Abstract
In this study, we analysed cocoa (a dried and fully fermented seed of Theobroma cacao L.) from two Amazonian cultivars and a commercial sample of the Amazonian variety EETP801, grown under sustainable organic conditions, in comparison to CCN51 cocoa grown on a neighbouring commercial farm using standard practises and a European commercial cacao powdered beverage. The overall metabolite profile of the 70% aq acetone sample cocoa extracts was analysed using high-performance TLC analyses (HPTLC), and the xanthine alkaloids were analysed using quantitative liquid chromatography–UV photodiode array (HPLC-DAD) analyses. The volatile fraction in the headspace of the freshly ground cocoa was subjected to solid phase micro-extraction and analysed by gas chromatography–mass spectrometry (HS-SPME/GC-MS). Total polyphenol content was determined by the Folin–Ciocalteu method. Despite the reduced production of cocoa by the EETP801 cultivar in comparison with the CCN51 cultivar, the obtained produce is significantly richer in theobromine (130 mg vs. 170 mg per g of cacao), with CCN51 having a double concentration of theophylline (12.6 vs. 6.5 mg per g of cacao). Qualitatively, the two Amazonian cocoa samples had a similar polyphenolic composition (per the HPTLC fingerprint). HS-SPME/GC-MS analyses revealed that all the samples show a spontaneous emission profile mainly rich in non-terpene derivatives, of which hydrocarbons and pyrazines are the most abundant groups. The most represented volatile organic compound is n-tridecane for both EETP801 and CCN51. The variability in the artisan fermentation and roasting processes influenced certain aspects of the volatile composition as reflected by the trimethyl pyrazine/tetramethyl pyrazine ratio, which was zero in EETP-801 and lower than 1 in CCN51. Acetic acid was absent in CCN51 but significant (c.a. 5.5.%) in EETP801 and the commercial samples. The cultivar EETP801 is a viable option for a more ecologically conscious sector of the cocoa beverages consumer group.
1. Introduction
The earliest evidence of cocoa drink consumption dates back to the Early Formative Period (1900–900 BC) in Mesoamerica. The Mokayan people on the Pacific coast of Chiapas, Mexico, were consuming cacao drinks by 1900 BC. However, the earliest written references to cocoa drinks come from the Mayan civilisation, who called it “xocolātl” (meaning “bitter water”) [1]. Today, it is among the most traded commodities in the drinks industry, along with tea and coffee [2,3].
The development of cocoa beverages still faces challenges due to the peculiarities of its composition, dominated by lipids and polyphenols. The main issues during production are sedimentation and layer formation, but marbling and curdling might also occur [4]. The content of stimulant alkaloids and cardiovascular protecting/antioxidant/antidiabetic polyphenols [5,6], as well as the sustainability of the production of cocoa beverages [7], have added pressure to these challenges as the consumers’ base grow more conscious of the health value and environmental footprint of their choices [8].
There are many steps and factors in the cocoa beverage production process—mainly fermentation and roasting—that can affect the content of “bioactive” polyphenols. To compensate for this loss, there is an increased interest in replacing them by adding polyphenols from other herbal sources. It is an important requirement to expand academic studies to enrich products with bioactive components during mixing and production without affecting the quality parameters such as the aroma and colour of cocoa-based beverages [4,9].
The chocolate industry has changed to a much higher demand for certified cocoa (Rainforest Alliance, UTZ, FairTrade) [10]. Companies like Mars have stated that by 2020, all their cocoa will be certified sustainable [11]. By this time, demand for cocoa is predicted to surpass the available supply by more than one million tons [7]. There is, therefore, a need for new sustainable and fair-trade sources of cocoa.
Ecuador is a region where cocoa is farmed by different communities such as Afro-Ecuadorian, Mestizo and Indigenous. All of them face the dilemma of the sustainability performance between fine flavour cultivars (collectively known as “Complejo Nacional” cultivars) vs. the most productive but less flavoured cultivars such as CCN-51 [12]. In 2011, The Iamoe Center started an experiment growing a native Amazonian cultivar of cocoa, commonly named “Nacional 801” or “Fino Pichilingue” and classified by the International Cocoa Germplasm Database as “EET-801” or “EETP-801” [13] relatively dispersed within a defined plot under the rainforest canopy. Aspects of its morphology are shown in Figure 1.

Figure 1.
Above: EETP801 pods from Iamoe Centre (Dayuma, Ecuador) at two maturity stages. Below: characteristic purple CCN51 pods from a neighbouring plantation (Dayuma, Ecuador) (Credits: Dr. Viteri).
