Mixture Design and Kano Model for a Functional Chickpea and Hibiscus Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
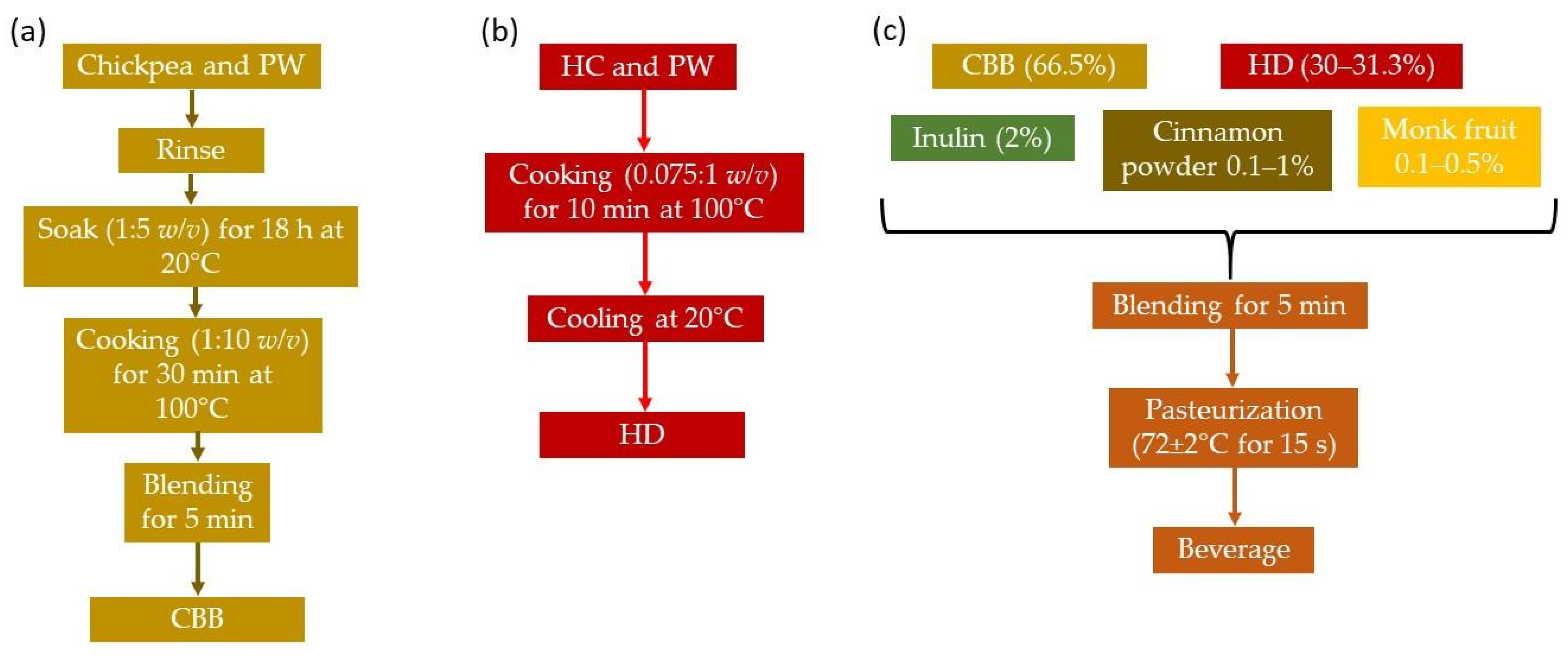
2.2. Raw Materials
2.3. Beverage Formulation
2.4. Antioxidant Capacity Assays
2.4.1. Trolox Equivalent Antioxidant Capacity (TEAC)
2.4.2. Ferric Reducing Antioxidant Power Assay (FRAP)
2.4.3. The Antioxidant Radical Absorbance Capacity (ORAC)
2.5. Determination of the Total Content of Phenolic Groups
2.6. Sensory Analysis
2.7. Beverage Optimization
2.8. Nutritional Content
2.9. Kano Model
2.10. Statistical Analysis
2.11. Ethics Declaration
3. Results
3.1. Beverage Optimization
3.2. Optimized Beverage Characterization
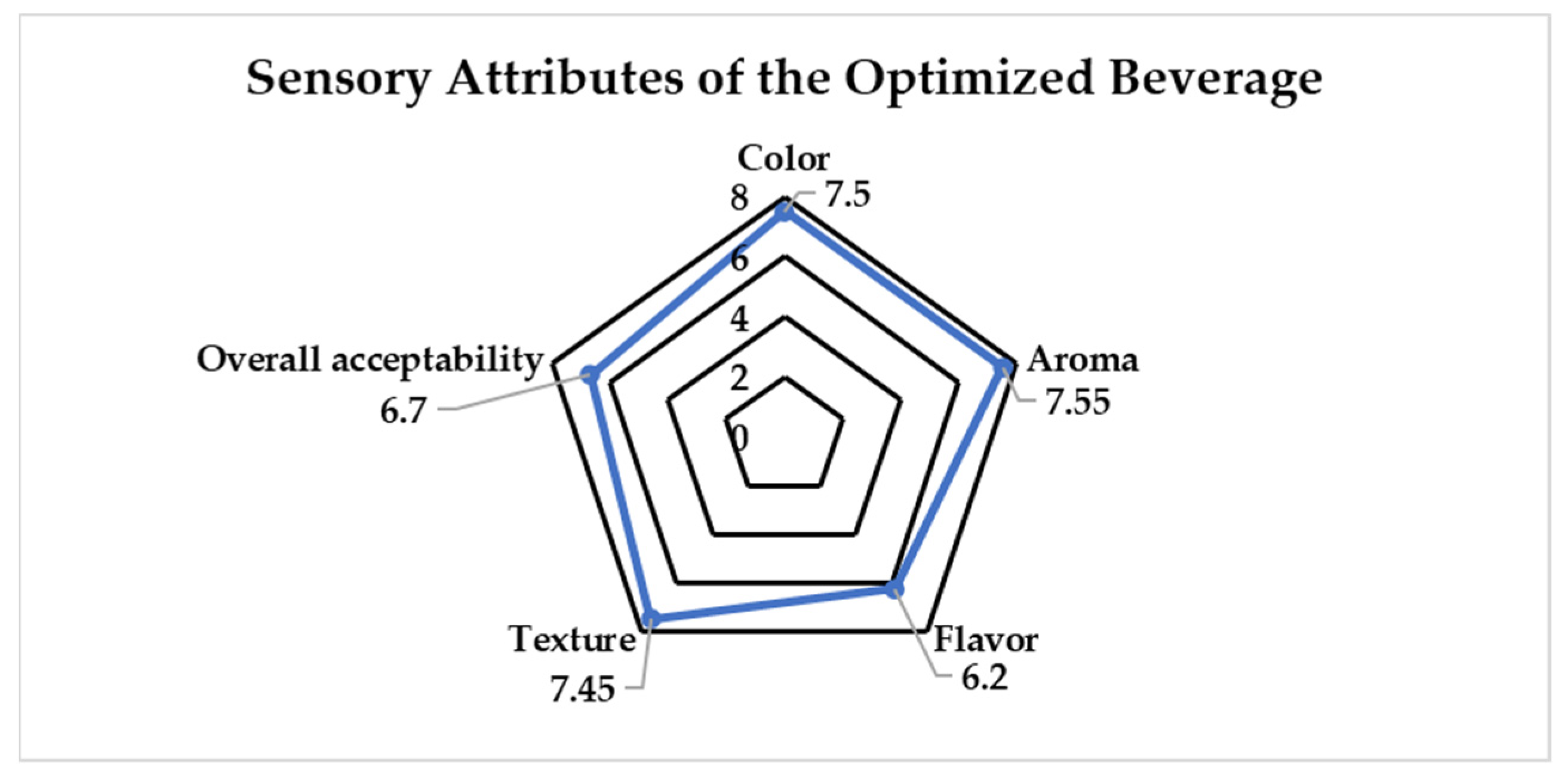
3.3. Sensory Analysis
3.4. Kano Model
4. Discussion
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| °C | Degrees Celsius |
| nm | Nanometer |
| mL | Milliliter |
| µM | Micromolar |
| µL | Microliter |
| N | Normality |
| cm | Centimeter |
| pH | Hydrogen potential |
| K+ | Potassium |
| Na+ | Sodium |
| min | Minutes |
| h | Hours |
| s | Seconds |
References
- Vlaicu, P.A.; Untea, A.E.; Varzaru, I.; Saracila, M.; Oancea, A.G. Designing Nutrition for Health—Incorporating Dietary By-Products into Poultry Feeds to Create Functional Foods with Insights into Health Benefits, Risks, Bioactive Compounds, Food Component Functionality and Safety Regulations. Foods 2023, 12, 4001. [Google Scholar] [CrossRef]
- Carvalho, F.; Lahlou, R.A.; Pires, P.; Salgado, M.; Silva, L.R. Natural Functional Beverages as an Approach to Manage Diabetes. Int. J. Mol. Sci. 2023, 24, 16977. [Google Scholar] [CrossRef]
- López-Cardoso, F.; Ontiveros-Apodaca, N.; Gutiérrez-Grijalva, E.P.; Arámburo-Gálvez, J.G.; Figueroa-Salcido, O.G.; Leyva-López, N.; Gastélum-Chavira, A.D.; Cárdenas-Torres, F.I.; Heredia, J.B. Functional food consumption preference in Culiacán, Mexico. Salud Publica Mex. 2024, 67, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ignat, M.V.; Salanță, L.C.; Pop, O.L.; Pop, C.R.; Tofană, M.; Mudura, E.; Coldea, T.E.; Borșa, A.; Pasqualone, A. Current Functionality and Potential Improvements of Non-Alcoholic Fermented Cereal Beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Ishak, Z.; Maarof, S.; Mohamad, N.I.; Kasim, N.; Noor, N.M.F.C.M. Potential Application of Spices and Pineapple as Healthy Beverages. Asian Food Sci. J. 2024, 23, 47–57. [Google Scholar] [CrossRef]
- Ghoshal, G.; Kansal, S.K. 2—The Emerging Trends in Functional and Medicinal Beverage Research and Its Health Implication. In Functional and Medicinal Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 11, pp. 41–71. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S.; Muniandy, P. 5—Potential Health-Promoting Effects of Probiotics in Dairy Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 173–204. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods: Commercial trends, research, and health implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z. 7—Medicinal Properties and Functional Components of Beverages. In Functional and Medicinal Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 11, pp. 235–284. [Google Scholar] [CrossRef]
