Abstract
This study investigated the presence and concentration of selected phthalates in municipal tap waters and purified waters sourced from domestic water purifiers and municipal reverse osmosis-based supplies. Five target compounds: Diethyl phthalate (DEP), Diisobutyl phthalate (DiBP), Butyl octyl phthalate (BOP), Dibutyl phthalate (DBP), and bis(2-ethylhexyl) phthalate (DEHP) were identified and quantified in the samples using the solid-phase microextraction gas chromatography-mass spectrometry (SPME-GC/MS) method. The analytical protocol demonstrated good sensitivity, precision, and accuracy, with low limits of detection and quantification, making it suitable for routine monitoring applications. Phthalates were detected in all samples, including both inlet and treated water, highlighting their widespread occurrence. The results show a significant percentage of reduction in total phthalate concentrations (from 4% to 53%; 30% on average) in purified water samples compared to untreated inlet water, thereby indicating the potential efficacy of such systems in reducing organic pollutants. Risk assessment based on the EFSA guidelines showed that the estimated daily intakes for all detected phthalates remained well below tolerable daily intake limits for both adults and toddlers. The findings underscore the importance of monitoring phthalates in drinking water and support the implementation of regular maintenance strategies for filtration devices. The analytical approach developed may be adopted as a cost-effective tool for water quality assessment and offers promising potential for broader application in public health and commercial water treatment systems.
1. Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous compounds that interfere with the normal functioning of the endocrine system, potentially leading to adverse health effects in organisms [1]. These substances can alter hormone synthesis, transport, and metabolism, often by mimicking or blocking natural hormones at their receptors [2]. EDCs are ubiquitous in modern life and are found in food, air, water, clothing, furniture, and a wide range of consumer products [3]. Among the many classes of EDCs, phthalates—or phthalic acid esters (PAEs)—stand out as particularly widespread plasticizers used in industrial and consumer goods. The European Union has identified over 400 substances with endocrine-disrupting potential, of which 194 are classified as Category 1, indicating robust evidence of endocrine-disrupting activity in intact organisms [4,5]. Although some of these compounds are listed as priority hazardous [6] substances under the Water Framework Directive, many, including several phthalates, remain insufficiently regulated. Recognizing the potential public health risks, the EU has recently updated its legislation to strengthen the classification and regulation of EDCs, including PAEs, introducing new hazard categories [7]. Concurrently, the European Food Safety Authority (EFSA) has reinforced the use of the tolerable daily intake (TDI) as a benchmark for evaluating chronic exposure to such chemicals [4,5].
PAEs are a class of synthetic organic compounds primarily used as plasticizers to enhance the flexibility and workability of polymeric (plastic) materials. Phthalates are not covalently bonded to the plastic matrix, which facilitates their release into air, water, and food [8,9]. They are commonly found in polyvinyl chloride (PVC), food contact materials (FCMs), takeaway food containers, nail polish, adhesives, and paints. Due to their widespread use in consumer products, including water distribution systems, phthalates are a major source of human exposure.
The EFSA has conducted comprehensive risk assessments for several phthalates, including Dibutyl phthalate (DBP), Benzyl butyl phthalate (BBP), bis(2-ethylhexyl) phthalate (DEHP), Diisononyl phthalate (DINP), and Diisodecyl phthalate (DIDP).
For DBP, BBP, DEHP, and DINP, a TDI of 50 µg/kg body weight/day has been established, based on their effects on fetal testosterone levels. DIDP, which does not interfere with testosterone production, has a higher TDI of 150 µg/kg body weight/day, based on liver toxicity data [4,9]. Phthalates have been linked to a range of health outcomes, including reproductive toxicity (e.g., reduced testosterone, sperm quality, and fertility); developmental reproductive system abnormalities in male infants; and adverse metabolic effects such as obesity, insulin resistance, and thyroid dysfunction [10]. Emerging research also suggests links to cognitive impairment, ADHD, and increased risks of hormone-related cancers [11].
