Caffeine and Polyphenolic Compound Recovery Optimization from Spent Coffee Grounds Utilizing Pressurized Liquid Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Spent Coffee Grounds Material
2.3. Design of the Experiment
2.4. Bioactive Compound Determination
2.4.1. Spectrophotometric Evaluation of Total Polyphenolic Content (TPC)
2.4.2. Chromatographic Analysis of Individual Phenolic Acids, Flavonoids, and Caffeine
2.5. Ferric-Reducing Antioxidant Power (FRAP) Evaluation of Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
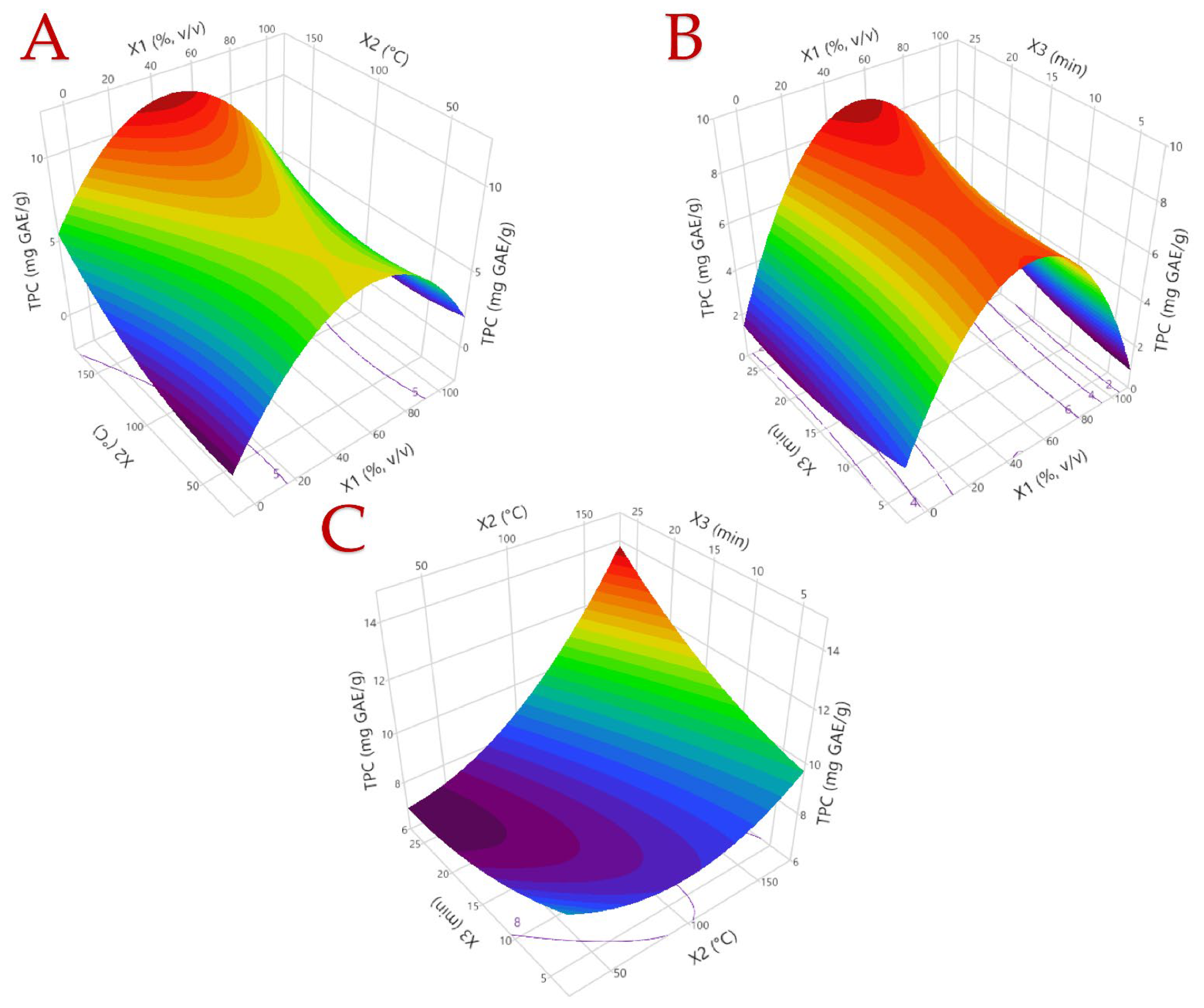
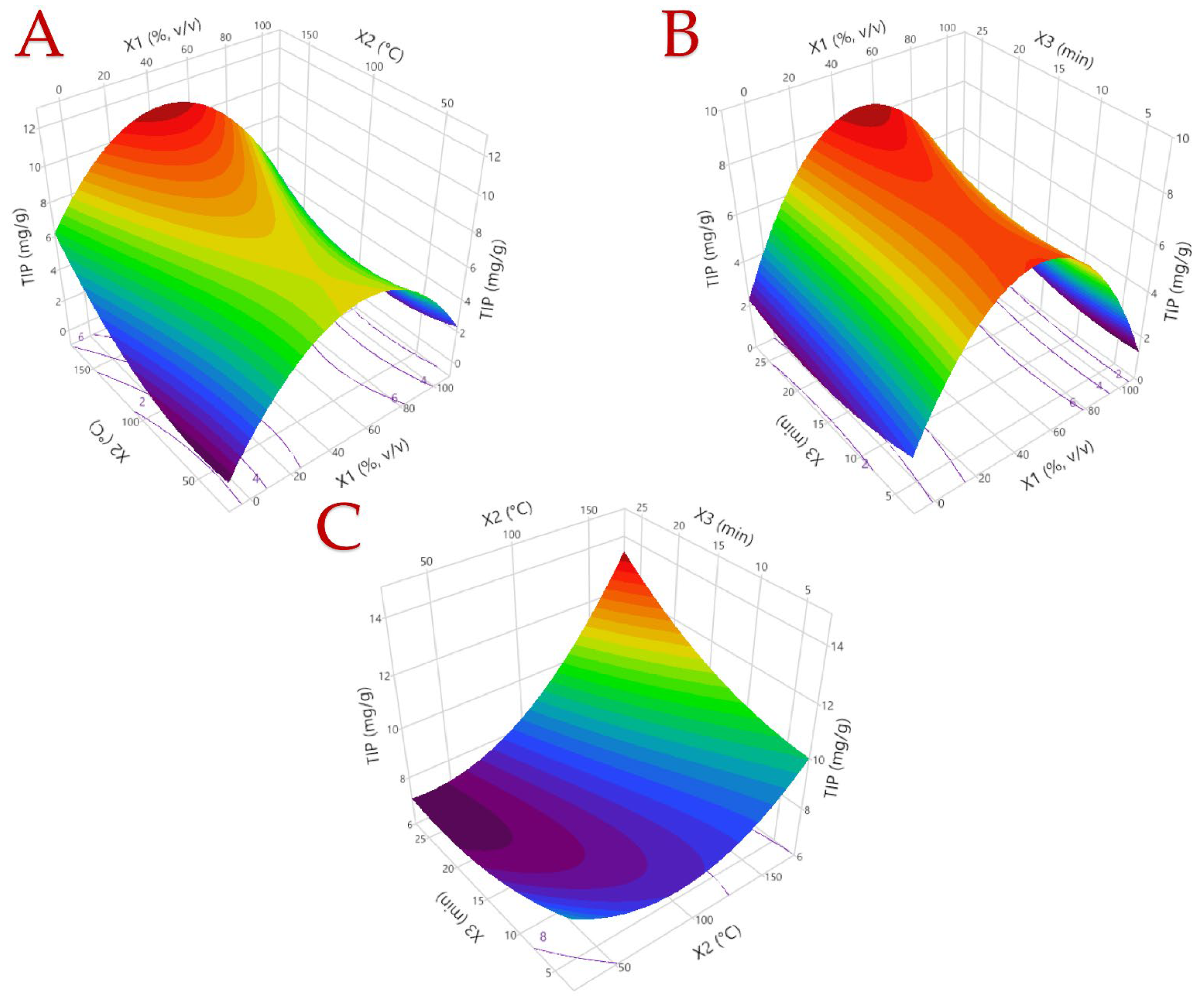
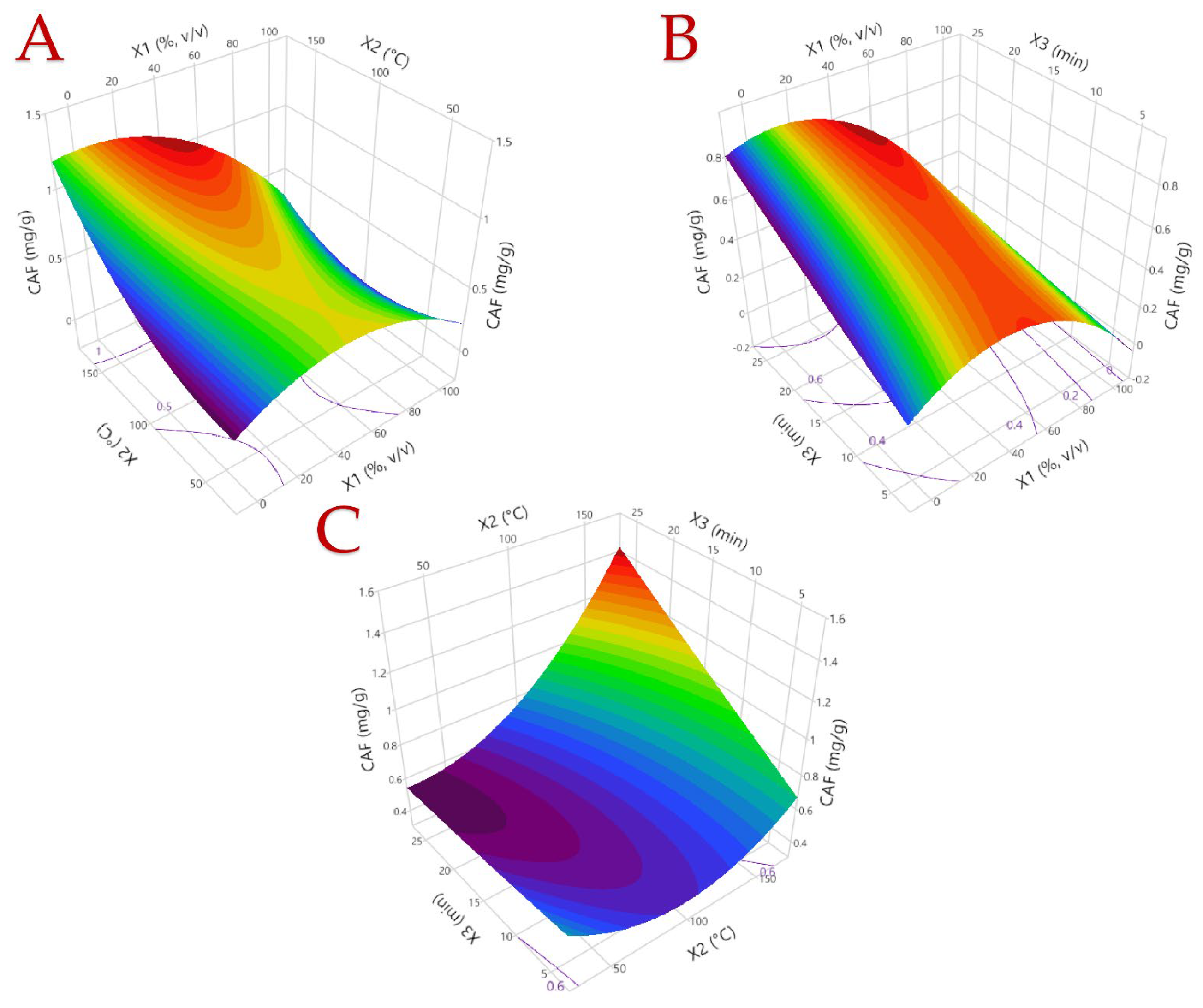
