Comparison and Study on Flavor and Quality Characteristics of Different Grades of Tianshanhong (TSH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tea Samples
2.3. Sensory Evaluation
2.4. Detection and Analysis of Non-Volatile Compounds by UPLC-MS
2.4.1. Catechin and Caffeine Analysis
2.4.2. Free Amino Acid Analysis
2.5. Detection and Analysis of Volatile Compounds by GC–MS
2.5.1. Sample Preparation and Treatment
2.5.2. GC-MS Conditions
2.6. Statistical Analysis
3. Results
3.1. The Sensory Evaluation of Different Grades of TSH
3.2. The Analysis of Catechins and Caffeine Content Between Different Grades of TSH
3.3. The Analysis of AA Content Between Different Grades of TSH
3.4. The Analysis of Volatile Between Different Grades of TSH
3.5. The Association Analysis of Non-Volatile and Volatile Compounds Between Different Grades of TSH
4. Discussion
4.1. The Selection of Non-Volatile Compounds and Their Correlation Between Different TSH Grades
4.2. The Selection of Volatile Compounds and Their Correlation Between Different TSH Grades
5. Conclusions
- (1)
- TSH samples generally exhibit linear correlations with the contents of non-volatile compounds (including amino acids and ester-type catechins). Thea, Arg, and GABA show significant differences across grades and serve as potential biomarker components for MKH.
- (2)
- Methyl salicylate and linalool were the most abundant volatile compounds in higher-grade TSH, contributing to the formation of delicate sweetness and floral notes. These compounds not only showed significant correlations with tea grades but also played crucial roles in developing the persistent sweet aroma with floral characteristics in premium-grade TSH.
- (3)
- The cultivar, harvesting season, and maturity of fresh tea leaves were closely associated with TSH grades and flavor profiles, which may represent the primary factors contributing to the differential accumulation of flavor metabolites across various grades.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSH | Tianshanhong black tea |
| AAs | Amino acids |
References
- Xu, Y.M.; Qiao, F.B.; Huang, J.K. Black tea markets worldwide: Are they integrated? J. Integr. Agric. 2022, 21, 552–565. [Google Scholar] [CrossRef]
- Ma, J.Q.; Wang, Y.J.; Li, J.Y.; Zhang, J.X.; Wei, Y.M.; Yan, Y.X.; Wang, H.P.; Pan, Y.; Xiong, Z.C.; Wang, R.J.; et al. Aroma formation mechanism by the drying step during Congou black tea processing: Analyses by HP-SPME and SAFE with GC-MS. LWT 2024, 198, 116019. [Google Scholar] [CrossRef]
- Wen, M.C.; Hu, W.; Li, L.; Long, P.P.; Han, Z.S.; Ke, J.P.; Deng, Z.Y.; Zhu, M.T.; Zhang, L. Developed metabolomics approach reveals the non-volatile color-contributing metabolites during Keemun congou black tea processing. Food Chem. 2025, 463, 141222. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Jolvis Pou, K.R. A Review of Withering in the Processing of Black Tea. J. Biosyst. Eng. 2016, 41, 365–372. [Google Scholar] [CrossRef]
- Wang, L.L.; Yang, Y.Q.; Xie, J.L.; Miao, Y.W.; Wang, Q.W.; Jiang, Y.W.; Deng, Y.L.; Tong, H.R.; Yuan, H.B. Evaluation of aroma quality in premium and defective black teas using electronic nose combined with GC-MS technology. Food Sci. 2024, 45, 274–282. (In Chinese) [Google Scholar]
- Xue, T.T. Research on Ningde Tea Industry Development and Targeted Poverty Alleviation Innovation Model. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. (In Chinese). [Google Scholar]