One significant pathway for phthalate exposure is through drinking water. In recent decades, the occurrence of PAEs in drinking water has become a topic of growing concern, especially regarding bottled and municipal water [12,13,14,15,16,17,18,19,20,21,22,23,24] that can be polluted by plasticizers residues that could be present in plastic bottles and piping. Italy, for instance, has the highest per capita consumption of bottled water in Europe, approximately 252 L per year, far exceeding the EU average of 88 L. In Italy, bottled (mineral) water consumption is widespread, and in 2021, two-thirds of Italian families consumed at least one liter of mineral water per day [25]. Nonetheless, in response to environmental concerns and the desire to reduce plastic waste, many consumers are now turning to household drinking water purification systems. These systems, more accurately referred to as “refinement” or “treatment” systems, employ various technologies, including activated carbon, ion exchangers, mechanical filters, water softeners, ultraviolet disinfection, reverse osmosis, ozonation, and chlorination. Their primary goal is to reduce turbidity, residual chlorine, and other unwanted substances in municipal water [18,19,20,21,22,23]. Filter jugs represent the most widely adopted solution (13.3%), followed by systems designed to remove chlorine and other chemical residues (9.8%) and those offering refrigeration or carbonation (4.1%). It is important to recognize, however, that no single system is universally effective, and all require regular maintenance and monitoring to ensure optimal performance [24].
This study aims to identify and quantify the presence of phthalates in drinking water supplied by various household purification systems and municipal distribution points known as “water houses”. These water houses, installed in several Italian municipalities, provide public drinking water, often chilled and carbonated, and are typically funded through public resources. In some cases, the water is distributed free of charge, while, in others, a fee is applied.
To achieve this goal, we used solid-phase microextraction followed by gas chromatography-mass spectrometry (SPME-GC/MS). This analytical technique is fast, cost-effective, solvent-free, and well suited for the detection of trace-level organic contaminants. The results are compared with those from untreated municipal water to assess the effectiveness of these systems in reducing human exposure to phthalates.
2. Materials and Methods
2.1. Chemicals and Materials
Dibutyl phthalate (DBP) was purchased from Carlo Erba (Cornaredo, Italy), and di-Bis(2-ethylhexyl) phthalate (DEHP) and ultrapure water were obtained from Sigma-Aldrich (Milan, Italy). The SPME fibers, divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS), were purchased from Supelco (Merck KGaA, Darmstadt, Germany). The 40 mL SPME screw top vials with metal caps were purchased from Gerstel (Baltimore, MD, USA).
2.2. Samples
To evaluate the effectiveness of the purification devices, a total of 36 water samples were collected: 13 samples were obtained from household water purifiers (HWP), 5 from municipal water houses (MWH), and the remaining 18 samples represented the corresponding inlet water (IW) for each device. The samples collected (about 1 L) were stored in brown glass bottles at 4 °C until analysis, performed within 1–3 days on the unmodified sample.
2.3. Standards and Calibration Solutions Preparation
Individual standards (Table 1 and Figure 1) were not available for each investigated compound. To overcome this limitation, we employed relative quantification methods. Diethyl phthalate (DEP), diisobutyl phthalate (DiBP), and butyl octyl phthalate (BOP) were quantified and referred to dibutyl phthalate (DBP). For bis(2-ethylhexyl) phthalate (DEHP), a standard stock solution was prepared.

Table 1.
List of PAEs determined in this study and their selected physicochemical properties.
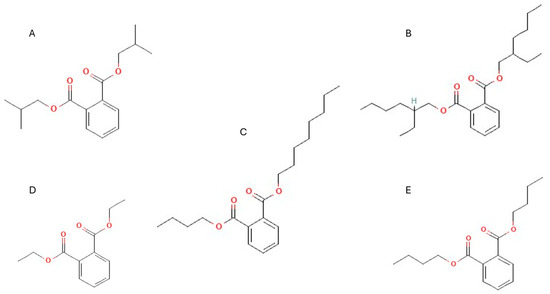
Figure 1.
Molecular structure of (A) diisobutyl phthalate (DiBP), (B) bis(2-ethylhexyl) phthalate (DEHP), (C) butyl octyl phthalate (BOP), (D) diethyl phthalate (DEP), and (E) dibutyl phthalate (DBP).