It was therefore our aim to examine the composition of this Amazonian cocoa in comparison with the more productive but less sustainable cultivar CCN51 in terms of volatiles and polyphenols.
2. Materials and Methods
2.1. Plant Materials
Cocoa beans, defined as the dried and fully fermented seed of Theobroma cacao L. from two Amazonian cultivars and a commercial sample, were sampled.
Seeds of the EETP-801 cultivar (also known as “Fino Pichilingue”) [14] were acquired from the Ecuadorian National Institute for Agricultural Research (INIAP) and grown under organic conditions in a shaded area of primary rainforest (Lat 0, 41′ South, Lon 76, 50′ West, Altitude 260 m). Only organic kitchen waste, coffee grounds and rice hulls were used to support the growth of the plants. Not any kind of fertiliser or pesticide was applied. They yielded the first fruits after 15 months only. The seeds were collected, dried, roasted, peeled and ground within the premises of the Iamoe Centre (Kupi Village, Dayuma Parish, Francisco de Orellana Province, Ecuador) following traditional, fully manual processing techniques.
Cocoa samples from the high-yield cultivar CCN-51 (INIAP, Quito, Ecuador) grown under non-organic conditions in a neighbouring, deforested area of Iamoe Centre were collected by Dr. Rocío Alarcón and Dr. M. Viteri with the owner’s permission.
A commercial pure defatted cocoa powder (Chocolate Valor S.A., Villajoyosa, Spain) was bought in the supermarket and used for comparison with the Amazonian samples. The manufacturer’s labelling declares a 16% fat content and no added sugars. It did not claim to be organic.
2.2. Extraction
Cocoa samples of 0.25 g were extracted in 5 mL acetone and water (70:30 v/v) in a 15 mL test tube shaken for 20 min.
After the extract was separated by centrifugation (1600 rpm for 10 min), it was filtered with a 0.22 µm pore filter and aliquoted into 1.5 mL glass vials. The extract was used in the subsequent analyses.
2.3. Phenol Content Determination by the Folin–Ciocalteu Method
The samples were dissolved in methanol 4.12 mg/5 mL. An amount of 50 µL of each filtered extraction solution was transferred to a 15 mL test tube with 3,5 mL of water. After swirling the contents, 250 µL of Folin–Ciocalteu’s phenol reagent was added, and the solutions were swirled again. After 8 min, 750 µL of sodium carbonate solution was filled up to 5 mL exactly with water. UV absorption was measured 2 h after the carbonate solution was added, at 610 nm.
The percentage of total phenols in extracts was calculated using the following equation:
where A denotes absorption, and C denotes concentration. The absorbances of blank experiments were subtracted from the absorbances of samples. Blanks were prepared the same way as samples by changing the Folin–Ciocalteu reagent with water.
Total phenols % = 100 × (Asample × Astandard)/(Astandard × Csample)
2.4. High-Performance Thin Layer Chromatography (HPTLC)
Extracts were diluted to a concentration of 50 mg/mL in methanol. Control compounds were prepared at a concentration of 1 mg/mL, also diluted in methanol. A CAMAG Linomat 5 was used to apply 5 μL of the samples onto TLC silica gel 60 F254 aluminium sheets. The plates were developed using a CAMAG ADC2 automatic developing chamber. The method included 30 s pre-drying, a 10 min humidity control using magnesium chloride to 48.3% relative humidity and a 20 min saturation time using saturation pads, all performed at 25.2 °C. The mobile phase used was ethyl acetate/formic acid/water (82:9:9). During development, the solvent front was allowed to migrate 80 mm before a drying time of 5 min. For derivatisation, we used the natural products reagent (NPR) followed by PEG 4000. All visualisation and analyses were performed using a CAMAG TLC visualiser both before and after derivatisation.
2.5. High-Performance Liquid Chromatography (HPLC)
The HPLC unit used in this study was an Agilent 1200 series. The HPLC conditions used were Evo Biphenyl (Phenomenex, Macclesfield, UK) 150 × 4.6 mm column, a flow rate of 1 mL/min, an injection volume of 10 µL for samples and 5 µL for standards, column oven temperature of 35 °C and a gradient mobile phase as follows: 0 min—90% acid water (0.001% H3PO4) 10% acetonitrile and 30 min—10% acid water (0.001% H3PO4) 90% acetonitrile.