- Zhu, Y.; Fan, X.; Yu, W.; Liu, C.; Wang, P.; Wei, M.; Lu, M.; Lv, S.; Cai, C.; Ye, N. Genetic variation of tea plant in Ningde and its adjacent regions revealed by single nucleotide polymorphism genotyping. Agron. J. 2023, 115, 2829–2842. [Google Scholar] [CrossRef]
- Zheng, K.L.; Fang, C.; Wu, H.X. Quality and processing technology of Tianshanhong Congou black tea. China Tea 2011, 33, 28–29. (In Chinese) [Google Scholar]
- Ouyang, J.; Jiang, R.G.; An, H.M.; Ou, X.C.; Wang, J.; Xie, H.; Fu, W.J.; Zhang, J.; Chen, H.Y.; Liu, Q.; et al. Decoding the specific minty-like aroma of ‘Rucheng baimaocha’ (Camellia pubescens) black tea. Food Chem. X 2025, 26, 102253. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, X.H.; Qu, D.; Huang, W.; He, L.J.; Wan, T.; Zhang, L.J.; Wang, Q.; Hu, C.Y. Determination of geographical origin of Southern Shaanxi Congou black teas using sensory analysis combined with gas chromatography-ion mobility spectrometry. Foods 2024, 13, 3904. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, S.S.; Shen, R.; Huang, J.X.; Beatrice, B.M.; Chen, X.P.; Xie, G.F. Comparative study of volatile compounds and metabolic pathways of Congou black tea from four regions based on sensory evaluation and HS-SPME/GC-MS. Microchem. J. 2024, 205, 111276. [Google Scholar] [CrossRef]
- Long, P.P.; Su, S.X.; Han, Z.S.; Granato, D.; Hu, W.; Ke, J.P.; Zhang, L. The effects of tea plant age on the color, taste, and chemical characteristics of Yunnan Congou black tea by multi-spectral omics insight. Food Chem. X 2024, 21, 101190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Qiao, X.Y.; Wu, H.L.; Cao, J.X.; Chen, D. Study on Aroma Compounds of Black Teas from Five Regions in Sri Lanka by HS-SPME/GC-MS. Food Res. Dev. 2016, 37, 128–133. [Google Scholar]
- Wang, Q.S.; Qin, D.D.; Huang, G.Z.; Jiang, X.H.; Fang, K.X.; Wang, Q.; Ni, E.D.; Li, B.; Pan, C.D.; Li, H.J.; et al. Identification and characterization of the key volatile flavor compounds in black teas from distinct regions worldwide. Food Chem. 2022, 390, 133207. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.N.; Wang, Z.H.; Peng, H.; Li, W.J.; Yang, P.X. UPLC-QTOF/MS-based non-targeted metabolomics coupled with the quality component, QDA, to reveal the taste and metabolite characteristics of six types of Congou black tea. LWT 2023, 185, 115197. [Google Scholar] [CrossRef]
- Niu, Y.W.; Zhang, Y.; Xiao, Z.B.; Zhu, J.C.; Zhang, F.M.; Chen, F. Release effect of aroma compounds of Keemun black tea brewed with deuterium-depleted water with different deuterium content. LWT 2023, 173, 114382. [Google Scholar] [CrossRef]
- Yue, C.N.; Yang, P.X.; Qin, D.D.; Cai, H.L.; Wang, Z.H.; Li, C.; Wu, H.L. Identification of volatile components and analysis of aroma characteristics of Jiangxi Congou black tea. Int. J. Food Prop. 2020, 23, 2160–2173. [Google Scholar] [CrossRef]