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Vladimir-Knežević, S.; Butorac, D.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Funct. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Olatunji, O.J.; Nirmal, N.P.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Maulida, I.D.; Al Marsam, M.R.; Purnama, I.; Mutamima, A. A novel beverage with functional potential incorporating cascara (Coffea arabica), roselle (Hibiscus sabdariffa), and red ginger (Zingiber officinale Rosc. var. rubrum) extracts: Chemical properties and sensory evaluation. Discov. Food 2024, 4, 94. [Google Scholar] [CrossRef]
- Cruz, A.G.; Cadena, R.S.; Walter, E.H.; Mortazavian, A.M.; Granato, D.; Faria, J.A.; Bolini, H.M. Sensory Analysis: Relevance for Prebiotic, Probiotic, and Synbiotic Product Development. Compr. Rev. Food Sci. Food Saf. 2010, 9, 358–373. [Google Scholar] [CrossRef]
- Kraus, A. Development of functional food with the participation of the consumer. Motivators for consumption of functional products. Int. J. Consum. Stud. 2014, 39, 2–11. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Vallath, A.; Shanmugam, A.; Rawson, A. Prospects of future pulse milk variants from other healthier pulses—As an alternative to soy milk. Trends Food Sci. Technol. 2022, 124, 51–62. [Google Scholar] [CrossRef]
- Sharma, N.; Yeasmen, N.; Dubé, L.; Orsat, V. A review on current scenario and key challenges of plant-based functional beverages. Food Biosci. 2024, 60, 104320. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Nwachukwu, I.C.; Ezeoke, C.S.; Woke, R.C.; Adegbite, O.A.; Olawole, T.D.; Martins, O.C. Production of a New Plant-Based Milk from Adenanthera pavonina Seed and Evaluation of Its Nutritional and Health Benefits. Front. Nutr. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-De-Almeida, C.A.; Ferraz, I.S.; Ued, F.d.V.; Almeida, A.C.F.; Del Ciampo, L.A. Impact of soy consumption on human health: Integrative review. Braz. J. Food Technol. 2020, 23, e2019129. [Google Scholar] [CrossRef]
- Vallath, A.; Shanmugam, A.; Rawson, A. Evaluation of physicochemical and organoleptic properties of plant based beverage developed from chickpea. Pharma Innov. J. 2021, 10, 1871–1875. [Google Scholar]
- Wang, S.; Chelikani, V.; Serventi, L. Evaluation of chickpea as alternative to soy in plant-based beverages, fresh and fermented. LWT 2018, 97, 570–572. [Google Scholar] [CrossRef]
- Chiu, H.; Liao, Y.; Shen, Y.; Han, Y.; Golovinskaia, O.; Venkatakrishnan, K.; Hung, C.; Wang, C. Improvement on blood pressure and skin using roselle drink: A clinical trial. J. Food Biochem. 2022, 46, e14287. [Google Scholar] [CrossRef]
- Herranz-López, M.; Olivares-Vicente, M.; Encinar, J.A.; Barrajón-Catalán, E.; Segura-Carretero, A.; Joven, J.; Micol, V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients 2017, 9, 907. [Google Scholar] [CrossRef]