2.6. Headspace (HS) Solid Phase Micro-Extraction (SPME) of Volatiles
Each sample, once ground, was put in a glass vial closed with aluminium foil and left to equilibrate at room temperature for 30 min. Supelco SPME (Solid Phase Micro-Extraction) (Merck KGaA, Darmstadt, Germany) devices coated with polydimethylsiloxane (PDMS, 100 μm) were used to sample the headspace. SPME sampling was performed using the same new fibre, preconditioned according to the manufacturer’s instructions, for all the analyses. Sampling was accomplished in an air-conditioned room (22 ± 1 °C) to guarantee a stable temperature. After the equilibration time, the fibre was exposed to the headspace for one hour for each sample. Once sampling was finished, the fibre was withdrawn into the needle and transferred to the injection port of the GC-MS system. The desorption conditions were identical for all the samples. Furthermore, blanks were performed before each first SPME extraction and randomly repeated during each series. Quantitative comparisons of relative peak areas were performed between the same chemicals in different samples.
2.7. Gas Chromatography—Mass Sepctrometry (GC-MS) Analysis of Volatiles
GC-MS analyses were performed using a Varian CP-3800 apparatus equipped with a DB-5 capillary column (30 m × 0.25 mm i.d., film thickness of 0.25 μm) coupled with a Varian Saturn 2000 ion-trap mass detector. The oven temperature was programmed to rise from 60 °C to 240 °C at 3 °C/min; injector temperature, 250 °C; transfer-line temperature, 240 °C; carrier gas, He (1 mL/min).
The identification of the constituents was based on the comparison of their retention times (tR) with those of pure reference samples and their linear retention indices (LRIs) determined relative to the tR of a series of n-alkanes. The mass spectra were compared with those listed in the commercial libraries NIST 14 and ADAMS, a homemade mass-spectral library built from pure substances and components of known mixtures, and MS literature data [15,16,17,18,19,20,21].
2.8. Statistical Analyses
Statistical analyses were performed with GraphPad Prism v. 5 using a one-way Analysis of Variance (ANOVA) and a multiple comparison test (Tukey’s HSD). Other basic statistical calculations (averages, SD, SEM, etc.) and figures were performed with the help of Excel (Microsoft, Redmond, WA, USA).
3. Results
3.1. Total Phenol Content
The content of phenols calculated as per the Folin–Ciocalteu’s method showed that the CCN51 cultivar had a significantly higher content (p < 0.01) followed by EETP801, which was also significantly higher (p < 0.01) than the commercial sample. The latter had a lower content, probably due to the industrial processing (Figure 2).
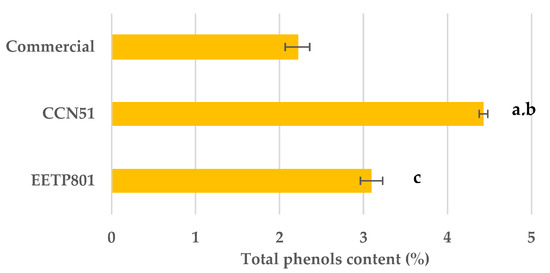
Figure 2.
Total phenols content measured by the Folin–Ciocalteu method. (a) CCN51 vs. commercial (p < 0.01); (b) CCN51 vs. EETP801 (p < 0.01); (c) EETP801 vs. commercial (p < 0.01) according to Tukey HSD.
3.2. HPTLC Anlysis of Cocoa Samples
The overall fingerprint of the pure Amazonian cocoa samples EETP801 and CCN51 was obtained using high-performance thin-layer chromatography (Figure 3). The standards used included kaempferol, catechin gallate, epicatechin, epigallocatechin gallate, gallic acid, chlorogenic acid, rutin, ferulic acid and hesperidin. Only catechin was unambiguously identified in the cocoa samples.
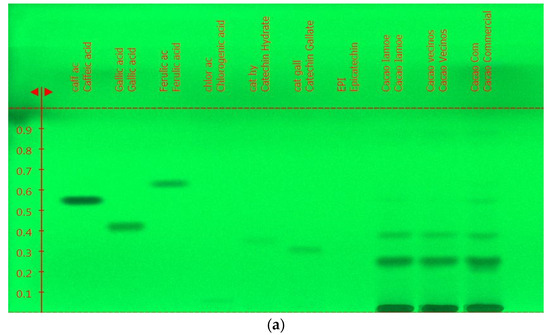
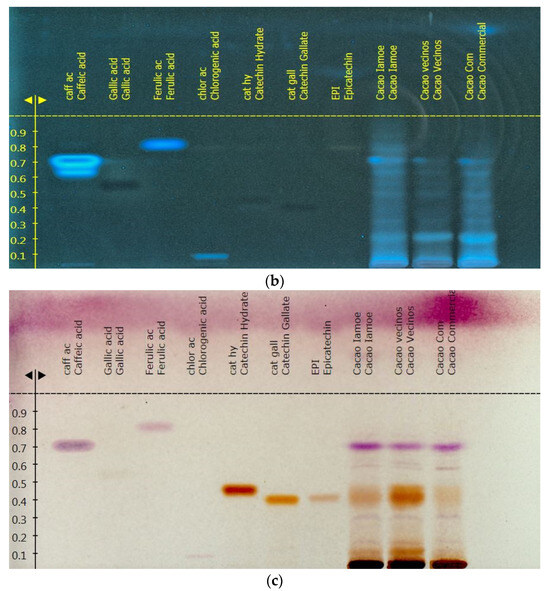
Figure 3.