- Yue, C.N.; Li, W.J.; Li, C.; Wang, Z.H.; Peng, H.; Yang, P.X. Differential characterization of volatile components and aroma sensory properties of different types of Hehong tea (Congou black tea). Flavour Fragr. J. 2022, 38, 61–72. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Wu, Q.Y.; Chen, L.Q.; Ruan, S.L.; Yang, Z.Y.; Sun, Y.; Aikebaier, R. Comparison of Different Grades of Innovative Tanyang Congou Black Tea (Minkehong) Based on Metabolomics and Sensory Evaluation. Horticulturae 2024, 10, 374. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Y.; Li, J.; Lyu, S.; Wang, Z.; Xie, F.; Luo, Y.; Zhang, F.; Chen, Z.; Sun, W. Relationship between the grade and the characteristic flavor of PCT (Panyong Congou Black Tea). Foods 2022, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.M.; Chen, F.Y.; Liu, G.Y.; Zhang, Q.; Ye, J.H.; Lei, W.X.; Jia, X.L.; He, H.B. Comparative analysis on the quality of Wuyi Rougui (Camellia sinensis) tea with different grades. Food Sci. Technol. 2022, 42, 115321. [Google Scholar] [CrossRef]
- Zeng, L.; Fu, Y.Q.; Liu, Y.Y.; Huang, J.S.; Chen, J.X.; Yin, J.F.; Jin, S.; Sun, W.J.; Xu, Y.Q. Comparative analysis of different grades of Tieguanyin oolong tea based on metabolomics and sensory evaluation. LWT 2023, 174, 114423. [Google Scholar] [CrossRef]
- Fu, X.S.; Xu, L.; Yu, X.P.; Ye, Z.H.; Cui, H.F. Robust and automated internal quality grading of a Chinese green tea (Longjing) by near-infrared spectroscopy and chemometrics. J. Spectrosc. 2013, 2013, 139347. [Google Scholar] [CrossRef]
- Chen, L.Q.; Zhou, Z.W.; Cai, Y.W.; Zhang, Z.H.; Jiang, S.T.; Aikebaier, R.; Chen, Y. Analysis of main chemical components in nine white tea reference materials based on UPLC-MS/MS technology. J. Agric. Sci. Technol. 2024, 26, 50–62. (In Chinese) [Google Scholar] [CrossRef]
- Yue, W.J.; Sun, W.J.; Rao, R.S.P.; Ye, N.X.; Yang, Z.B.; Chen, M.J. Non-targeted metabolomics reveals distinct chemical compositions among different grades of Bai Mudan white tea. Food Chem. 2018, 277, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.P.; Lin, Z.; Zhang, Y.; Liang, Y.R. Comparison of chemical composition and antioxidant activity of Pu-erh tea with different grades. J. Tea Sci. 2013, 33, 386–395. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.D. Primary processing technology of high-aroma Tianshanhong Congou black tea. Fujian Tea 2014, 36, 36–37. (In Chinese) [Google Scholar]
- GB/T 8313-2018; Method for the Determination of Tea Polyphenols and Catechins in Tea. China Standards Press: Beijing, China, 2018. (In Chinese)
- GB/T 23776-2018; Method for Sensory Evaluation of Tea. China Standards Press: Beijing, China, 2018. (In Chinese)
- Chen, S.; Li, M.H.; Zheng, G.Y.; Wang, T.T.; Lin, J.; Wang, S.S.; Wang, X.X.; Chao, Q.L.; Cao, S.X.; Yang, Z.B.; et al. Metabolite Profiling of 14 Wuyi Rock Tea Cultivars Using UPLC-QTOF MS and UPLC-QqQ MS Combined with Chemometrics. Chem. Chem. 2018, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Thibert, V.; Legeay, P.; Chapuis-Hugon, F.; Pichon, V. Molecularly imprinted polymer for the selective extraction of cocaine and its metabolites, benzoylecgonine and ecgonine methyl ester, from biological fluids before LC-MS analysis. J. Chromatogr. B 2014, 949–950, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.Y.; Han, F.R.; Ge, C.L.; Mao, W.K.; Chen, L.; Hu, H.P.; Chen, G.G.; Lang, Q.L.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-H.; Lee, S.-S.; Zhu, Y.-M.; Jin, Z.-Q.; Wu, F.-B.; Qiu, C.-W. Comparative metabolomic profiling reveals key secondary metabolites associated with high quality and nutritional value in broad bean (Vicia faba L.). Molecules 2022, 27, 8995. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, Q.S.; Qin, D.D.; Fang, K.X.; Zhu, H.Y.; Jiang, X.H.; Chen, D.; Wu, H.L. Research progress on black tea quality and related biochemical factors. Food Sci. 2020, 41, 246–253. (In Chinese) [Google Scholar]