Individual stock solutions of DBP and DEHP were prepared in hexane (Sigma-Aldrich-Milan, Italy) at 1100 mg/L and 630 mg/L, respectively. Phthalates were quantified using an external calibration curve, with five calibration points ranging from 0.2 to 25 μg/L (0.2, 1, 5, 10, and 25 μg/L) for DBP and from 0.9 to 25 μg/L (0.9, 1, 5, 10, and 25 μg/L) for DEHP. Calibration solutions were prepared by diluting individual stock solutions with ultrapure water that had been previously analyzed to ensure the absence of phthalates.
2.4. Volatile Organic Compounds by SPME-GC/MS Analysis
The SPME technique is applied to investigate many trace organic compounds. It is based on the principle of adsorption/desorption of the analytes, using a fused silica fiber coated with a thin polymeric film, and aims to adsorb volatile (and non-volatile) compounds from gaseous, liquid, or solid matrices [26,27,28,29]. The experimental conditions for the extraction phase, temperature, choice of SPME fiber, desorption, and subsequent GC/MS analyses were optimized by means of several experimental tests.
2.4.1. Extraction Procedure
Aliquots of 10 mL of the water samples were placed in a 40 mL vial with a silicon septum. The analytes were extracted for 30 min at 25 °C under continuous magnetic stirring using the solid-phase microextraction (SPME) technique.
Although phthalates are quite volatile and thermally stable, their extraction was performed using the direct immersion mode (DI-SPME) using a triphasic divinylbenzene/carboxen/polydimethylsiloxane fiber (DVB/CAR/PDM) [26]. DI-SPME is an extraction technique in which the SPME fiber, coated with an adsorbent phase, is directly immersed into the liquid sample. In this configuration, the fiber is subjected to controlled agitation or directional flow, which enhances mass transfer and improves extraction efficiency and reproducibility. Before use, the fiber was conditioned in a GC injector at 270 °C for 0.5 h. After the extraction time, the fiber was retracted into the hollow needle of the syringe, removed from the vial, manually introduced into the GC injector, and left to desorb for 3 min at 260 °C [26]. All samples were analyzed in triplicate.
2.4.2. Instrumentation and Chromatographic Conditions
Analyses were performed using a Thermo Fisher Scientific (Waltham, MA, USA) TSQ 8000 triple-quadrupole mass spectrometer and a Thermo Fisher Scientific Trace 1310 gas chromatographic apparatus.
Samples were analyzed using a TG XLBMS column (20 m × 0.18 mm ID × 0.18 μm, Thermo Scientific, Waltham, MA, USA, GC column), and the injector port was maintained at 260 °C. The column carrier gas was helium (99.9995%) at a constant flow rate of 1.0 mL/min. The oven (Thermo Fisher Scientific-Waltham, MA, USA) temperature was held at 50 °C for 3 min, increased to 300 °C at a rate of 40 °C/min, and held for 10 min. Mass analysis was performed under electron ionization (EI)-positive conditions with an ionization energy of 70 eV, and the source temperature was set to 300 °C. Mass spectra have been acquired in full-scan acquisition mode, in the range of 35 to 500 m/z. Each analyte was identified using the extracted ion chromatogram, retention time, when the corresponding reference standard was available (Table S1), and the corresponding mass spectrum was compared with the NIST library 2018 database.
2.5. Analytical Parameters
The GC/MS method was evaluated in terms of repeatability, reproducibility, linearity, limit of detection (LOD), and limit of quantification (LOQ) values.
Repeatability is the measure of precision under the same experimental conditions and in a small-time interval (intra-day). Reproducibility is defined as the variation of the response of an analytical method under the same experimental conditions but among different analytical set times (inter-day).
Precision was calculated as the relative standard deviation (%RSD), and accuracy was expressed in terms of percentage of relative error (%RE).
The LOD and LOQ were calculated following the directives of the American Chemical Society’s Committee on Environmental Analytical Chemistry and based on blank samples treated in the same way as the water samples. The LOD and LOQ values were estimated using the regression parameters of the calibration curves and were calculated according to the following dependence: LOD = 3 × σb/m and LOQ = 10 × σb/m, where σb is the standard deviation of the blank signal, and m is the slope of the calibration curve. The sample concentrations were calculated by means of the calibration curves for DEHP and DBP; for the analytes for which the corresponding standard was not available, the calibration curve of DBP was adopted as a reference.