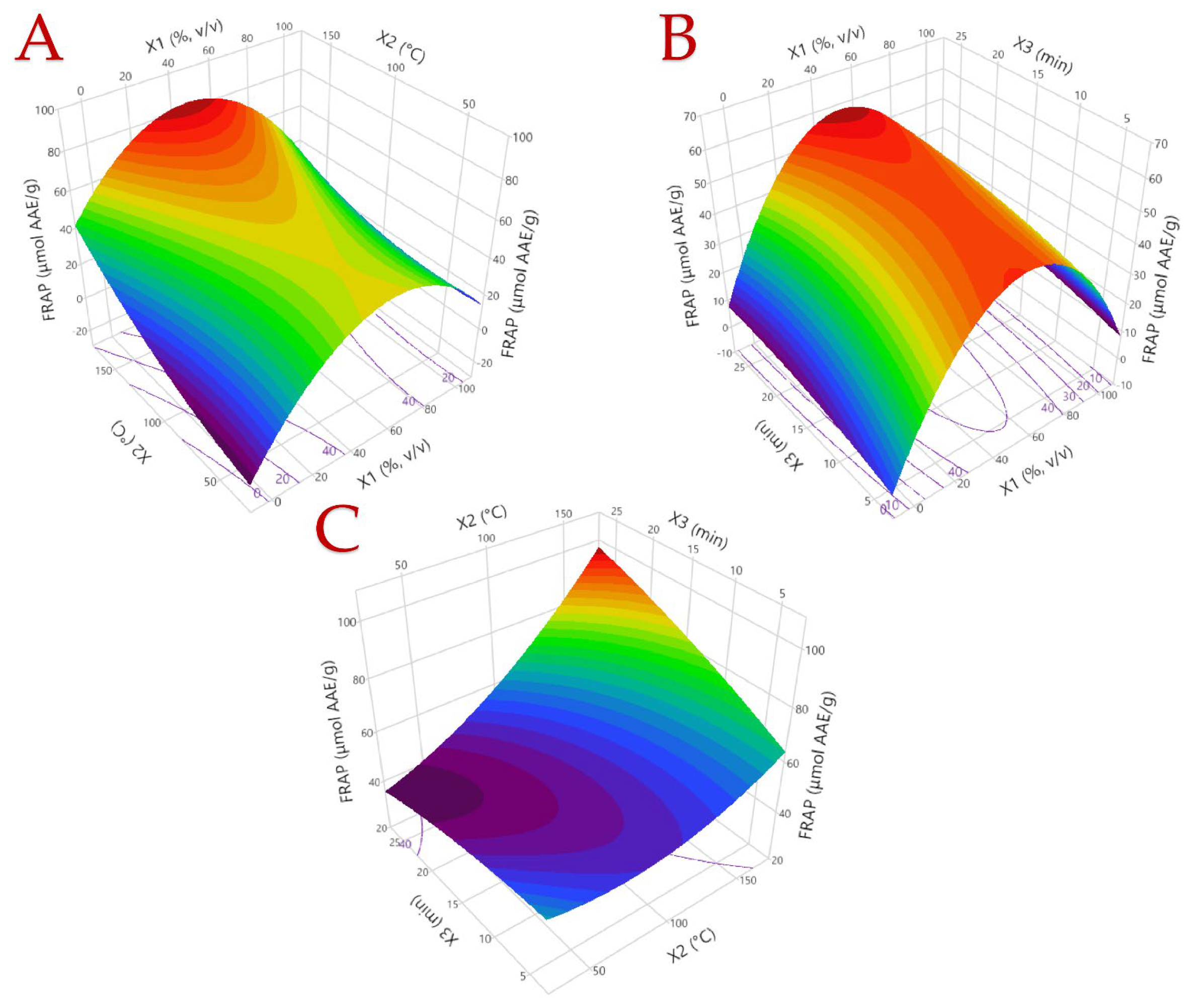
3.1. Optimization of the Extraction Procedure
3.2. Model Analysis
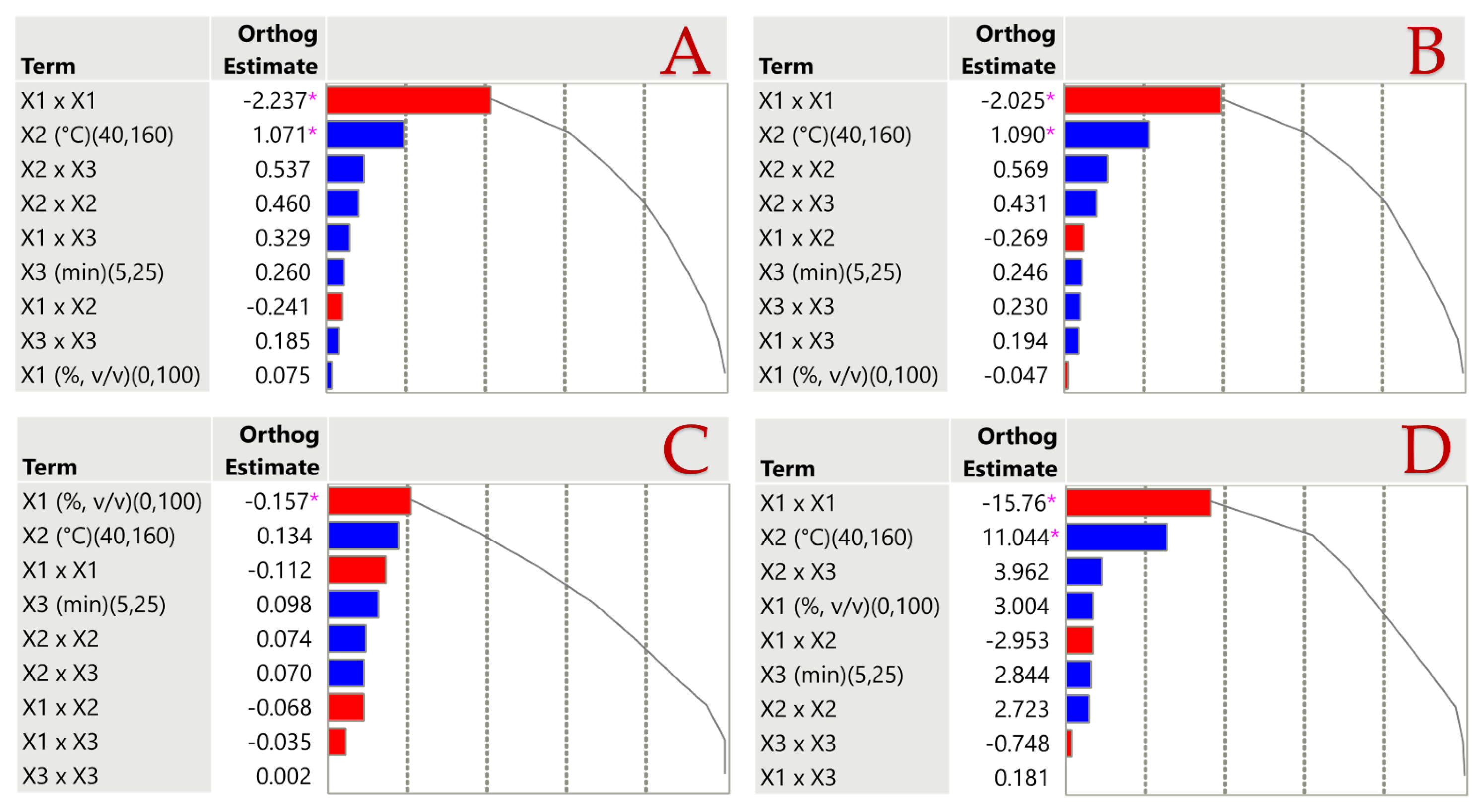
3.3. Statistical Analysis of Extraction Parameters Using Pareto Plots
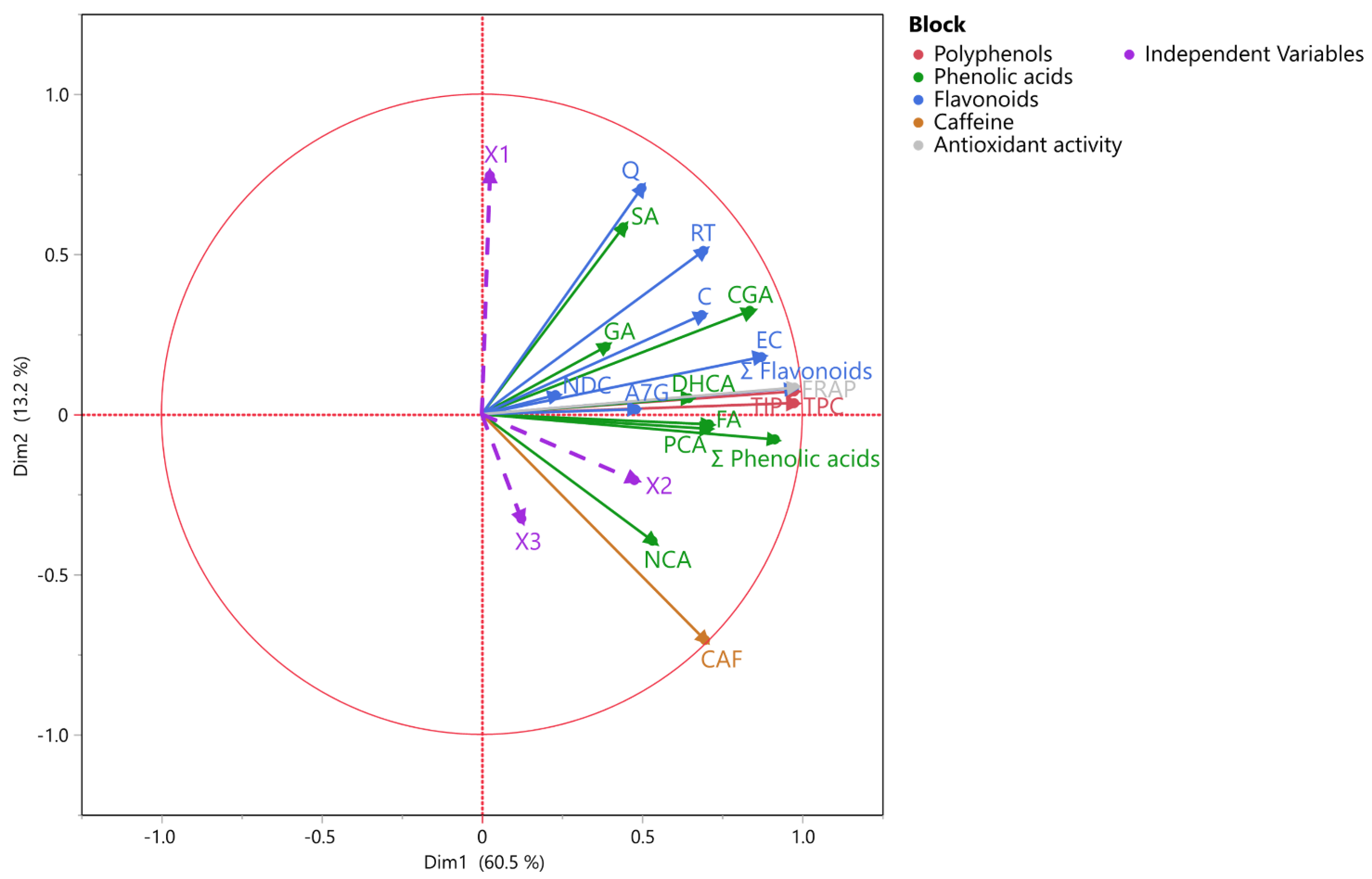
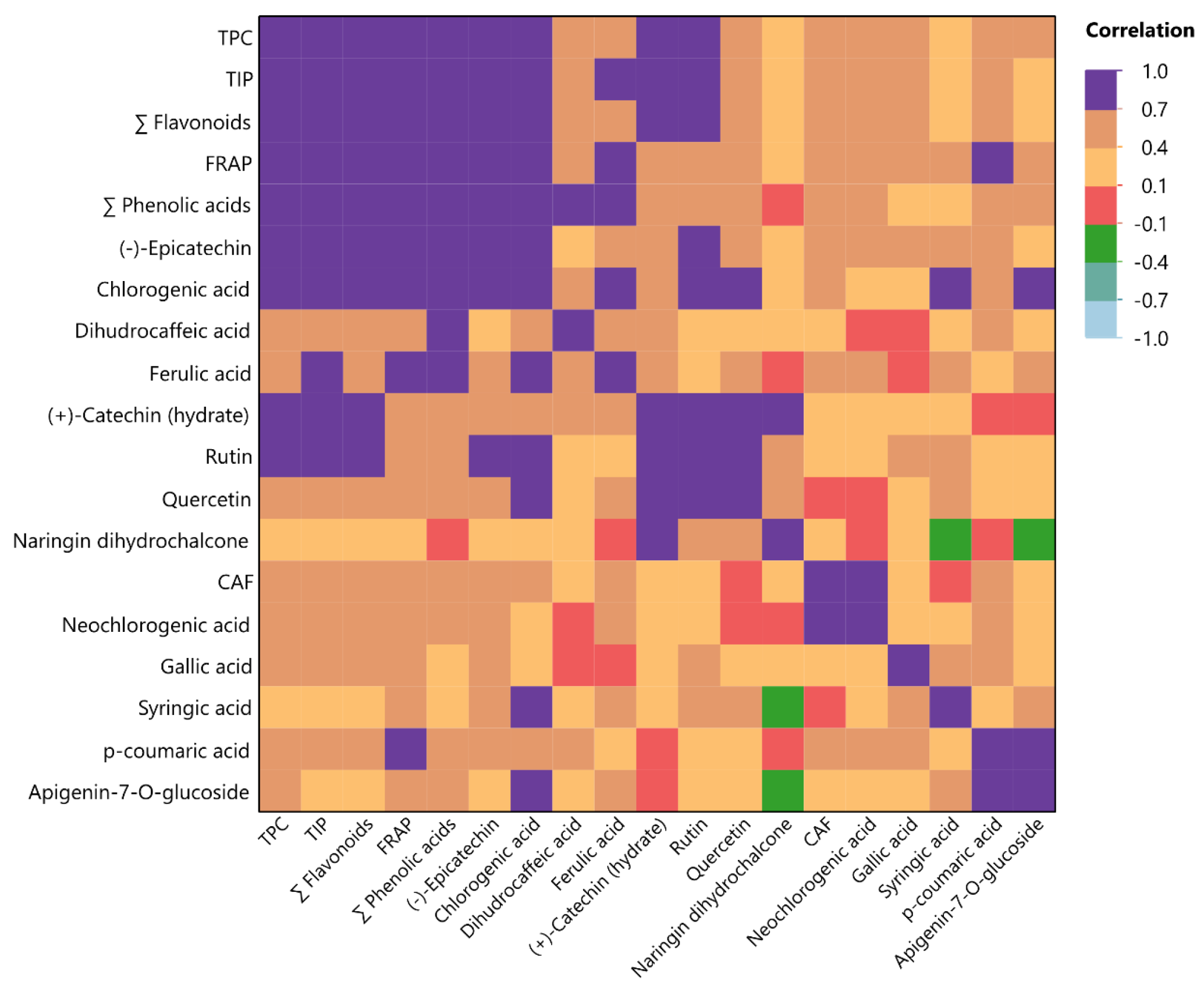
3.4. Analysis of Multiple Factors (MFA) and Multivariate Correlation (MCA)
3.5. Partial Least Squares (PLS) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Design Point | Independent Variables | Responses | ∑ Phenolic Acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 (C, %) | X2 (T, °C) | X3 (t, min) | GA | NCA | DHCA | CGA | SA | PCA | FA | ||