- Li, C.; Jiang, X.F.; Cao, H.H.; Zhang, J.G.; Yu, Z.; Ni, D.J.; Dong, C.W.; Jiang, H.Y. Flavor characteristics of Hehong tea (Congou black tea) at different quality grades. Chin. Agric. Sci. Bull. 2023, 39, 151–157. (In Chinese) [Google Scholar]
- Su, D.; Zhu, J.Y.; Li, Y.C.; Qin, M.X.; Lei, Z.D.; Zhou, J.T.; Yu, Z.; Chen, Y.Q.; Zhang, D.; Ni, D.J. Effect of drying temperature on sensory quality, flavor components, and bioactivity of Lichuan black tea processed by Echa No. 10. Molecules 2025, 30, 361. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Tang, Y.; Tan, Z.P.; Ren, J.C.; He, Y.; Huang, H.S. Detection of black tea fermentation quality based on optimized deep neural network and hyperspectral imaging. Infrared Phys. Technol. 2024, 143, 105625. [Google Scholar] [CrossRef]
- Fu, X.M.; Liao, Y.Y.; Cheng, S.H.; Deng, R.F.; Yang, Z.Y. Stable isotope-labeled precursor tracing reveals that L-alanine is converted to L-theanine via L-glutamate not ethylamine in tea plants in vivo. J. Agric. Food Chem. 2021, 69, 15354–15361. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Liu, X.Z.; Ye, M.J.; Xu, J.F.; Gao, H.Y.; Zheng, G.J. Determination of free amino acid components in tea by ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2019, 37, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Chen, J.Y.; Yuan, H.B.; Wang, L.L.; Yang, Y.Q.; Jiang, Y.W. Evaluation of non-volatile differential components in Dianhong tea infusions across different grades using electronic tongue and metabolomics. J. Chin. Inst. Food Sci. Technol. 2024, 24, 354–368. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.J.; Hua, J.J.; Jiang, Y.W.; Wang, J.J.; Yuan, H.B. Effects of full-fire heat transfer method on quality components, color and taste of Congou black tea. Food Sci. 2020, 41, 148–157. (In Chinese) [Google Scholar]
- Xiao, H.; Xiao, W.X. Formation mechanism and enrichment technology of γ-aminobutyric acid in tea. Fujian Tea 2003, 2, 33–35. (In Chinese) [Google Scholar]
- Chen, L.; Shi, Y.X.; Sun, J.Y.; Wang, H.J.; Wang, Y.F.; Shu, Z.F.; He, W.Z.; Dong, C.W.; Xu, P. Deciphering the flavor profile and seasonal variation of black tea processed from cultivar ‘Baiye 1’. Food Res. Int. 2025, 208, 116181. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Ye, Z.B.; Wu, Y.D.; Yu, T.Z.; Zhao, W.; Qi, Z.H.; Jiang, Y.Q.; Liu, Q.Y.; Lu, G.F.; Ning, J.M. Evaluation of taste quality of Keemun congou black tea during ripening and the effect of this quality on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase. Food Chem. X 2025, 26, 102264. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiao, Y.; Jiang, X.; Li, C.; Sun, W.; Chen, Y.; Yu, Z.; Ni, D. Effects of Sun Withering Degree on Black Tea Quality Revealed via Non-Targeted Metabolomics. Foods 2023, 12, 2430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Yang, J.H.; Liu, Y.Q.; Wang, H.; Xu, Y.J.; Huang, J.Q.; Lei, P.D. Analysis of the Contribution of Stems and Leaves to the Quality of Keemun Black Tea. Food Sci. 2023, 44, 220–228. [Google Scholar]