2.6. Risk Assessment Methodology
To determine the potential human health risks associated with phthalate exposure through drinking water, we adopted a risk assessment approach based on the comparison between the estimated daily intake (DI) and the EFSA tolerable daily intake (TDI), which is set at 50 µg/kg body weight (bw)/day for both DBP and DEHP [4,5]. The DI (µg/kg bw/day) was calculated for each sample using the following equation:
where C is the phthalate concentration in the water sample (µg/L), IR is the daily ingestion rate (2 L/day for adults and 1.5 L/day for toddlers), and WB is the body weight (60 kg for adults and 12 kg for toddlers) [30,31].
To determine C, in the case of samples with analyte concentrations below the LOD, we hypothesized the worst-case scenario considering a concentration of the sample numerically equal to the LOD value. For samples with an analyte concentration between the LOQ and LOD, an estimate value numerically equal to half of the LOQ level was considered.
These values were then compared to the EFSA TDI, and a risk quotient (RQ) was determined as the ratio between DI and TDI according to
An RQ value below 1 indicates an exposure level below the threshold of concern, suggesting no significant health risk.
3. Results and Discussion
3.1. Pollutant Evaluation in the Samples
Due to the low concentration levels of phthalates in drinking water, a sample extraction, preconcentration, and pretreatment step is always required to improve analytical sensitivity during quantification. Several analytical approaches for the preparation of drinking water and tap water samples have been reported in the literature [7,8,13,17], including liquid–liquid extraction (LLE), solid-phase extraction (SPE), solid-phase microextraction (SPME), and liquid-phase microextraction (LPME). Among chromatographic techniques, liquid chromatography (LC) with UV detection or diode array detection (DAD), as well as GC/MS, have been employed. In addition, non-chromatographic methods such as immunoassay-based techniques and electrochemical sensors have been proposed for the analysis of phthalate esters in beverages. In our study, all 36 water samples were analyzed using SPME in direct immersion mode [32] with a commercially available DVB/CAR/PDMS fiber. This fiber showed higher extraction efficiency for the target phthalates, primarily due to the carboxen porous sorbent layer, which is suitable for the extraction of smaller polar molecules [13,26]. Our experimental conditions for the GC/MS analyses were optimized to achieve the best chromatographic separation and maximum sensitivity. The primary advantage of employing direct immersion SPME as a sample pretreatment technique prior to GC/MS analysis lies in its operational simplicity, which significantly reduces the risk of secondary contamination during sample handling. In addition, direct immersion enhances the extraction efficiency for polar compounds and minimizes the formation of artifacts associated with fiber coating saturation. This approach also requires minimal sample volumes, limits operator exposure to toxic organic solvents, and supports the use of environmentally friendly (green) extraction procedures. To evaluate the performance of the developed method, several validation parameters were assessed, including linearity, LOD, LOQ, intra- and inter-day precision, and recovery (Table 2). The calibration curves demonstrated excellent linearity in the calibrated range, with correlation coefficients (R2) exceeding 0.993.

Table 2.
Analytical validation parameters determined for the evaluation of the GC/MS method.
Method accuracy was evaluated using fortified samples prepared by spiking a blank matrix (bi-distilled water) with known quantities of DPB and DEHP. The accuracy was expressed in relative error (RE%). The calculated analytical parameters confirmed the reliability of the proposed approach. The low values of the LOD and LOQ suggest that the proposed technique is suitable for rapid screening of phthalates in drinking water.
Moreover, recovery consistently above 90% for both the analytes, checked on different days from independent analysis of the same drinking water, highlighted the method’s accuracy.
The following phthalates were identified and quantified in the water samples: DEP, DiBP, BOP, DBP, and DEHP (Figure 2), and the corresponding levels determined for household water purifiers and municipal water houses are reported in Figure 3 and Figure 4, respectively.