Representative HPTLC analysis of cocoa samples (mobile phase ethyl acetate/eater/formic acid/acetic acid (100:11:11:27): (a) no derivatisation, UV light (235 nm); (b) no derivatisation, UV light (366 nm); (c) derivatisation with 5% NPR + PEG4000, Vis light (380 to 750 nm). The three last lanes on the right are ETTP801 (“Cacao Iamoe”), CCN51 (“Cacao vecinos”) and commercial sample (“Cacao Commercial”).
3.3. Analysis of the Volatile Metabolites of the Amazonian Samples
In terms of volatiles, all the samples show a spontaneous emission profile (Table 1), mainly rich in non-terpene derivatives, of which hydrocarbons and pyrazines are the most abundant groups. For EETP801 and CCN51, n-tridecane is the most represented volatile organic compound, whilst 2,3,5,6-tetramethyl pyrazine (TMP) is the most abundant volatile in the headspace of the commercial chocolate (Figure 4).

Table 1.
Volatile compounds identified in cocoa samples by HS-SPME/GC-MS.
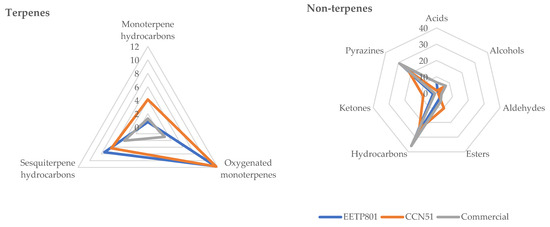
Figure 4.
Comparison of the main chemical families found in the volatile profile of the cacao samples.
3.4. Quantitative Analysis of the Xanthine Alkaloids in Cocoa Samples
Xanthine alkaloids, the stimulant bioactive ingredients of cocoa, were separated and quantified by HPLC-UV, showing the following order of elution: theophylline (Rt = 2.1 min), theobromine (Rt = 3.1 min) and caffeine (Rt = 5.3 min).
All the samples showed theobromine as their most prominent one, followed by theophylline and caffeine, with EETP801 showing the highest content in theobromine (Figure 5).
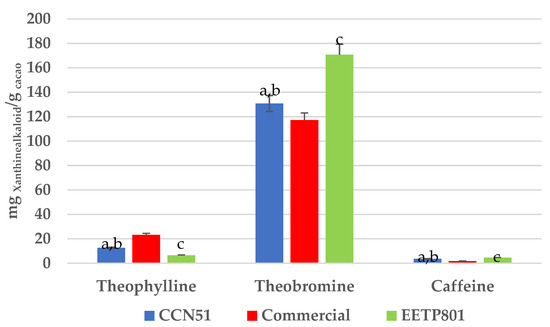
Figure 5.
Concentration of xanthine alkaloids (mg alkaloid per gram of cocoa) in the samples. All values within each series are significantly different from each other (p = 0.01), according to Tukey HSD. (a) CCN51 vs. commercial (p < 0.01); (b) CCN51 vs. EETP801 (p < 0.01); (c) EETP801 vs. commercial (p < 0.01) according to Tukey HSD.
4. Discussion
The total polyphenol content measured by Folin–Ciocalteu using caffeic acid as standard showed that it is slightly higher in CCN51 than in EETP801, at 4.42% and 3.09%, respectively, and significantly higher than in commercial cocoa, at 2.21%.
In terms of volatiles, all the samples show a spontaneous emission profile mainly rich in non-terpene derivatives, of which hydrocarbons and pyrazines are the most abundant groups. For EETP801 and CCN51, n-tridecane is the most represented volatile organic compound, whilst 2,3,5,6-tetramethyl pyrazine (TMP) is the most abundant volatile in the headspace of Valor chocolate.