- Wu, Q.Y.; Zhou, Z.W.; Zhang, Y.N.; Huang, H.Q.; Ou, X.X.; Sun, Y. Identification of key components responsible for the aromatic quality of jinmudan black tea by means of molecular sensory science. Foods 2023, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Xia, T. Secondary Metabolism of Tea Plant, 1st ed.; Science Press: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Wan, X.C. Biochemistry of Tea; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Liu, C.; Wang, C.; Zheng, T.T.; Zhao, M.M.; Gong, W.Y.; Wang, Q.M.; Yan, L.; Zhang, W.J. Characterization of key odor-active compounds in sun-dried black tea by sensory and instrumental-directed flavor analysis. Foods 2022, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Liu, Y.N.; Fan, Y.L.; Xu, T.X.; Zhang, L.; Li, J.; Yao, L.P.; Gao, Y.X. Comparative analysis of aroma component variations in Ninghong Golden Hao tea across different storage years. Mod. Food Sci. Technol. 2023, 39, 271–280. (In Chinese) [Google Scholar]
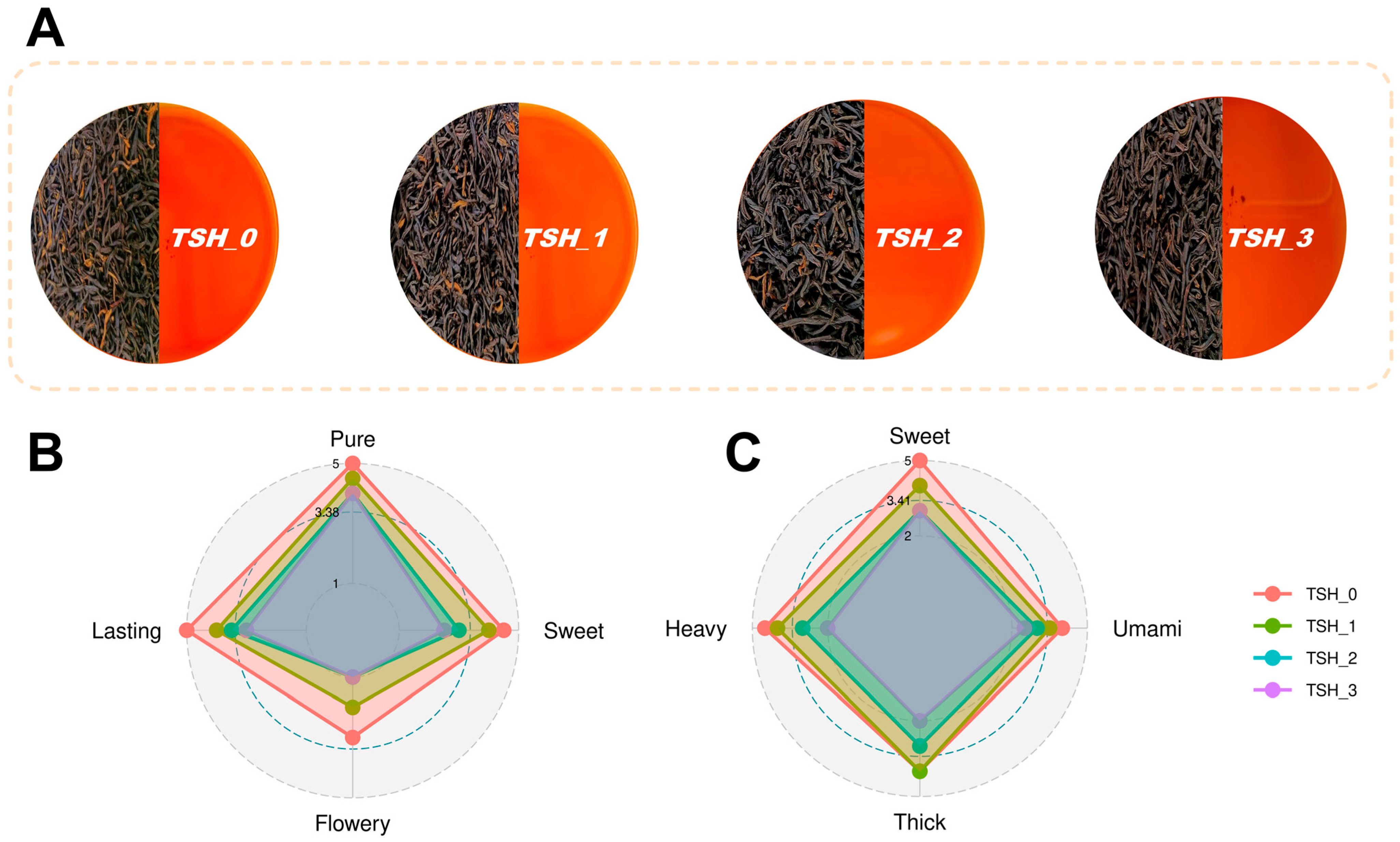
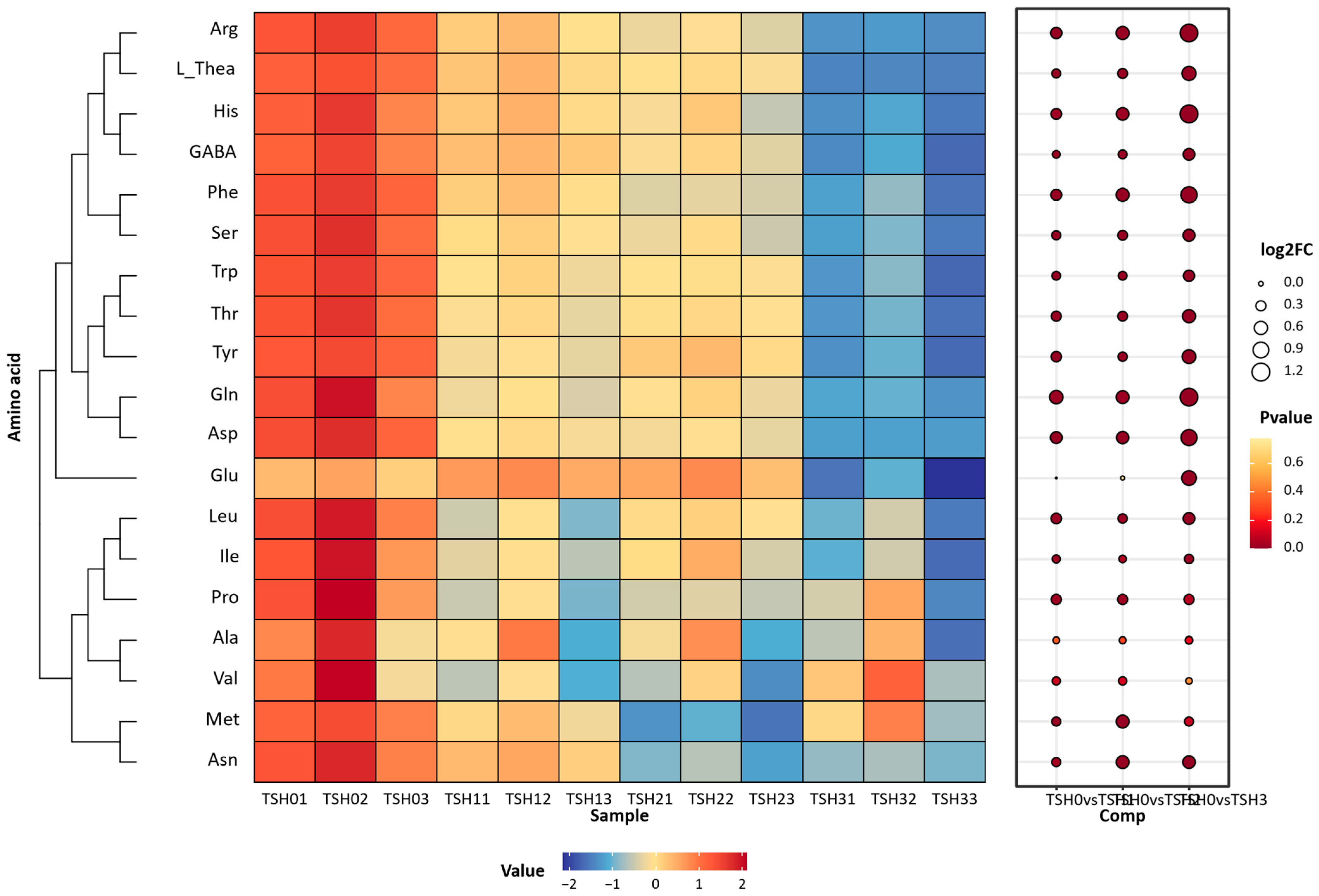
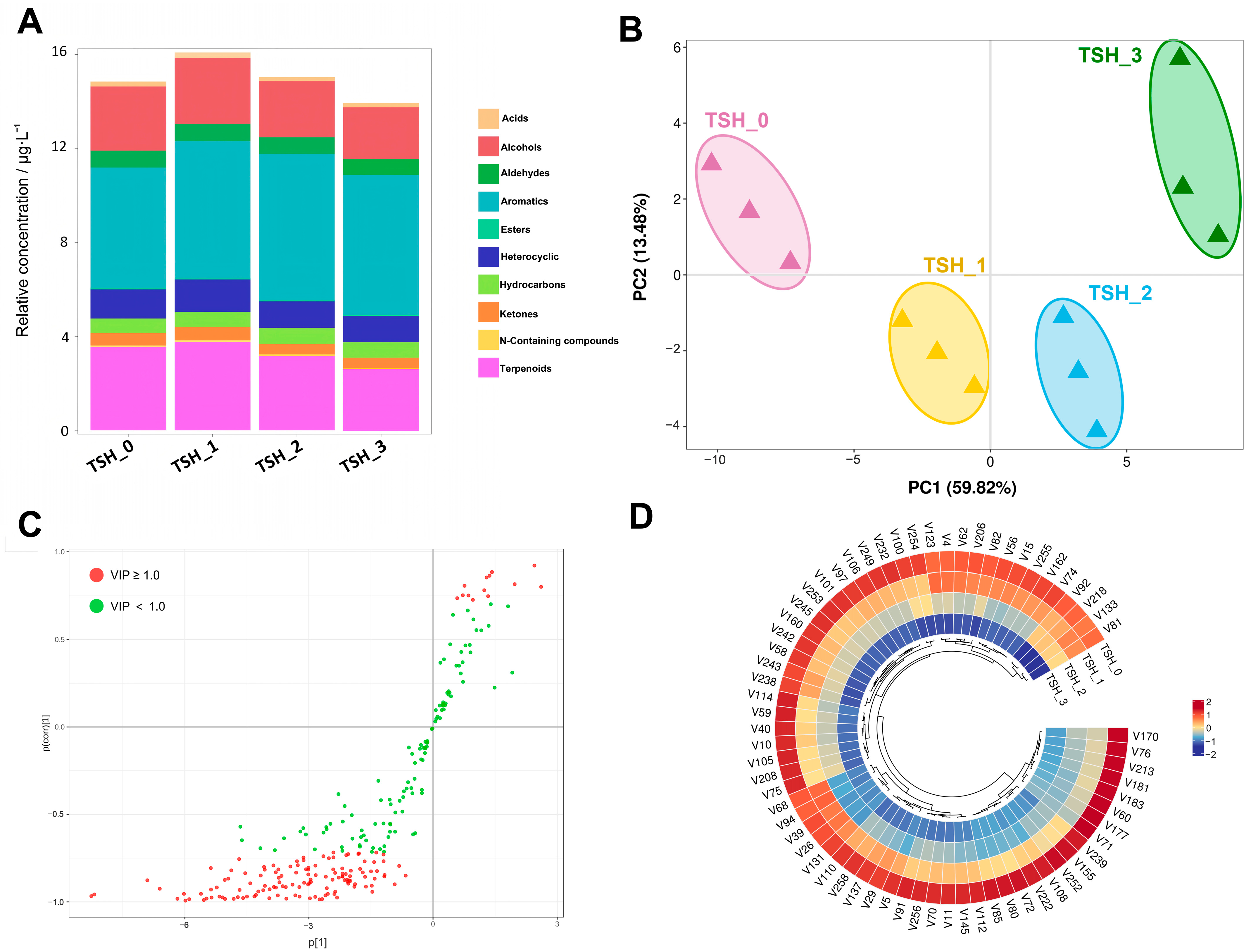

| Compound/mg/g | TSH-0 | TSH-1 | TSH-2 | TSH-3 |
|---|---|---|---|---|
| EGCG | 56.60 ± 4.01 a | 47.81 ± 1.13 b | 50.28 ± 1.07 b | 49.99 ± 0.80 b |
| ECG | 84.30 ± 5.09 a | 68.56 ± 2.13 b | 64.60 ± 3.76 b | 58.14 ± 2.45 c |
| C | 11.12 ± 0.66 a | 10.95 ± 0.25 a | 10.82 ± 0.28 a | 10.52 ± 0.40 a |
| EC | 8.47 ± 0.42 a | 8.06 ± 0.50 a | 8.39 ± 0.69 a | 7.60 ± 0.76 a |
| EGC | 3.49 ± 0.20 b | 3.94 ± 0.55 b | 5.97 ± 0.65 a | 6.57 ± 0.36 a |
| Caffeine | 27.90 ± 1.45 a | 28.32 ± 1.62 a | 25.38 ± 0.63 a | 28.07 ± 0.69 a |
| No | Compounds | RI | Nist_RI | Class | Odor Description | Relative Mean Content (μg/g) | |||
|---|---|---|---|---|---|---|---|---|---|
| TSH-0 | TSH-1 | TSH-2 | TSH-3 | ||||||
| V4 | Hexenoic acid | 964 | 995.61 | Acids | Fruity, fat | 0.0194 ± 0.0007 | 0.0191 ± 0.0024 | 0.0152 ± 0.0006 | 0.0108 ± 0.0011 |
| V5 | (Aminooxy)-acetic acid | 895 | 1015.83 | Acids | Vinegar | 0.0736 ± 0.0039 | 0.0649 ± 0.0057 | 0.0534 ± 0.0026 | 0.0511 ± 0.0093 |
| V10 | (3R,6S)-2,2,6-Trimethyl-6-vinyltetrahydro-2H-pyran-3-ol | 1255 | 1174.86 | Alcohols | Citrus, green | 0.4491 ± 0.0048 | 0.375 ± 0.0111 | 0.3396 ± 0.0017 | 0.2897 ± 0.0132 |
| V11 | α-Terpineol | 1143 | 1193.42 | Alcohols | Pine, iris, teil | 0.0325 ± 0.001 | 0.0281 ± 0.0014 | 0.025 ± 0.0003 | 0.0227 ± 0.0012 |
| V15 | 2,6-Cyclooctadien-1-ol | 1112 | 1069.81 | Alcohols | — | 0.0056 ± 0.0001 | 0.0053 ± 0.0002 | 0.0045 ± 0.0005 | 0.0041 ± 0.0003 |
| V26 | 2-Chloro-(E)-cyclopentanol | 954 | 864.30 | Alcohols | — | 0.0091 ± 0.0001 | 0.0084 ± 0.0004 | 0.0048 ± 0.0003 | 0.0045 ± 0.0006 |
| V29 | Phenylethyl alcohol 60 μg/kg | 1136 | 1113.84 | Alcohols | Fruity, rose, sweet, | 1.1668 ± 0.0096 | 1.0623 ± 0.0529 | 0.879 ± 0.0203 | 0.8041 ± 0.069 |
| V39 | α-Ethylidene-benzeneacetaldehyde | 1265 | 1275.90 | Aldehydes | — | 0.0301 ± 0.0015 | 0.0278 ± 0.0015 | 0.0175 ± 0.0004 | 0.0174 ± 0.0016 |
| V40 | 4-(1,1-Dimethylethyl)-benzenepropanal | 1508 | 1218.47 | Aldehydes | Flowery | 0.0056 ± 0.0002 | 0.0046 ± 0.0001 | 0.0042 ± 0.0001 | 0.0036 ± 0.0001 |
| V56 | 1-Ethyl-4-methoxy-benzene | 1082 | 1089.60 | Aromatics | — | 0.0286 ± 0.0009 | 0.0267 ± 0.001 | 0.0222 ± 0.0002 | 0.0191 ± 0.0043 |
| V58 | 4-Ethenyl-1,2-dimethyl-benzene | 1110 | 1090.17 | Aromatics | Aromatic | 0.0097 ± 0.0003 | 0.0085 ± 0.0002 | 0.008 ± 0.0002 | 0.0066 ± 0.0007 |
| V59 | Butoxy-benzene | 1168 | 1221.64 | Aromatics | — | 0.0038 ± 0.0001 | 0.0029 ± 0.0001 | 0.0027 ± 0.0001 | 0.0022 ± 0.0001 |