- Tsai, P.-J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Lin, H.H.; Huang, H.P.; Huang, C.C.; Chen, J.H.; Wang, C.J. Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Mol. Carcinog. 2005, 43, 86–99. [Google Scholar] [CrossRef]
- Delpino, F.M.; dos Santos, F.S.; Flores, T.R.; Cerqueira, H.S.; Santos, H.O. The effects of blueberry and cranberry supplementation on blood pressure in patients with cardiovascular diseases: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2023, 38, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Choobkar, N.; Daraei Garmakhany, A.; Aghajani, A.R.; Ataee, M. Response surface optimization of pudding formulation containing fish gelatin and clove (Syzygium aromaticum) and cinnamon (Cinnamomum verum) powder: Effect on color, physicochemical, and sensory attributes of the final pudding product. Food Sci. Nutr. 2022, 10, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Polansky, M.M.; Anderson, R. Whole Cinnamon and Aqueous Extracts Ameliorate Sucrose-Induced Blood Pressure Elevations in Spontaneously Hypertensive Rats. J. Am. Coll. Nutr. 2006, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, D.G.; Kitsios, A.-P.; Koutoulis, A.S.; Malisova, O.; Karabagias, I.K. Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties. Antioxidants 2024, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Ban, Q.; Liu, Z.; Yu, C.; Sun, X.; Jiang, Y.; Cheng, J.; Guo, M. Physiochemical, rheological, microstructural, and antioxidant properties of yogurt using monk fruit extract as a sweetener. J. Dairy Sci. 2020, 103, 10006–10014. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Zhang, H.; Wang, Y.; Hu, P. Development of a Process for Separation of Mogroside V from Siraitia grosvenorii by Macroporous Resins. Molecules 2011, 16, 7288–7301. [Google Scholar] [CrossRef]
- Ban, Q.; Cheng, J.; Sun, X.; Jiang, Y.; Zhao, S.; Song, X.; Guo, M. Effects of a synbiotic yogurt using monk fruit extract as sweetener on glucose regulation and gut microbiota in rats with type 2 diabetes mellitus. J. Dairy Sci. 2020, 103, 2956–2968. [Google Scholar] [CrossRef]
- Buchilina, A.; Aryana, K. Physicochemical and microbiological characteristics of camel milk yogurt as influenced by monk fruit sweetener. J. Dairy Sci. 2021, 104, 1484–1493. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Wimalasiri, K.M.; Silva, K.F.; Ajlouni, S. Comparison of Properties of New Sources of Partially Purified Inulin to Those of Commercially Pure Chicory Inulin. J. Food Sci. 2015, 80, C950–C960. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Rashid, S. Functional and therapeutic potential of inulin: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 59, 1–13. [Google Scholar] [CrossRef]
- Ávila-Fernández, Á.; Galicia-Lagunas, N.; Rodríguez-Alegría, M.E.; Olvera, C.; López-Munguía, A. Production of functional oligosaccharides through limited acid hydrolysis of agave fructans. Food Chem. 2011, 129, 380–386. [Google Scholar] [CrossRef]
- Galvan, D.; Effting, L.; Cremasco, H.; Conte-Junior, C.A. Recent Applications of Mixture Designs in Beverages, Foods, and Pharmaceutical Health: A Systematic Review and Meta-Analysis. Foods 2021, 10, 1941. [Google Scholar] [CrossRef]