| 1 | 0 (50) | 0 (100) | 0 (15) | 0.15 | 0.25 | 0.78 | nd | 0.56 | 0.31 | 0.15 | 2.20 |
| 2 | −1 (0) | 0 (100) | 1 (25) | 0.08 | 0.33 | 0.52 | 0.24 | 0.23 | 0.17 | 0.13 | 1.70 |
| 3 | −1 (0) | −1 (40) | 0 (15) | 0.15 | 0.23 | 0.51 | 0.42 | 0.24 | 0.15 | 0.09 | 1.80 |
| 4 | 1 (100) | 0 (100) | 1 (25) | 0.12 | nd | 0.59 | 0.71 | 0.52 | 0.18 | 0.16 | 2.27 |
| 5 | −1 (0) | 0 (100) | −1 (5) | nd | 0.27 | 0.58 | 0.41 | 0.35 | 0.12 | 0.13 | 1.86 |
| 6 | 0 (50) | 1 (160) | −1 (5) | nd | 0.32 | 1.04 | 1.09 | 0.60 | 0.27 | 0.18 | 3.49 |
| 7 | 0 (50) | 0 (100) | 0 (15) | 0.09 | 0.28 | 0.99 | 0.83 | 0.52 | 0.14 | 0.21 | 3.06 |