Pyrazines are important aroma-active compounds in chocolate volatile emissions. Apart from tri- (TrMP) and tetra-methyl pyrazines (TMP), which are produced during the fermentation process by bacterial enzymes [15,16], pyrazines are developed during the Maillard reactions, which take place during the roasting phase of cocoa seeds. Their total abundance reflects the degree of fermentation and the overall aroma potential [17]. Tetramethyl pyrazine was not detected in the Jamoe sample, whilst it represents 9.60 and 10.04% in CCN51 and commercial samples, respectively (see Table 1). TMP confers positive characteristics to chocolate flavour: it is described as a green aroma contributor to cocoa, with coffee- and cocoa-like attributes [15,18,19]. The second most represented pyrazine in the samples is 2,3,5-trimethyl pyrazine, representing 6.45, 4.84 and 5.64% in EETP801 and CCN51 and commercial samples, respectively. It is a positive aroma contributor described as green, rhum-, cocoa- and roasted nuts-like [15,18]. The TMP/TrMP ratio is an important parameter for evaluating the degree of roasting: the higher the ratio, the better the roasting conditions [20]. Therefore, CCN51 and commercial samples have undergone better roasting conditions than the EETP801 sample.
Other typical and positive attributes of chocolate come from the ester’s bouquet of chocolate headspace: they are aroma-active compounds with low odour thresholds and confer fruity and floral notes to the final product [15]. 2-Phenylethyl acetate is detected in all the samples, accounting for 1.45, 2.61 and 2.69% in EETP801 and CCN51 and commercial samples, respectively. Its aroma contribution is perceived as positive, as it has rose, honey and tobacco-like notes [21]. Ethyl acetate was only detected in the CCN51 sample, accounting for 7.58%. Its aroma contribution is defined as sweet and fruity [21], so its presence is desirable. It is developed during the fermentation as a product of esterification from acetic acid and ethanol [22]: in the CCN51 sample, acetic acid was not detected, presumably because it has all been converted in ethyl acetate through this esterification reaction and/or volatilised during the roasting phases.
Aldehydes, particularly those derived from Strecker degradation like benzaldehyde and phenyl acetaldehyde, are relevant in the perception of a desirable cocoa aroma [17,23]. Benzaldehyde confers a bitter final aroma to chocolate [15]: in EETP801 and CCN51, it was detected in low amounts (0.77 and 0.63%, respectively), whilst its relative abundance is more significant in the commercial sample, where it accounts for 2.06%. According to Bonvehí [15], the presence of aliphatic aldehydes is favourable, as they confer fruity and flowery notes to the final product: nonanal accounts for 1.16 and 1.01% in EETP801 and commercial samples, respectively, whilst decanal is only detected in CCN51, where it accounts for 0.4%. Aldehyde’s behaviour is like that of pyrazines, as their relative abundance is lower in less fermented chocolate: the commercial sample seems more fermented than EETP801 and CCN51.
The only relevant non-terpene ketone detected is 2-nonanone, whose relative abundance is particularly significant in the CCN51 sample, where it accounts for 7.10%. Its aroma contribution is positive, as it is described as fresh and sweet [15].
Volatile acids are critical for the consumer appreciation of the final product: high amounts (over 1%) of these compounds are undesirable, as they confer unpleasant rancid notes to chocolate [15]. Among these, acetic acid is the most important contributor, as it generally is the most abundant one and tastes more acidic than the other acids [24]. They are produced during the fermentation phase and are then released during the drying and roasting phases. Acetic acid relative abundance is significant in EETP801 and the commercial samples, as it accounts for 5.56 and 5.52%, respectively. The reasons could be different: (i) the raw seeds were particularly rich in acid content to begin with, which is generally the case for low-quality varieties of cocoa (like other cocoa cultivars such as Trinitario and Forastero); (ii) the drying phase was conducted with forced air drying and/or at high temperatures, which hardened the husks and did not permit the volatile acids release; (iii) incomplete or inadequate roasting. Acetic acid was not detected in the CCN51 sample headspace. Another negative contributor to chocolate taste is 2-methylbutanoic acid, in which the aroma notes are described as rancid and sweaty [15,25,26]. It was detected only in EETP801 and CCN51 samples but with low relative abundance, as it accounts for 0.39 and 0.55%, respectively.
The HPLC-UV analyses of the characteristic stimulant xanthine alkaloids of cocoa beverages showed that the organically grown EETP801 is significantly richer in theobromine. The samples were similar in their contents of theophylline and caffeine. Theobromine is a sought-after bioactive ingredient in the wellness industry, both as nutraceutical and cosmeceutical [9].