- Hill, T.; Lewicki, P.; Lewicki, P. Statistics: Methods and Applications: A Comprehensive Reference for Science, Industry, and Data Mining; StatSoft, Inc.: Tulsa, OK, USA, 2006. [Google Scholar]
- Anastácio, A.; de Carvalho, I.S. Development of a beverage benchtop prototype based on sweet potato peels: Optimization of antioxidant activity by a mixture design. Int. J. Food Sci. Nutr. 2016, 67, 496–506. [Google Scholar] [CrossRef]
- Santos, J.S.; Deolindo, C.T.P.; Hoffmann, J.F.; Chaves, F.C.; do Prado-Silva, L.; Sant’ANa, A.S.; Azevedo, L.; Carmo, M.A.V.D.; Granato, D. Optimized Camellia sinensis var. sinensis, Ilex paraguariensis, and Aspalathus linearis blend presents high antioxidant and antiproliferative activities in a beverage model. Food Chem. 2018, 254, 348–358. [Google Scholar] [CrossRef]
- Liu, X. Research on Function Optimization of Electric Vehicle Charging Stations Based on User Demand Analysis: An Empirical Study Using the Kano Model and AHP Method. Sustainability 2024, 16, 10783. [Google Scholar] [CrossRef]
- Mkpojiogu, E.O.C.; Hashim, N.L. Understanding the relationship between Kano model’s customer satisfaction scores and self-stated requirements importance. SpringerPlus 2016, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Daffa, V.K.; Romulo, A. Application of the Kano model to identify important sensory attributes of bubble tea drinks accompanied with boba pearl made from purple sweet potato and sugar palm fruit. IOP Conf. Ser. Earth Environ. Sci. 2024, 1338, 012028. [Google Scholar] [CrossRef]
- Setiawan, L.; Romulo, A. Utilization of Kano Model and Quality Function Deployment to Identify Key Attributes for Developing Sorghum Functional Beverages. IOP Conf. Ser. Earth Environ. Sci. 2024, 1413, 012076. [Google Scholar] [CrossRef]
- Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. Foods 2020, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Orsat, V. Optimization of extraction parameters for preparation of Cicer arietinumn-based beverage using Response Surface Methodology. J. Food Process. Preserv. 2022, 46, e16428. [Google Scholar] [CrossRef]
- Yenrina, R.; Anggraini, T.; Effendi, M.A.B. The characteristics of rosella flower (Hibiscus sabdariffa) functional drink addition with red ginger (Zingiber officinale) extract. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 012060. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Villegas-Ochoa, A.M.; Sáyago-Ayerdi, S.G.; Wall-Medrano, A.; González-Córdova, A.; González-Aguilar, A.G. Formulation and characterization of an optimized functional beverage from hibiscus (Hibiscus sabdariffa L.) and green tea (Camellia sinensis L.). Food Sci. Technol. Int. 2019, 25, 547–561. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; la Peña, M.M.-D.; Welti-Chanes, J.; Guerrero-Beltrán, J.Á. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Salas-Millán, J.Á.; Conesa-Bueno, A.; Aguayo, E. A novel antidiabetic lactofermented beverage from agro-industrial waste (broccoli leaves): Process optimisation, phytochemical characterisation, and shelf-life through thermal treatment and high hydrostatic pressure. Food Biosci. 2024, 59, 103999. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Watts, B.M.; Ylimaki, G.; Jeffery, L.; Elías, L. Métodos Sensoriales Básicos Para la Evaluación de Alimentos; CIID: Ottawa, ON, Canada, 1992; pp. 8–10. [Google Scholar]
