content, Chemical Composition, and Sensory Properties of Cider. Beverages 2025, 11, 95. https://doi.org/10.3390/beverages11040095
Castro LF, Affonso AD, Perry KP. Effect of Apple Pomace Addition During Fermentation on the Phenolic Content, Chemical Composition, and Sensory Properties of Cider. Beverages. 2025; 11(4):95. https://doi.org/10.3390/beverages11040095
Chicago/Turabian StyleCastro, Luis F., Abigail D. Affonso, and Kate P. Perry. 2025. "Effect of Apple Pomace Addition During Fermentation on the Phenolic Content, Chemical Composition, and Sensory Properties of Cider" Beverages 11, no. 4: 95. https://doi.org/10.3390/beverages11040095
APA StyleCastro, L. F., Affonso, A. D., & Perry, K. P. (2025). Effect of Apple Pomace Addition During Fermentation on the Phenolic Content, Chemical Composition, and Sensory Properties of Cider. Beverages, 11(4), 95. https://doi.org/10.3390/beverages11040095