| 8 | 1 (100) | −1 (40) | 0 (15) | nd | nd | 0.27 | nd | 0.69 | 0.10 | nd | 1.06 |
| 9 | 0 (50) | −1 (40) | 1 (25) | 0.18 | 0.30 | 0.74 | 0.68 | 0.30 | 0.15 | 0.14 | 2.48 |
| 10 | 0 (50) | 1 (160) | 1 (25) | 0.21 | 0.52 | 0.74 | 1.17 | 0.76 | 0.32 | 0.18 | 3.91 |
| 11 | 0 (50) | −1 (40) | −1 (5) | 0.08 | 0.31 | 0.65 | 1.18 | 0.31 | 0.19 | nd | 2.72 |
| 12 | −1 (0) | 1 (160) | 0 (15) | nd | 0.62 | 0.63 | 0.88 | 0.45 | nd | 0.22 | 2.80 |
| 13 | 1 (100) | 0 (100) | −1 (5) | nd | nd | 0.54 | nd | 0.77 | 0.17 | nd | 1.48 |
| 14 | 0 (50) | 0 (100) | 0 (15) | nd | 0.30 | 0.81 | 1.18 | 0.67 | 0.25 | 0.19 | 3.40 |
| 15 | 1 (100) | 1 (160) | 0 (15) | nd | nd | 0.38 | 0.60 | 0.45 | 0.20 | 0.14 | 1.77 |
| Design Point | Independent Variables | Responses | ∑ Flavonoids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 (C, %) | X2 (T, °C) | X3 (t, min) | C | EC | RT | A7G | NDC | Q | ||
| 1 | 0 (50) | 0 (100) | 0 (15) | 0.56 | 0.75 | 0.48 | 0.18 | 0.68 | 0.41 | 3.06 |
| 2 | −1 (0) | 0 (100) | 1 (25) | 0.28 | 0.26 | 0.19 | 0.05 | nd | 0.16 | 0.94 |
| 3 | −1 (0) | −1 (40) | 0 (15) | 0.63 | 0.62 | nd | nd | nd | nd | 1.25 |
| 4 | 1 (100) | 0 (100) | 1 (25) | 0.72 | nd | 0.64 | nd | 0.51 | 0.56 | 2.42 |
| 5 | −1 (0) | 0 (100) | −1 (5) | 0.44 | 0.44 | 0.37 | 0.02 | 0.33 | 0.23 | 1.82 |