- García-Ramón, F.; Sotelo-Méndez, A.; Alvarez-Chancasanampa, H.; Norabuena, E.; Sumarriva, L.; Yachi, K.; Huamán, T.G.; Vega, M.N.; Cornelio-Santiago, H.P. Influence of Peruvian Andean grain flours on the nutritional, rheological, physical, and sensory properties of sliced bread. Front. Sustain. Food Syst. 2023, 7, 1202322. [Google Scholar] [CrossRef]
- Filla, M.C.; Garcia, S.; Prudencio, S.H. Mixed Beverage of Fruits and Vegetables: Effect of Refrigerated Storage on Antioxidant Capacity and Acceptance. J. Culin. Sci. Technol. 2017, 16, 237–253. [Google Scholar] [CrossRef]
- Peerkhan, N.; Pandey, M.; Bhandari, Y. Formulation of prebiotic, low glycemic index millet soups using foxtail, barnyard and kodo millet. Discov. Food 2024, 4, 62. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: San Diego, CA, USA, 1980. [Google Scholar]
- Megazyme, K. Sucrose, D-Fructose and D-Glucose Assay Procedure; Megazyme International: Bray, Ireland, 2018. [Google Scholar]
- Wimarnaya, V.W.; Fauza, G.; Prasetyo, H.; Muhammad, D.R.A.; Affandi, D.R.; Ariviani, S. Analysis of Customer Needs for Food Products Using Kano Model, A Case Study of Steamed Brownies. IOP Conf. Ser. Earth Environ. Sci. 2021, 828, 012057. [Google Scholar] [CrossRef]
- Surya, L.a.R. Development of ready-to-drink almond milk coffee as a novel alternative of vegan food. Earth Environ. Sci. IOP Publ. 2023, 1241, 012083. [Google Scholar]
- Allwin; Aurellia, C.A.; Romulo, A. The Utilization of The Kano Model for Development of Edible Spoon. E3S Web Conf. 2023, 388, 01016. [Google Scholar] [CrossRef]
- Belmonte-Herrera, B.H.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Valenzuela-Melendres, M.; Tortoledo-Ortiz, O.; González-Aguilar, G.A. Optimization and In Vitro Digestion of a Guava (Psidium guajava), Mamey (Pouteria sapota) and Stevia (Stevia rebaudiana) Functional Beverage. Foods 2023, 13, 142. [Google Scholar] [CrossRef]
- Sattar, S.; Ahmad, T.; Nisa, M.; Imran, M.; Holmes, M.; Maycock, J.; Nadeem, M.; Khan, M.K. Microwave processing impact on physicochemical and bioactive attributes of optimized peach functional beverage. J. Food Process. Preserv. 2019, 43, e13952. [Google Scholar] [CrossRef]
- Arya, S.; Shakya, N.K. High fiber, low glycaemic index (GI) prebiotic multigrain functional beverage from barnyard, foxtail and kodo millet. LWT 2021, 135, 109991. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Falcone, P.M. Application of mixture design methodology for development of high antioxidant fruity functional beverage. Food Sci. Nutr. 2022, 10, 2245–2254. [Google Scholar] [CrossRef]