The use of different varieties (or clones) of cocoa started in response to the devastating incidence of “witches’ broom” disease in Ecuador by 1960, caused by the fungi Moniliophthora perniciosa. The highly productive and adaptable CCN 51 clone was obtained from the crosses (IMC-67 X ICS-95) * “Canelo” (Oriente 1). It rapidly spread to neighbouring countries, but research continued to try and improve some challenging traits particularly the lack of the valuable fine-flavour profile of “Nacional type” (such as the “EET-xxx” clone series). The EETP clone series, such as EETP-800 and EETP-801, are cultivars recently released by the INIAP from the cross between CCN 51 × EET-233. Interestingly, EETP-801 has shown a low susceptibility to frosty pod rot disease and a medium incidence of witch’s broom infection. The high endurance of INIAP clones and productivity between 1 to 1.2 t/ha in comparison to CCN51 productivity oscillated between 1.5 and 2 t/ha, indicating its promising value. Recently, the breeders expected an increase in productivity up to 2 t/ha, keeping the quality of nacional cacao [13,27].
In our case, we cannot provide a meaningful calculation of the yield per hectare as the tress are grown ecologically dispersed under the canopy of the rainforest. Previous studies have shown that this “informal setting, small-scale cocoa farming” may achieve a satisfactory level of economic and environmental robustness, but it is not exempt from difficulties [28]. This is exactly the sustainability aspect of our approach, and the difficulty is that harvesting must be performed manually by the local people, knowing where the trees are located. Growing EETP801 in this manner is more sustainable as it can thrive under the natural canopy, whilst CCN51 needs to grow in full sunlight to reach its highest potential [27]. Therefore, farmers do not hesitate to opt for deforestation of their plots thus leading to soil degradation [29] and loss of pollinators [30]. In our experiment, we planted 400 EETP801 seedlings 13 years ago. At the age of 3 years, it gave its first harvest. About 50 plants have died for different reasons in the past 13 years (drought and strong winds cause trees to fall on them). The average amount of cocoa beans produced per year is ca 45 kg in a single annual harvest. This variety does not produce two harvests per year as CCN51 does [27].
The interest of a “health-conscious beverages consumer base” is warranted for the EETP801 variety as it has a significantly higher content in theobromine. This xanthine alkaloid is endowed with numerous potential health benefits [31]. Gao et al. [32] reported that daily intake of theobromine was associated with better cognitive performance. Furthermore, Lee et al. [33] stated that theobromine has been demonstrated to act as an immune response stimulator and might have protective effects against inflammatory disorders. In addition, Camps-Bossacoma et al. [34] observed that theobromine in cocoa plays an immunoregulatory role in systemic and intestinal antibody concentrations. Wei et al., [35] study results showed theobromine improved dyslipidemia, decreased body weight and fat mass, mitigated liver injury and significantly reduced hepatic triglyceride level in mice with obesity. Noteworthy, the combination of theobromine and caffeine in cacao beverages seems to have the expected benefits of methylxanthines with lower secondary effects such as insomnia or anxiety [36].
Before scaling up the ecological agroforestry production of the EETP801 variety, we need to address some limitations of the present study, such as the influence of environmental factors on the potential phytochemical variability of the product during a longer time frame, plus further fine-tuning in the traditional processing methods. In line with the findings of Silva et al., there is a need for standardisation in the pre-processing of cocoa to truly understand variations in the quality of the beans among producing regions [37].
Last but not least, the degree of acceptance/integration of this commercial activity in the surrounding Indigenous community is a key factor in the resilience to deforestation driven by the community’s changing needs and pressures from external stakeholders. Hopefully, EETP801 agroforestry may help stabilise the frontiers of Amazonian forest in the buffer zone of the Yasuni Park, a designated UNESCO Biosphere Reserve, and the welfare of communities adjacent to the Waorani Ethnic Reserve.
5. Conclusions
Despite the lower cocoa yield of the EETP801 cultivar compared to CCN51, the EETP801 cultivar’s produce boasts a higher theobromine content. While the overall polyphenolic composition of EETP801 is qualitatively similar to CCN51, the latter exhibits a richer quantity of these metabolites. Both Amazonian cacao varieties exhibit a superior fragrance profile compared to the commercial sample, although the artisan fermentation and roasting processes introduced some variability in volatile composition. EETP801 may represent a viable and sustainable option for the ecologically conscious cocoa beverage consumer that truly contributes to local communities in resource-limited areas of the planet if properly and sensibly scaled up.