| 6 | 0 (50) | 1 (160) | −1 (5) | 0.86 | 2.04 | 1.26 | 0.08 | nd | 0.78 | 5.02 |
| 7 | 0 (50) | 0 (100) | 0 (15) | 0.95 | 1.64 | 1.11 | 0.07 | 0.69 | 0.80 | 5.26 |
| 8 | 1 (100) | −1 (40) | 0 (15) | 0.66 | nd | 0.80 | nd | nd | nd | 1.46 |
| 9 | 0 (50) | −1 (40) | 1 (25) | 1.14 | 1.81 | 1.14 | 0.01 | 1.10 | nd | 5.20 |
| 10 | 0 (50) | 1 (160) | 1 (25) | 0.92 | 3.70 | 1.72 | 0.15 | 0.63 | 0.80 | 7.92 |
| 11 | 0 (50) | −1 (40) | −1 (5) | 0.92 | 2.00 | 1.24 | nd | nd | 0.83 | 4.99 |
| 12 | −1 (0) | 1 (160) | 0 (15) | 0.89 | 2.57 | 0.80 | 0.10 | nd | 0.42 | 4.78 |
| 13 | 1 (100) | 0 (100) | −1 (5) | 0.74 | nd | 1.22 | nd | nd | 0.80 | 2.76 |
| 14 | 0 (50) | 0 (100) | 0 (15) | 0.71 | 2.62 | 0.98 | 0.09 | nd | 0.50 | 4.90 |
| 15 | 1 (100) | 1 (160) | 0 (15) | 0.62 | nd | 1.13 | 0.01 | 0.79 | 0.64 | 3.20 |
References
- Badr, A.N.; El-Attar, M.M.; Ali, H.S.; Elkhadragy, M.F.; Yehia, H.M.; Farouk, A. Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins 2022, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Dattatraya Saratale, G.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef]
- International Coffee Organization. International Coffee Organization. Coffee Report and Outlook—December 2023; ICO: London, UK, 2023; Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 16 December 2024).