| V60 | Butylated hydroxytoluene | 1668 | 1517.22 | Aromatics | — | 0.0528 ± 0.0044 | 0.0363 ± 0.0024 | 0.0336 ± 0.0025 | 0.0323 ± 0.0034 |
| V62 | Naphthalene | 1231 | 1187.97 | Aromatics | Pungent, dry, tarry | 0.0766 ± 0.0012 | 0.0741 ± 0.0021 | 0.0621 ± 0.0023 | 0.0521 ± 0.0022 |
| V68 | Toluene | 794 | 751.23 | Aromatics | Sweet | 0.0078 ± 0.0002 | 0.0076 ± 0.0001 | 0.006 ± 0.0003 | 0.0059 ± 0.0003 |
| V70 | 1,3-oxazole-4-carboxylic acid, 4,5-dihydro-5-(1-methylethyl)-, ethyl ester | 1232 | 1375.78 | Esters | — | 0.0046 ± 0.0001 | 0.0038 ± 0.0004 | 0.0033 ± 0.0005 | 0.0028 ± 0.0003 |
| V72 | 1H-Indene-1-methanol, acetate | 1456 | 1336.18 | Esters | Aromatic | 0.0023 ± 0.0002 | 0.0016 ± 0.0001 | 0.0012 ± 0.0004 | 0.0011 ± 0.0001 |
| V74 | (E,Z)-2-Butenoic acid, 3-hexenyl ester | 1199 | 1234.46 | Esters | Green, fruity | 0.0066 ± 0.0004 | 0.0057 ± 0.0003 | 0.0042 ± 0.0004 | 0.003 ± 0.0004 |
| V75 | 2-Cyclohexyl-1,3-dioxolane-4,5-dicarboxylic acid, dimethyl ester | 1855 | 1517.70 | Esters | — | 0.002 ± 0.0001 | 0.0015 ± 0.0001 | 0.0014 ± 0.0001 | 0.0011 ± 0.0004 |
| V76 | 2-Ethylbutyric acid, tetrahydrofurfuryl ester | 1349 | 1349.75 | Esters | — | 0.0022 ± 0.0002 | 0.0016 ± 0.0001 | 0.0015 ± 0.0001 | 0.0014 ± 0.0001 |
| V80 | 2-propenoic acid, 2-methyl-, 4-formylphenyl ester | 1528 | 1389.34 | Esters | — | 0.013 ± 0.001 | 0.0095 ± 0.0008 | 0.0076 ± 0.0001 | 0.0072 ± 0.001 |
| V81 | (Z)-3-Hexen-1-ol, formate | 989 | 852.53 | Esters | Green, waxy, fresh, fruity | 0.0524 ± 0.0025 | 0.0505 ± 0.0019 | 0.0459 ± 0.0018 | 0.0322 ± 0.0003 |
| V82 | (Z)-3-Hexen-1-ol, propanoate | 1091 | 1231.66 | Esters | Green, fresh, fruity | 0.0205 ± 0.0001 | 0.019 ± 0.001 | 0.0154 ± 0.0002 | 0.0099 ± 0.0014 |
| V85 | 5-Methyl-2-(1-methylethenyl)-4-hexen-1-ol, acetate | 1270 | 1382.98 | Esters | Fresh, green | 0.0346 ± 0.003 | 0.026 ± 0.0021 | 0.021 ± 0.0015 | 0.0202 ± 0.0018 |
| V91 | Carbonic acid, monoamide, N-(2-pentyl)-N-hexyl-, ethyl ester | 1565 | 1551.98 | Esters | — | 0.0083 ± 0.0009 | 0.0063 ± 0.0005 | 0.0051 ± 0.0003 | 0.0042 ± 0.0004 |
| V92 | (Z)-3-Hexenyl iso-butyrate | 1126 | 1185.29 | Esters | Green, sweet, fruity | 0.0149 ± 0.0003 | 0.0125 ± 0.0006 | 0.0117 ± 0.0004 | 0.0047 ± 0.0009 |
| V94 | Diisobutyl dimethylpyrophosphonate | - | 1229.71 | Esters | — | 0.0028 ± 0.0001 | 0.0028 ± 0.0001 | 0.002 ± 0.0002 | 0.0019 ± 0.0001 |
| V97 | Ethyl 6-methylpyridine-2-carboxylate | 1267 | 1036.78 | Esters | — | 0.0716 ± 0.0028 | 0.0568 ± 0.0024 | 0.0449 ± 0.0025 | 0.0284 ± 0.0024 |
| V100 | (Z)-Hexanoic acid, 3-hexenyl ester | 1389 | 1380.72 | Esters | Green, natural, cognac, herbal | 0.0707 ± 0.0053 | 0.0534 ± 0.005 | 0.0483 ± 0.0016 | 0.0241 ± 0.0052 |
| V101 | Hexanoic acid, methyl ester | 884 | 923.30 | Esters | Cabbage, rubbery | 0.0064 ± 0.0003 | 0.0054 ± 0.0002 | 0.0049 ± 0.0001 | 0.004 ± 0.0001 |
| V105 | Methyl 5-methyl-4H-1,2,3-triazole-4-carboxylate | - | 1403.21 | Esters | Herbal, floral | 0.0147 ± 0.0016 | 0.009 ± 0.0008 | 0.0076 ± 0.0003 | 0.0038 ± 0.0007 |
| V106 | Methyl salicylate | 1281 | 1197.72 | Esters | Mint, green | 2.2248 ± 0.0253 | 1.8931 ± 0.0638 | 1.6134 ± 0.0476 | 1.2785 ± 0.1079 |
| V108 | 1,6-Dimethyl-4-(1-methylethyl)-naphthalene | 1706 | 1688.29 | Esters | Aromatic | 0.009 ± 0.0009 | 0.0068 ± 0.0005 | 0.0051 ± 0.0001 | 0.0049 ± 0.0005 |