Author Contributions
Conceptualization, J.M.P.; methodology, R.D.l.P.-A., R.A. (Roberta Ascrizzi), J.M.P. and G.F.; formal analysis, R.A. (Roberta Ascrizzi), J.M.P. and G.F.; resources, R.A. (Rocio Alarcon) and M.V.; writing—original draft preparation, J.M.P., R.A. (Roberta Ascrizzi) and G.F.; writing—review and editing, J.M.P.; visualisation, J.M.P.; supervision, J.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon demand.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Diby, L.; Kahia, J.; Kouamé, C.; Aynekulu, E. Tea, Coffee, and Cocoa. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 420–425. ISBN 978-0-12-394808-3. [Google Scholar]
- International Monetary Fund. Primary Commodities: Market Developments & Outlook; World Economic and Financial Surveys; International Monetary Fund: Washington, DC, USA, 1986; ISBN 978-1-4519-4304-7. [Google Scholar]
- Coe, S.D.; Coe, M.D. The True History of Chocolate; Thames & Hudson: New York, NY, USA, 1996; ISBN 978-0-500-29474-1. [Google Scholar]
- Barišić, V.; Icyer, N.C.; Akyil, S.; Toker, O.S.; Flanjak, I.; Ačkar, Đ. Cocoa Based Beverages—Composition, Nutritional Value, Processing, Quality Problems and New Perspectives. Trends Food Sci. Technol. 2023, 132, 65–75. [Google Scholar] [CrossRef]
- González-Garrido, J.A.; García-Sánchez, J.R.; López-Victorio, C.J.; Escobar-Ramírez, A.; Olivares-Corichi, I.M. Cocoa: A Functional Food That Decreases Insulin Resistance and Oxidative Damage in Young Adults with Class II Obesity. Nutr. Res. Pract. 2023, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, J.; Yang, J.; Lou, H.; Zhao, B.; Chi, J.; Tang, W. Dark Chocolate Intake and Cardiovascular Diseases: A Mendelian Randomization Study. Sci. Rep. 2024, 14, 968. [Google Scholar] [CrossRef]
- Kongor, J.E.; Owusu, M.; Oduro-Yeboah, C. Cocoa Production in the 2020s: Challenges and Solutions. CABI Agric. Biosci. 2024, 5, 102. [Google Scholar] [CrossRef]
- Kim, N.; Lee, K. Environmental Consciousness, Purchase Intention, and Actual Purchase Behavior of Eco-Friendly Products: The Moderating Impact of Situational Context. Int. J. Environ. Res. Public Health 2023, 20, 5312. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Sarkar, T.; Chakraborty, R.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M.; Rengasamy, K.R.R. Dark Chocolate: An Overview of Its Biological Activity, Processing, and Fortification Approaches. Curr. Res. Food Sci. 2022, 5, 1916–1943. [Google Scholar] [CrossRef] [PubMed]
- Rainforest Alliance. Rainforest Alliance Certified Cocoa; Rainforest Alliance: New York, NY, USA, 2022. [Google Scholar]
- Halliday, J. Mars Pledges Sustainable Cocoa Only by 2020. Available online: https://www.foodnavigator.com/Article/2009/04/10/Mars-pledges-sustainable-cocoa-only-by-2020/ (accessed on 13 December 2024).
- Heredia-R, M.; Blanco-Gutiérrez, I.; Esteve, P.; Puhl, L.; Morales-Opazo, C. Assessment of Sustainability in Cocoa Farms in Ecuador: Application of a Multidimensional Indicator-Based Framework. Int. J. Agric. Sustain. 2024, 22, 2379863. [Google Scholar] [CrossRef]
- EETP-801. Available online: https://icgd.reading.ac.uk/all_data.php?nacode=33607&&tables=planting_mat-country (accessed on 14 December 2024).
- INIAP. Materiales de siembra: EETP-801. Available online: https://tecnologia.iniap.gob.ec/wp-content/uploads/2023/11/80111.pdf (accessed on 27 December 2024).