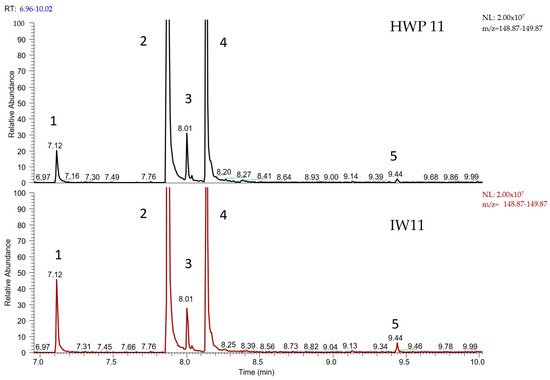
Figure 2.
Extracted ion GC/MS chromatogram of purified water sample HWP11 and the corresponding inlet water (IW11), showing a m/z 149 signal. The compounds eluted in the following order (increasing retention times from left to right): (1) diethyl phthalate at 7.12 min, (2) diisobutyl phthalate at 7.94 min, (3) butyl octyl phthalate at 8.01 min, (4) dibutyl phthalate at 8.21 min, and (5) di(2-ethylhexyl) phthalate at 9.44 min.
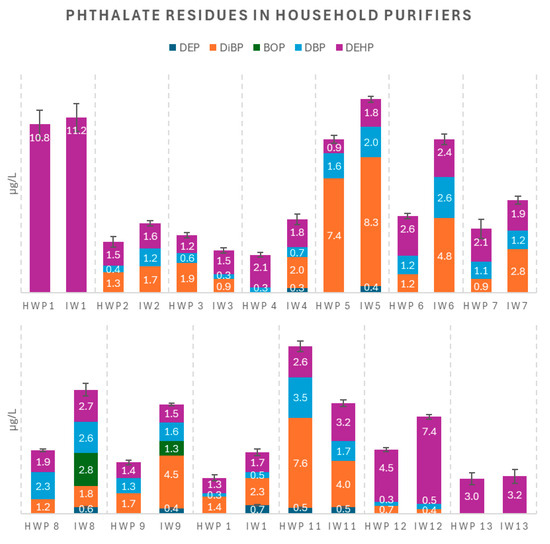
Figure 3.
Phthalate residue levels (µg/L) in household water purifiers (HWPs) and corresponding inlets (IWs); error bars are related to the total phthalate residue.
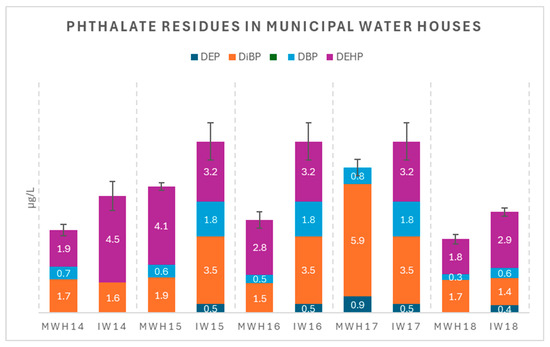
Figure 4.
Phthalate residue levels (µg/L) in municipal water houses (MWHs) and corresponding inlets (IWs); error bars are related to the total phthalate residue.
Table S2 reports the concentrations of five phthalates detected in 13 purified water samples from household water purifiers (HWPs) and 13 inlet water (IW) samples along with the corresponding standard errors individually determined for each analyte. The total phthalate concentrations in these samples ranged from 2.26 to 14.22 µg/L. Table S3 presents the concentrations of the same phthalates (along with the corresponding standard errors, individually determined for each analyte) determined in the five purified water samples from municipal water houses (MWHs) and their respective inlet water (IW) samples, with total phthalate concentrations ranging from 3.21 to 8.21 µg/L.
All five phthalates were contemporarily detected in only two inlet water samples (IW8 and IW9). BOP was lacking in the remaining inlet samples and all purified water samples. The highest phthalate concentration was observed in sample IW1 (DEHP at 11.23 µg/L), followed by samples HWP1 (DEHP at 10.83 µg/L) and IW5 (DBP at 8.27 µg/L). The two isomers of butyl phthalate, DiBP and DBP, were present in most of the analyzed samples, except IW1, HWP1, and HWP13, in which their concentrations were below the limit of detection (LOD).