| V110 | Phosphoric acid, diundecyl ethyl ester | - | 985.60 | Esters | Sweet, fruity tropical | 0.0025 ± 0.0001 | 0.0021 ± 0.0001 | 0.0017 ± 0.0001 | 0.0013 ± 0.0001 |
| V112 | (E)-Geranic acid methyl ester | 1252 | 1324.14 | Esters | Floral, citrus | 0.023 ± 0.0015 | 0.0187 ± 0.0011 | 0.0154 ± 0.0001 | 0.0139 ± 0.0012 |
| V114 | Phosphoric acid, di-2-propenyl ester | - | 1271.61 | Esters | Herbal, floral | 0.0069 ± 0.0002 | 0.005 ± 0.0003 | 0.0043 ± 0.0004 | 0.0034 ± 0.0003 |
| V123 | 6-Hydroxy-2(1H)-pyridinone | 977 | 990.49 | Heterocyclic compound | — | 0.0107 ± 0.0002 | 0.0107 ± 0.0004 | 0.0081 ± 0.0002 | 0.0057 ± 0.0002 |
| V131 | 2-Hydrazinopyridine | 1266 | 1092.47 | Heterocyclic compound | — | 0.0321 ± 0.0004 | 0.0287 ± 0.0011 | 0.0195 ± 0.002 | 0.0194 ± 0.0008 |
| V133 | 1-Isopropyl-3-methyl-2-pyrazoline | 957 | 1038.02 | Heterocyclic compound | — | 0.0036 ± 0.0001 | 0.0035 ± 0.0002 | 0.0032 ± 0.0003 | 0.0021 ± 0.0002 |
| V137 | 3-[4-(2-Methylpropyl)phenyl]butan-2-one | 1511 | 1502.51 | Terpenoids | Aromatic | 0.0061 ± 0.0006 | 0.005 ± 0.0002 | 0.0035 ± 0.0001 | 0.0027 ± 0.0001 |
| V145 | 5-(4-Nitropyrazol-1-yl)-2H-1,2,3,4-tetrazole | - | 1530.14 | Heterocyclic compound | — | 0.003 ± 0.0003 | 0.0024 ± 0.0001 | 0.0018 ± 0.0001 | 0.0016 ± 0.0002 |
| V155 | N-(2-Methylpropionyl)-cyclobutylamine | 1175 | 1344.88 | Heterocyclic compound | — | 0.0089 ± 0.0006 | 0.007 ± 0.0001 | 0.0064 ± 0.0005 | 0.0059 ± 0.0003 |
| V160 | N-(3-Ethylpentyn-3-yl)pyrrolidine | 1222 | 1142.44 | Heterocyclic compound | Bread | 0.0029 ± 0.0002 | 0.0022 ± 0.0001 | 0.0018 ± 0.0001 | 0.0011 ± 0.0001 |
| V162 | 2,2-Dimethyl-oxazolidine | 869 | 991.04 | Heterocyclic compound | — | 0.0052 ± 0.0002 | 0.0045 ± 0.0002 | 0.0033 ± 0.0002 | 0.0023 ± 0.0003 |
| V71 | 2-Hexyl-1H-benzimidazole | 1858 | 1516.65 | Heterocyclic compound | — | 0.0062 ± 0.0004 | 0.0037 ± 0.0003 | 0.0036 ± 0.0001 | 0.0036 ± 0.0004 |
| V170 | 2,6,10-Trimethyltridecane | 1419 | 1461.07 | Hydrocarbons | — | 0.034 ± 0.0016 | 0.0261 ± 0.0014 | 0.0256 ± 0.0011 | 0.0238 ± 0.0011 |
| V177 | 2,6,10-Trimethyl-dodecane | 1320 | 1569.65 | Hydrocarbons | — | 0.0054 ± 0.0006 | 0.0032 ± 0.0002 | 0.0029 ± 0.0003 | 0.0029 ± 0.0003 |
| V181 | 2,6,11,15-Tetramethyl-hexadecane | 1753 | 1495.67 | Hydrocarbons | — | 0.0485 ± 0.0029 | 0.0366 ± 0.0007 | 0.0346 ± 0.0014 | 0.0327 ± 0.0034 |
| V183 | Tetradecane | 1413 | 1398.52 | Hydrocarbons | Waxy | 0.0356 ± 0.0019 | 0.0249 ± 0.0009 | 0.0223 ± 0.001 | 0.0215 ± 0.0009 |
| V206 | 2,2-Dimethylnon-5-en-3-one | 1174 | 1236.57 | Ketone | — | 0.0095 ± 0.0001 | 0.0089 ± 0.0005 | 0.0075 ± 0.0002 | 0.0062 ± 0.0001 |
| V208 | (Z)-2-Cyclopenten-1-one, 3-methyl-2-(2-pentenyl)- | 1338 | 1403.78 | Ketone | Strong, caramel | 0.0202 ± 0.0024 | 0.0126 ± 0.0011 | 0.0107 ± 0.0003 | 0.0054 ± 0.0013 |
| V213 | 3-Isopropylidene-tricyclo[4.3.1.1(2,5)]undecan-10-one | 1432 | 1389.83 | Ketone | — | 0.0047 ± 0.0002 | 0.0031 ± 0.0001 | 0.0029 ± 0.0002 | 0.0025 ± 0.0006 |
| V218 | 6-Methyl-5-hepten-2-one | 938 | 986.31 | Ketone | Herbal, green, citrus, musty, lemon grass | 0.0475 ± 0.0024 | 0.045 ± 0.0016 | 0.0438 ± 0.0011 | 0.0338 ± 0.0009 |
| V222 | 1-(5-Methyl-1-phenyl-1H-pyrazol-4-yl)-ethanone | 1703 | 1634.98 | Ketone | — | 0.0026 ± 0.0004 | 0.0018 ± 0.0002 | 0.0012 ± 0.0001 | 0.0011 ± 0.0002 |