- Fakhri, L.A.; Ghanbarzadeh, B.; Falcone, P.M. Development of a Novel Low-Calorie Lime Juice-Based Prebiotic Beverage Using a Combined Design Optimization Methodology. Foods 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Diniz do Nascimento, L.; De Moraes, A.A.B.; Da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.D.A.; De Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, H.; Francis, N.; Santhakumar, A.B.; Blanchard, C.L.; Chinkwo, K.A. The antioxidant and anticancer properties of chickpea water and chickpea polyphenol extracts in vitro. Cereal Chem. 2023, 100, 895–903. [Google Scholar] [CrossRef]
- Shang, H.-M.; Zhou, H.-Z.; Yang, J.-Y.; Li, R.; Song, H.; Wu, H.-X.; Agbor, G. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Cao, W.; Yuan, Y.; Wang, Y.; Zhang, X.; Chen, Y.; Yiasmin, M.N.; Tristanto, N.A.; Hua, X. Recent advancements in mogrosides: A review on biological activities, synthetic biology, and applications in the food industry. Food Chem. 2024, 449, 139277. [Google Scholar] [CrossRef]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free. Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening Methods to Measure Antioxidant Activity of Sorghum (Sorghum bicolor) and Sorghum Products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- Giavoni, M.; Villanueva-Suárez, M.J.; De la Peña-Armada, R.; Garcia-Alonso, A.; Mateos-Aparicio, I. Pasteurization Modifies the Sensorial Attributes and Nutritional Profile of Orange Pulp By-Product. Foods 2022, 11, 1973. [Google Scholar] [CrossRef] [PubMed]
- Hani, N.F.M.; Zaiton, H.; Faridah, H.; Norlelawati, A. Physico-chemical properties and sensory acceptance of mixed drinks of red cabbage (Brassica oleracea L.) and roselle (Hibiscus sabdariffa L.) extracts. Int. Food Res. J. 2019, 26, 671–677. [Google Scholar]
- Rios-Corripio, G.; Welti-Chanes, J.; Rodríguez-Martínez, V.; Guerrero-Beltrán, J.Á. High hydrostatic pressure processing of fresh juice and a fermented beverage of black cherry (Prunus serotina). J. Agric. Food Res. 2023, 15, 100937. [Google Scholar] [CrossRef]
- Salar, F.J.; Periago, P.M.; Agulló, V.; García-Viguera, C.; Fernández, P.S. High Hydrostatic Pressure vs. Thermal Pasteurization: The Effect on the Bioactive Compound Profile of a Citrus Maqui Beverage. Foods 2021, 10, 2416. [Google Scholar] [CrossRef]
- Al, H. Organic acids composition of different parts of the medicinal plant–roselle (Hibiscus sabdariffa). Int. J. Biol. Pharm. Res. 2015, 6, 808–8013. [Google Scholar]
- Mesquita, M.C.; Mendonça, M.A.; Botelho, R.B.A.; Arruda, S.F.; Leandro, E.d.S.; Khaliq, G. Development of a plant-based dessert using araticum pulp and chickpea extract: Physicochemical, microbiological, antioxidant, and sensory characterization. PLoS ONE 2024, 19, e0307640. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Panel on the Definition of Dietary Fiber and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Appendix C Development and Evolution of Methods Used to Extract and Measure Dietary Fiber 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK223584/ (accessed on 2 May 2025).