Statistical analysis of our dataset (Table S4), evaluated by means of pairwise Student’s t-tests (at the p = 0.05 level), revealed a general decrease in the total concentration of phthalates (tcalc 95% = 4.38) in the purified water samples compared to their respective inlet samples, except for two household purifiers (HWP3 and HWP11). In these two cases, a marked increase of phthalates was observed in the purified waters with respect to the inlet samples.
This unexpected result warranted further investigation. In theory, the use of advanced filtration systems, such as activated carbon filters and reverse osmosis membranes, should significantly reduce the levels of these organic contaminants. On average, the total phthalate concentration was reduced by 20%, with the greatest reduction observed in sample pair IW9/HWP9, showing a 53% decrease. In the few cases where the phthalate concentrations were surprisingly increased in the purified water (HWP3 and HWP11), it was confirmed that the associated purification devices were equipped with worn-out activated carbon filters that had not been replaced within the six-month interval recommended by the manufacturers. Excluding these outliers from the dataset led to a more correct evaluation of the average removal efficiency, now raised to 30%.
The average removal efficiencies (%) of individual phthalates was also statistically assessed by means of the Student’s t-test (at p = 0.05 level), but no significative difference was evidenced among them, suggesting a non-structure-dependent removal mechanism. Interestingly even the simplest purification devices (HWP4 and HWP8), namely the Brita filters, based on an ion exchange resin and activated carbon, performed comparably to complex purification systems featuring multiple-stage filters and reverse osmosis filter devices in reducing phthalate contamination.
3.2. Assessment of Potential Risks for Human Health
To determine the potential risk for human health, related to phthalate exposure through drinking water, the TDI recommended by the EFSA [4,5] was used as a reference value. Based on our measured phthalate concentrations, the estimated DI and RQ were calculated for each water sample, and the results are presented in Table 3 for household water purifiers and in Table 4 for municipal water houses. It is possible to state that none of the samples analyzed exceeded this limit.

Table 3.
Estimated daily intakes (DIs) and risk quotient (RQ) for adults (body weight of 60 kg) and toddlers (body weight of 12 kg) considering a daily water consumption of 2 L for adults and 1.5 L for toddlers in the case of household water purifiers.

Table 4.
Estimated daily intakes (DIs) and risk quotient (RQ) for adults (body weight of 60 kg) and toddlers (body weight of 12 kg) considering a daily water consumption of 2 L for adults and 1.5 L for toddlers in the case of municipal water houses.
Specifically, the levels of DBP and DEHP, both of which have been identified by the EFSA as substances of concern due to their adverse effects on reproductive health, were well below their respective TDI values [8]. Since all RQ values are below 1 and, therefore, below the threshold of concern, our findings suggest that, under the tested conditions, the presence of phthalates in purified drinking water and in inlet water samples does not pose a significant risk to human health for either adults or toddlers.
4. Conclusions
The present study demonstrates the applicability and reliability of a SPME method coupled with GC/MS for detecting and quantifying selected phthalates in drinking water. The analytical method showed good sensitivity, precision, accuracy, and low limits of detection and quantification, making it suitable for routine monitoring purposes. Phthalates were detected in both the inlet and purified water samples collected from household and municipal filtration systems. While most purification devices effectively reduced the total phthalate concentrations with an average reduction of 30%, a few cases showed an unexpected increase in phthalate levels in the purified water. These anomalies were linked to inadequate maintenance, specifically the use of worn-out activated carbon filters. No significant differences were observed in the removal efficiency among the different phthalates, suggesting that the filtration step is likely non-selective and not strongly dependent on molecular structure. Interestingly, simple filtration systems such as Brita filters achieved comparable results to more complex multi-stage or reverse osmosis systems in reducing phthalate concentrations. Risk assessment based on the EFSA guidelines indicated that the estimated daily intake of phthalates from all water samples was well below the tolerable daily intake values for adults and toddlers. In particular, the levels of DBP and DEHP did not approach harmful thresholds. Overall, these findings emphasize the importance of regular maintenance of filtration devices to ensure optimal performance and the continued protection of public health. Further, the ubiquitous presence of phthalates in all samples (purified drinking water and inlet water) should be considered a warning against the indiscriminate use of plastic pipes, tanks and bottles containing phthalates, or other hormone-disrupting agents. The proposed analytical approach can serve as an effective tool for monitoring phthalate contamination and supporting water safety assessments.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/beverages11030092/s1: Table S1: Retention time (mins), molecular ions (m/z), and extracted ions (m/z) used for phthalates identification in water samples; Table S2: Phthalates concentration (μg/L) in inlet water (IW) and purified water by household water purifiers (HWPs); Table S3: Phthalates concentration (μg/L) in inlet water (IW) and purified water obtained by municipal water houses (MWHs); Table S4: Difference in phthalates concentration (μg/L) in inlet water (IW) with respect to the corresponding purified water (HWP or MWH), for a pairwise t-test (*). Theoretical t at p = 0.05 with 17 degrees of freedom is 2.110.