| V232 | N-Methyl-mercaptoacetamide | 1023 | 888.73 | Nitrogen compounds | — | 0.015 ± 0.0008 | 0.013 ± 0.0001 | 0.0118 ± 0.0007 | 0.0102 ± 0.0004 |
| V238 | (1S,4S,4aS)-1-Isopropyl-4,7-dimethyl-1,2,3,4,4a,5-hexahydronaphthalene | 1440 | 1355.45 | Terpenoids | — | 0.0118 ± 0.0008 | 0.0101 ± 0.0004 | 0.0082 ± 0.0002 | 0.0058 ± 0.0005 |
| V239 | α-Calacorene | 1547 | 1552.33 | Terpenoids | Woody | 0.0409 ± 0.0042 | 0.0289 ± 0.002 | 0.0239 ± 0.0002 | 0.0194 ± 0.0016 |
| V242 | β-Myrcene | 958 | 990.42 | Terpenoids | Fruity, tropical | 0.2159 ± 0.0053 | 0.1693 ± 0.0048 | 0.1442 ± 0.0049 | 0.0967 ± 0.008 |
| V243 | β-Ocimene | 976 | 1047.41 | Terpenoids | Apple, pear, fruity | 0.0956 ± 0.0031 | 0.0818 ± 0.0033 | 0.0665 ± 0.0026 | 0.0431 ± 0.0027 |
| V245 | 1-Methyl-4-(1-methylethyl)-1,3-cyclohexadiene | 998 | 1016.24 | Terpenoids | Lemon, citrus | 0.0171 ± 0.0007 | 0.0137 ± 0.0005 | 0.0123 ± 0.0005 | 0.0094 ± 0.0012 |
| V249 | (E)-2,6-Octadienal, 3,7-dimethyl- | 1174 | 1271.32 | Terpenoids | Floral, rose, soapy, citrus, dewy, pear | 0.0362 ± 0.0024 | 0.0283 ± 0.0021 | 0.0224 ± 0.0013 | 0.0152 ± 0.0034 |
| V252 | 2-Methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane | 1407 | 1429.69 | Terpenoids | — | 0.0092 ± 0.001 | 0.006 ± 0.0004 | 0.0052 ± 0.0001 | 0.0044 ± 0.0018 |
| V253 | Geraniol 40 | 1228 | 1254.08 | Terpenoids | Sweet, floral, fruity, rose, waxy, citrus | 2.1867 ± 0.1233 | 1.6762 ± 0.1179 | 1.4309 ± 0.0384 | 1.0119 ± 0.0831 |
| V254 | Linalool 10 | 1082 | 1099.12 | Terpenoids | Flowery | 0.8175 ± 0.0029 | 0.7165 ± 0.0228 | 0.7009 ± 0.0005 | 0.5576 ± 0.1032 |
| V255 | 1,2,3,4,4a,7-Hexahydro-1,6-dimethyl-4-(1-methylethyl)-naphthalene | 1440 | 1642.98 | Terpenoids | — | 0.0031 ± 0.0003 | 0.0027 ± 0.0002 | 0.002 ± 0.0001 | 0.0014 ± 0.0004 |
| V256 | 1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-Z)-naphthalene | 1469 | 1530.75 | Terpenoids | Thyme, herbal, woody, dry | 0.0364 ± 0.0033 | 0.0259 ± 0.001 | 0.0203 ± 0.0006 | 0.0141 ± 0.0009 |
| V258 | (E)-Calamenene | 1537 | 1531.55 | Terpenoids | Vanilla | 0.0702 ± 0.0059 | 0.0551 ± 0.0025 | 0.0406 ± 0.0008 | 0.0305 ± 0.0042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.-T.; Huang, X.-Z.; Huang, J.-F.; Wu, Q.-Y.; Wu, Y.; Deng, T.-T.; Xu, X.-X.; Liu, H.-X.; Chen, X.-H.; Zheng, S.-Z.; et al. Comparison and Study on Flavor and Quality Characteristics of Different Grades of Tianshanhong (TSH). Beverages 2025, 11, 111. https://doi.org/10.3390/beverages11040111
Xiao S-T, Huang X-Z, Huang J-F, Wu Q-Y, Wu Y, Deng T-T, Xu X-X, Liu H-X, Chen X-H, Zheng S-Z, et al. Comparison and Study on Flavor and Quality Characteristics of Different Grades of Tianshanhong (TSH). Beverages. 2025; 11(4):111. https://doi.org/10.3390/beverages11040111
Chicago/Turabian StyleXiao, Shu-Ting, Xian-Zhou Huang, Jian-Feng Huang, Qing-Yang Wu, Yang Wu, Ting-Ting Deng, Xian-Xian Xu, Hao-Xiang Liu, Xiao-Hui Chen, Shi-Zhong Zheng, and et al. 2025. "Comparison and Study on Flavor and Quality Characteristics of Different Grades of Tianshanhong (TSH)" Beverages 11, no. 4: 111. https://doi.org/10.3390/beverages11040111
APA StyleXiao, S.-T., Huang, X.-Z., Huang, J.-F., Wu, Q.-Y., Wu, Y., Deng, T.-T., Xu, X.-X., Liu, H.-X., Chen, X.-H., Zheng, S.-Z., & Zhou, Z.-W. (2025). Comparison and Study on Flavor and Quality Characteristics of Different Grades of Tianshanhong (TSH). Beverages, 11(4), 111. https://doi.org/10.3390/beverages11040111