- Bonvehí, J.S. Investigation of Aromatic Compounds in Roasted Cocoa Powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of Fermentation Time and Drying Temperature on Volatile Compounds in Cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Van Durme, J.; Ingels, I.; De Winne, A. Inline Roasting Hyphenated with Gas Chromatography–Mass Spectrometry as an Innovative Approach for Assessment of Cocoa Fermentation Quality and Aroma Formation Potential. Food Chem. 2016, 205, 66–72. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology; Wiley-Blackwell: Chichester, UK; Ames, IA, USA, 2010; ISBN 978-1-4051-9906-3. [Google Scholar]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of Volatile and Non-Volatile Compounds in Cocoa (Theobroma cacao L.) during Fermentation and Drying Processes Using Principal Components Analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Tran, P.D.; Van De Walle, D.; De Clercq, N.; De Winne, A.; Kadow, D.; Lieberei, R.; Messens, K.; Tran, D.N.; Dewettinck, K.; Van Durme, J. Assessing Cocoa Aroma Quality by Multiple Analytical Approaches. Food Res. Int. 2015, 77, 657–669. [Google Scholar] [CrossRef]
- Rodríguez, A.; San Andrés, V.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Gadea, J.; Rodrigo, M.J.; Zacarías, L.; Palou, L.; et al. Terpene Down-Regulation in Orange Reveals the Role of Fruit Aromas in Mediating Interactions with Insect Herbivores and Pathogens. Plant Physiol. 2011, 156, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tailoring Wine Yeast for the New Millennium: Novel Approaches to the Ancient Art of Winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Acierno, V.; Yener, S.; Alewijn, M.; Biasioli, F.; Van Ruth, S. Factors Contributing to the Variation in the Volatile Composition of Chocolate: Botanical and Geographical Origins of the Cocoa Beans, and Brand-Related Formulation and Processing. Food Res. Int. 2016, 84, 86–95. [Google Scholar] [CrossRef]
- Jinap, S.; Dimick, P.S.; Hollender, R. Flavour Evaluation of Chocolate Formulated from Cocoa Beans from Different Countries. Food Control 1995, 6, 105–110. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Identification of the Key Aroma Compounds in Cocoa Powder Based on Molecular Sensory Correlations. J. Agric. Food Chem. 2006, 54, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Frauendorfer, F.; Schieberle, P. Changes in Key Aroma Compounds of Criollo Cocoa Beans During Roasting. J. Agric. Food Chem. 2008, 56, 10244–10251. [Google Scholar] [CrossRef]
- Jaimez, R.E.; Barragan, L.; Fernández-Niño, M.; Wessjohann, L.A.; Cedeño-Garcia, G.; Sotomayor Cantos, I.; Arteaga, F. Theobroma cacao L. Cultivar CCN 51: A Comprehensive Review on Origin, Genetics, Sensory Properties, Production Dynamics, and Physiological Aspects. PeerJ 2022, 10, e12676. [Google Scholar] [CrossRef]
- Pokorny, B.; Robiglio, V.; Reyes, M.; Vargas, R.; Patiño Carrera, C.F. The Potential of Agroforestry Concessions to Stabilize Amazonian Forest Frontiers: A Case Study on the Economic and Environmental Robustness of Informally Settled Small-Scale Cocoa Farmers in Peru. Land Use Policy 2021, 102, 105242. [Google Scholar] [CrossRef]
- Andres, C.; Comoé, H.; Beerli, A.; Schneider, M.; Rist, S.; Jacobi, J. Cocoa in Monoculture and Dynamic Agroforestry. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2016; Volume 19, pp. 121–153. ISBN 978-3-319-26776-0. [Google Scholar]
- Groeneveld, J.H.; Tscharntke, T.; Moser, G.; Clough, Y. Experimental Evidence for Stronger Cacao Yield Limitation by Pollination than by Plant Resources. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 183–191. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Jia, L.; Zhang, Y.; Qin, R.; Xu, S.; Mei, Y. Health Benefits and Mechanisms of Theobromine. J. Funct. Foods 2024, 115, 106126. [Google Scholar] [CrossRef]
- Gao, L.; Ge, W.; Peng, C.; Guo, J.; Chen, N.; He, L. Association between Dietary Theobromine and Cognitive Function in a Representative American Population: A Cross-Sectional Study. J. Prev. Alzheimers Dis. 2022, 9, 449–457. [Google Scholar] [CrossRef]
- Lee, H.-W.; Choi, I.-W.; Ha, S.K. Immunostimulatory Activities of Theobromine on Macrophages via the Activation of MAPK and NF-κB Signaling Pathways. Curr. Issues Mol. Biol. 2022, 44, 4216–4228. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Theobromine Is Responsible for the Effects of Cocoa on the Antibody Immune Status of Rats. J. Nutr. 2018, 148, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wu, S.; Liu, J.; Zhang, X.; Guan, X.; Gao, L.; Xu, Z. Theobromine Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Hepatic Lipid Metabolism via mTOR Signaling Pathway in Vivo and in Vitro. Can. J. Physiol. Pharmacol. 2021, 99, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef]
- de Silva, N.M.J.; de Lima, C.L.S.; Meireles dos Santos, R.; Rogez, H.; de Souza, J.N.S. Exploring Variations in Quality Parameters of Theobroma cacao L. Beans from Eastern Amazonia. Heliyon 2024, 10, e39295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).