Author Contributions
Conceptualization, G.A. and C.L.; methodology, G.A. and C.L.; software, D.B.; validation, S.I. (Serena Indelicato); investigation, C.L.; data curation C.L. and D.B.; writing—original draft preparation, S.I. (Serena Indelicato) and C.L.; writing—review and editing, F.D. and S.I. (Sergio Indelicato); supervision, S.I. (Serena Indelicato) and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Talpade, J.; Shrman, K.; Sharma, R.K.; Gutham, V.; Singh, R.P.; Meena, N.S. Bisphenol a: An endocrine disruptor. J. Entomol. Zool. Stud. 2018, 6, 394–397. [Google Scholar]
- Locatelli, M.; Sciascia, F.; Cifelli, R.; Malatesta, L.; Bruni, P.; Croce, F. Analytical methods for the endocrine disruptor compounds determination in environmental water samples. J. Chromatogr. 2016, 1434, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M.; Harun, R. Drinking water consumption and association between actual and perceived risks of endocrine disrupting compounds. NPJ Clean Water 2022, 5, 25. [Google Scholar] [CrossRef]
- EFSA. European Food Safety Authority. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to bis (2-ethylhexyl) phthalate (DEHP) for use in food contact materials. EFSA J. 2005, 243, 1–2. [Google Scholar]
- EFSA. European Food Safety Authority. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a Request from the Commission Related to Di-Butylphthalate (DBP) for Use in Food Contact Materials. 2005. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/242 (accessed on 9 June 2025).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013L0039 (accessed on 9 June 2025).
- Domínguez-Morueco, N.; González-Alonso, S.; Valcárcel, Y. Phthalate occurrence in rivers and tap water from central Spain. Sci. Total Environ. 2014, 500–501, 139–146. [Google Scholar] [CrossRef]
- Zaki, G.; Shoeib, T. Concentrations of several phthalates’ contaminants in Egyptian bottled water: Effects of storage conditions and estimate of human exposure. Sci. Total Environ. 2018, 618, 142–150. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging. Appl. Sci. 2021, 11, 2130. [Google Scholar] [CrossRef]
- Singh, S.; Li, S.S.L. Phthalates: Toxicogenomics and inferred human diseases. Genomics 2011, 97, 148–157. [Google Scholar] [CrossRef]
- Zare Jeddi, M.; Rastkari, N.; Ahmadkhaniha, R.; Yunesian, M. Endocrine disruptor phthalates in bottled water: Daily exposure and health risk assessment in pregnant and lactating women. Environ. Monit. Assess. 2016, 188, 534. [Google Scholar] [CrossRef]
- Keresztes, S.; Tatár, E.; Czégény, Z.; Záray, G.; Mihucz, V.G. Study on the leaching of phthalates from polyethylene terephthalate bottles into mineral water. Sci. Total Environ. 2013, 458–460, 451–458. [Google Scholar] [CrossRef]
- Wator, K.; Rusiniak, P.; Kmiecik, E.; Bugno, R.; Ristic Vakanjac, V. Assessing health risks in bottled water: Chemical compounds and their impact on human health. Environ. Geochem. Health 2024, 46, 178. [Google Scholar] [CrossRef] [PubMed]
- Viktoryova, N.; Szarka, A.; Capilla-Flores, R.; Arrebola Liebanas, F.J. Solid-phase microextraction coupled to gas chromatography–Mass spectrometry as an advanced method for the determination of bioplasticizers in environmental and bottled water samples. J. Chromatogr. A 2025, 1751, 465943. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liu, Z.; Yin, H.; Dang, Z.; Wu, P.; Zhu, N.; Lin, Z.; Liu, Y. Migration and potential risk of trace phthalates in bottled water: A global situation. Water Res. 2018, 147, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Costa, R.; Sainara Maia Fernandes, T.; de Sousa Almeida, E.; Tomé Oliveira, J.; Arruda Carvalho Guedes, J.; Julião Zocolo, G.; Wagner de Sousa, F.; Ferreira do Nascimento, R. Potential risk of BPA and phthalates in commercial water bottles: A minireview. J. Water Health 2021, 19, 411–435. [Google Scholar] [CrossRef]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Phthalates in Beverages and Plastic Bottles: Sample Preparation and Determination. Food Anal. Methods 2018, 11, 48–61. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Sato, I. Effectiveness of household water purifiers in removing perfluoroalkyl substances from drinking water. Environ. Sci. Pollut. Res. 2021, 28, 11665–11671. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Elkhatib, R.; Al-Rajoudi, T.; Al-Qudaihi, G. Assessing the concentration of phthalate esters (PAEs) and bisphenol A (BPA) and the genotoxic potential of treated wastewater (final effluent) in Saudi Arabia. Sci. Total Environ. 2017, 578, 440–451Y. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfig, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeed, R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in Tehran, Iran. Environ. Res. J. 2019, 173, 469–479. [Google Scholar] [CrossRef]
- Timóteo Vieira, W.; Barbosa de Farias, M.; Spaolonzi, M.P.; Gurgel Carlos da Silva, M.; Gurgel Adeodato Vieira, M. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Lin, W.; Ye, C.; Guo, L.; Hu, D.; Yu, X. Analysis of microbial contamination of household water purifiers. Appl. Microbiol. Biotechnol. 2020, 104, 4533–4545. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Zhu, Z.; Qiu, Y.; Zhao, J.; Huang, Q.; Bergman, A. Human exposure to phthalate esters via ingestion of municipal drinking water from automatic water purifiers: Levels, sources, and risks. Environ. Sci. Water Res. Technol. 2022, 8, 2843–2855. [Google Scholar] [CrossRef]
- Boyraz, Y.K.; Demir, L.S.; Eken, K.; Tabara, M.F.; Evci, R.; Durduran, Y.; Uyar, M.; Şahin, T.K. Determination of usage frequency of household type water purifiers and effects on drinking water quality in Meram. Türk Hij. Deney. Biyol. Derg. 2019, 76, 149–156. [Google Scholar] [CrossRef]
- Italian Statistical Institute. Le Statistiche Dell’istat Sull’acqua Anni 2019–2021. Available online: https://www.istat.it/comunicato-stampa/le-statistiche-dellistat-sullacqua-anni-2019-2021/ (accessed on 9 June 2025).
- Alshehri, M.M.; Ouladsmane, M.A.; Aouak, T.A.; ALOthman, Z.A.; Ahmed, A.Y.B.H. Determination of phthalates in bottled waters using solid-phase microextraction and gas chromatography tandem mass spectrometry. Chemosphere 2022, 304, 135214. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace solid-phase microextraction: Fundamentals and recent advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Peng, S.; Huang, X.; Huang, Y.; Huang, Y.; Zheng, J.; Zhu, F.; Xu, J.; Ouyang, G. Novel solid-phase microextraction fiber coatings: A review. J. Sep. Sci. 2022, 45, 282–304. [Google Scholar] [CrossRef]
- Jin, H.; Shi, Y.; Cao, J. Recent advances and applications of novel advanced materials in solid-phase microextraction for natural products. Trends Anal. Chem. 2024, 178, 11785. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 2579. [Google Scholar]
- EFSA, European Food Safety Agency. Scientific opinion on dietary reference values for water. EFSA J. 2010, 8, 1459. [Google Scholar]
- Gionfriddo, E.; Souza-Silva, E.A.; Pawliszyn, J. Headspace versus direct immersion solid phase microextraction in complex matrixes: Investigation of analyte behavior in multicomponent mixtures. Anal. Chem. 2015, 87, 8448–8456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).