- Jung, S.E.; Shin, Y.H.; Severt, K.; Crowe-White, K.M. Determinants of a Consumer’s Intention to Consume Antioxidant-infused Sugar-free Chewing Gum: Measuring Taste, Attitude, and Health Consciousness. J. Food Prod. Mark. 2020, 26, 38–54. [Google Scholar] [CrossRef]
- Harwood, W.S.; Drake, M. Application of temporal penalty analysis for the optimization of sugar reduction in protein beverages. J. Sens. Stud. 2021, 36, e12644. [Google Scholar] [CrossRef]
- Rovai, D.; Watson, M.; Barbano, D.; Drake, M. Consumer acceptance of protein beverage ingredients: Less is more. J. Dairy Sci. 2025, 108, 1392–1407. [Google Scholar] [CrossRef]
- Vallath, A.; Shanmugam, A. Study on model plant based functional beverage emulsion (non-dairy) using ultrasound—A physicochemical and functional characterization. Ultrason. Sonochem. 2022, 88, 106070. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.C.; Filho, J.G.d.O.; Santana, A.C.A.; de Freitas, B.S.M.; Silva, F.G.; Takeuchi, K.P.; Egea, M.B. Optimization of soymilk fermentation with kefir and the addition of inulin: Physicochemical, sensory and technological characteristics. LWT 2019, 104, 30–37. [Google Scholar] [CrossRef]
- Sinela, A.M.; Mertz, C.; Achir, N.; Rawat, N.; Vidot, K.; Fulcrand, H.; Dornier, M. Exploration of reaction mechanisms of anthocyanin degradation in a roselle extract through kinetic studies on formulated model media. Food Chem. 2017, 235, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-Y.; Zhou, Z.-Y.; Zhao, J.-C.; Li, F.-H.; Zeng, K.-F.; Ming, J. The interactions between proteins and anthocyanins based on covalent/non-covalent binding: A review. Food Ferment. Ind. 2022, 48, 293–299. [Google Scholar]
- Sriharti; Andriansyah, R.C.E.; Agustina, W.; Indriati, A.; Litaay, C.; Luthfiyanti, R.; Mayasti, N.K.I.; Triyono, A.; Tribowo, R.I.; Purwandoko, P.B. Optimization of herbal tea drink formula based on aloe vera rind (Aloe barbadensis miller). Food Sci. Technol. 2022, 42, e69022. [Google Scholar] [CrossRef]
- Lakićević, S.H.; Karabegović, I.T.; Cvetković, D.J.; Lazić, M.L.; Jančić, R.; Popović-Djordjević, J.B. Insight into the Aroma Profile and Sensory Characteristics of ‘Prokupac’ Red Wine Aromatised with Medicinal Herbs. Horticulturae 2022, 8, 277. [Google Scholar] [CrossRef]
- Gunes-Bayir, A.; Bilgin, M.G.; Guclu, D.; Pogda, S.; Dadak, A. Preparation and evaluation of novel functional fermented dairy products containing propolis and cinnamon. J. Food Sci. Technol. 2021, 59, 2392–2401. [Google Scholar] [CrossRef]
- Ouyang, Y.; Behnke, C.; Almanza, B.; Ghiselli, R. The Influence of Food Aromas on Restaurant Consumer Emotions, Perceptions, and Purchases. J. Hosp. Mark. Manag. 2017, 27, 405–423. [Google Scholar] [CrossRef]
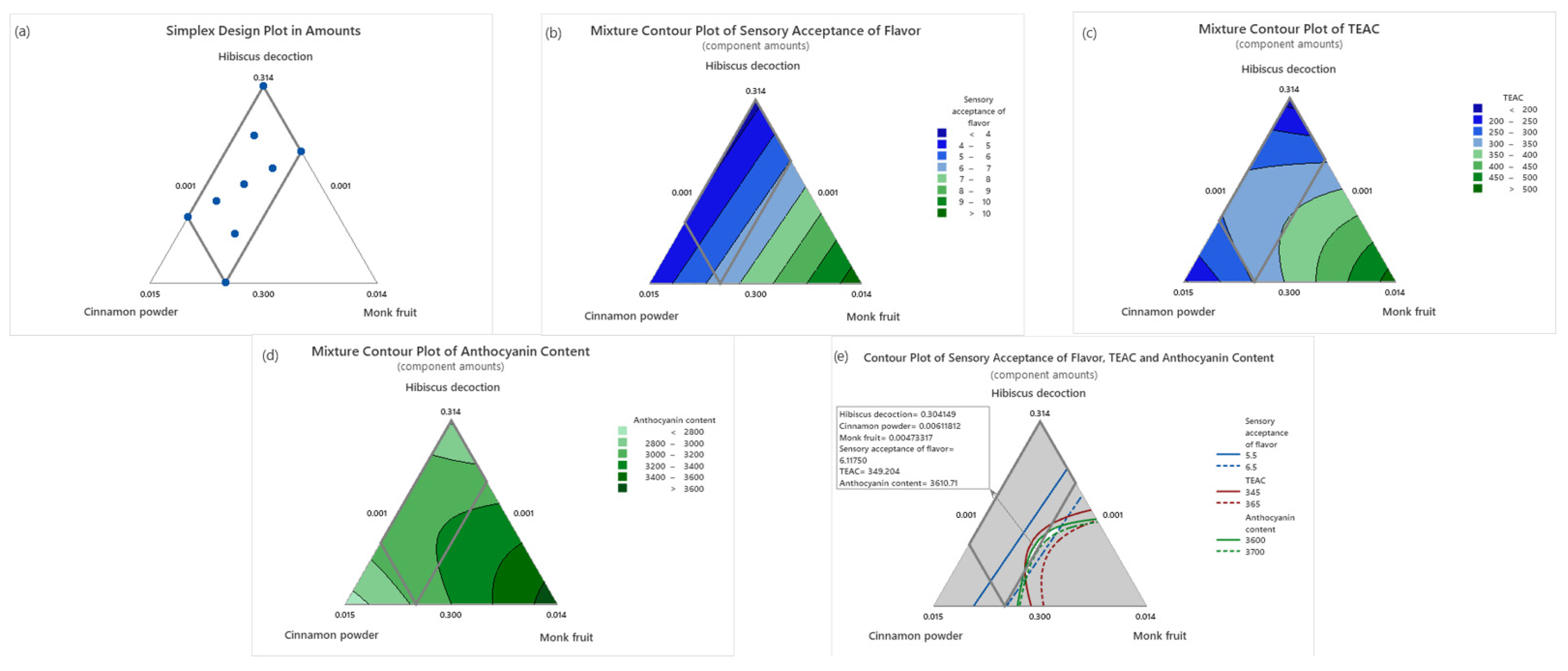
| Run Order | Process Variables | Response Variables | ||||
|---|---|---|---|---|---|---|
| HD (%) | Cinnamon Powder (%) | Monk Fruit (%) | Sensory Acceptance of Flavor 1 | TEAC 2 (µmol TE/100 mL) | Anthocyanin Content 2 (µgC3GE/100 mL) | |
| 1 | 31.0125 | 0.325 | 0.1625 | 4.3 ± 2 | 281 ± 36 | 3192 ± 113 |
| 2 | 30 | 1 | 0.5 | 6.1 ± 2.1 | 316 ± 39 | 3162 ± 34 |
| 3 | 31.35 | 0.1 | 0.05 | 3.6 ± 2.8 | 177 ± 16 | 2838 ± 120 |
| 4 | 30.9 | 0.1 | 0.5 | 5.9 ± 2.3 | 286 ± 25 | 2716 ± 16 |
| 5 | 30.5625 | 0.775 | 0.1625 | 5.0 ± 2.5 | 315 ± 19 | 3095 ± 73 |
| 6 | 30.45 | 1 | 0.05 | 3.9 ± 2.3 | 282 ± 23 | 2738 ± 67 |
| 7 | 30.7875 | 0.325 | 0.3875 | 5.8 ± 2.1 | 303 ± 19 | 3192 ± 52 |
| 8 | 30.6750 | 0.550 | 0.2750 | 5.1 ± 2.5 | 354 ± 29 | 3514 ± 122 |
| 9 | 30.3375 | 0.775 | 0.3875 | 6 ± 1.9 | 315 ± 20 | 3174 ± 57 |
| Variable | Predicted Value 1,* | 95% CI | Experimental Value 2,* | p |
|---|---|---|---|---|
| Sensory acceptance of flavor | 6.11 | (5.5, 6.6) | 6.2 | p > 0.05 |
| TEAC (µmol TE/100 mL) | 349.2 | (301.9, 356.5) | 329.2 | p > 0.05 |
| Anthocyanin content (µgC3GE/100 mL) | 3610.7 | (3515.6, 3618.4) | 3567 | p > 0.05 |
| Component | Content (g/100 g of Beverage) |
|---|---|
| Moisture | 93.15 ± 0.1 |
| Ash | 0.189 ± 0.004 |
| Crude protein | 0.92 ± 0.01 |
| Fat | 0.16 ± 0.04 |
| Insoluble dietary fiber | 0.73 ± 0.14 |
| Soluble dietary fiber | 0.49 ± 0.09 |
| Available carbohydrates (By difference) | 4.36 |
| Total starch | 1.72 ± 0.01 |
| Glucose | 0.10 ± 0.01 |
| Fructose | 0.12 ± 0.01 |
| Sucrose | 0.11 ± 0.006 |
| Attributes | A | O | M | I | R | Q | Category | SI | DI |
|---|---|---|---|---|---|---|---|---|---|
| Good flavor | 27 | 4 | 2 | 25 | 1 | 1 | A | 0.53 | 0.10 |
| Functional proprieties | 37 | 16 | 2 | 5 | 0 | 0 | A | 0.88 | 0.30 |
| Sweetened with monk fruit | 26 | 5 | 7 | 14 | 4 | 1 | A | 0.56 | 0.22 |
| Chickpeas as an ingredient for plant-based beverages | 26 | 3 | 1 | 26 | 4 | 0 | A * | 0.52 | 0.07 |
| Presence of natural sediments in the beverage | 14 | 2 | 3 | 26 | 12 | 3 | I | 0.36 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cardoso, F.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; de la Rocha, R.V.; Cabanillas-Bojórquez, L.A.; Camberos-Barraza, J.; Cárdenas-Torres, F.I.; Heredia, J.B. Mixture Design and Kano Model for a Functional Chickpea and Hibiscus Beverage. Beverages 2025, 11, 112. https://doi.org/10.3390/beverages11040112
López-Cardoso F, Leyva-López N, Gutiérrez-Grijalva EP, de la Rocha RV, Cabanillas-Bojórquez LA, Camberos-Barraza J, Cárdenas-Torres FI, Heredia JB. Mixture Design and Kano Model for a Functional Chickpea and Hibiscus Beverage. Beverages. 2025; 11(4):112. https://doi.org/10.3390/beverages11040112
Chicago/Turabian StyleLópez-Cardoso, Fernando, Nayely Leyva-López, Erick Paul Gutiérrez-Grijalva, Rosabel Vélez de la Rocha, Luis Angel Cabanillas-Bojórquez, Josué Camberos-Barraza, Feliznando Isidro Cárdenas-Torres, and José Basilio Heredia. 2025. "Mixture Design and Kano Model for a Functional Chickpea and Hibiscus Beverage" Beverages 11, no. 4: 112. https://doi.org/10.3390/beverages11040112
APA StyleLópez-Cardoso, F., Leyva-López, N., Gutiérrez-Grijalva, E. P., de la Rocha, R. V., Cabanillas-Bojórquez, L. A., Camberos-Barraza, J., Cárdenas-Torres, F. I., & Heredia, J. B. (2025). Mixture Design and Kano Model for a Functional Chickpea and Hibiscus Beverage. Beverages, 11(4), 112. https://doi.org/10.3390/beverages11040112