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil from Spent Coffee Grounds for Biofuel Production. Waste Biomass Valor. 2019, 10, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Agrawal, D.C.; Huang, W.-Y.; Hsu, H.-C.; Yang, S.-J.; Huang, S.-L.; Lin, Y.-S. Functionality Analysis of Spent Coffee Ground Extracts Obtained by the Hydrothermal Method. J. Chem. 2019, 2019, 4671438. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Campisi, B.; Vargas Peregrina, D.; Censi, R.; Khamitova, G.; Angeloni, S.; Caprioli, G.; Zannotti, M.; Ferraro, S.; Giovannetti, R.; et al. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants 2020, 9, 370. [Google Scholar] [CrossRef]
- Massaya, J.; Prates Pereira, A.; Mills-Lamptey, B.; Benjamin, J.; Chuck, C.J. Conceptualization of a spent coffee grounds biorefinery: A review of existing valorisation approaches. Food Bioprod. Process. 2019, 118, 149–166. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.-M.; Mourtzinos, I.; Goula, A.M. Recovery of phenolic compounds from spent coffee grounds through optimized extraction processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- Haile, M. Integrated volarization of spent coffee grounds to biofuels. Biofuels Res. J. 2014, 2, 65–69. [Google Scholar] [CrossRef]
- Bae, J.-H.; Park, J.-H.; Im, S.-S.; Song, D.-K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef]
- Costabile, A.; Sarnsamak, K.; Hauge-Evans, A.C. Coffee, type 2 diabetes and pancreatic islet function—A mini-review. J. Funct. Foods 2018, 45, 409–416. [Google Scholar] [CrossRef]
- Montenegro, J.; dos Santos, L.S.; de Souza, R.G.G.; Lima, L.G.B.; Mattos, D.S.; Viana, B.P.P.B.; da Fonseca Bastos, A.C.S.; Muzzi, L.; Conte-Júnior, C.A.; Gimba, E.R.P.; et al. Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE). Food Res. Int. 2021, 140, 110014. [Google Scholar] [CrossRef]
- AlAmri, O.D.; Albeltagy, R.S.; Akabawy, A.M.A.; Mahgoub, S.; Abdel-Mohsen, D.M.; Abdel Moneim, A.E.; Amin, H.K. Investigation of antioxidant and anti-inflammatory activities as well as the renal protective potential of green coffee extract in high fat-diet/streptozotocin-induced diabetes in male albino rats. J. Funct. Foods 2020, 71, 103996. [Google Scholar] [CrossRef]
- Sedaghat, G.; Mirshekar, M.A.; Amirpour, M.; Montazerifar, F.; Miri, S.; Shourestani, S. Sub-chronic administration of brewed coffee on rat behavior and cognition and oxidative stress Alzheimer’s disease model. Clin. Nutr. Exp. 2019, 28, 62–73. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef]
- Zhu, M.; Jatoi, A. Colorectal Cancer, Crohn-Like Lymphoid Reactions, Survival—And the Power of a Good Cup of Coffee! Mayo Clin. Proc. 2022, 97, 15–17. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Álvarez, R.; Muñoz-Durango, K. Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Açıkalın, B.; Sanlier, N. Coffee and its effects on the immune system. Trends Food Sci. Technol. 2021, 114, 625–632. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Martinez, A.S.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Araujo, M.N.; dos Santos, K.C.; do Carmo Diniz, N.; de Carvalho, J.C.; Corazza, M.L. A biorefinery approach for spent coffee grounds valorization using pressurized fluid extraction to produce oil and bioproducts: A systematic review. Bioresour. Technol. Rep. 2022, 18, 101013. [Google Scholar] [CrossRef]
- Shang, Y.-F.; Xu, J.-L.; Lee, W.-J.; Um, B.-H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Vargas-González, M.; Pedreschi, F.; Pérez-Correa, J.R. The Antioxidant and Safety Properties of Spent Coffee Ground Extracts Impacted by the Combined Hot Pressurized Liquid Extraction–Resin Purification Process. Molecules 2018, 23, 21. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Mello, F.M.P.A.; Cabral, F.A.; Meirelles, A.J.A. High-pressure fractionation of spent coffee grounds oil using green solvents. J. Supercrit. Fluids 2020, 157, 104689. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Mantiniotou, M.; Lalas, S.I. Optimization of Ultrasonication Probe-Assisted Extraction Parameters for Bioactive Compounds from Opuntia macrorhiza Using Taguchi Design and Assessment of Antioxidant Properties. Appl. Sci. 2024, 14, 10460. [Google Scholar] [CrossRef]
- Thoo, Y.; Ng, S.Y.; Khoo, M.; Mustapha, W.; Ho, C. A binary solvent extraction system for phenolic antioxidants and its application to the estimation of antioxidant capacity in Andrographis paniculata extracts. Int. Food Res. J. 2013, 20, 1103–1111. [Google Scholar]
- Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1–19. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Zhong, J.; Tang, N.; Asadzadeh, B.; Yan, W. Measurement and Correlation of Solubility of Theobromine, Theophylline, and Caffeine in Water and Organic Solvents at Various Temperatures. J. Chem. Eng. Data 2017, 62, 2570–2577. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Khan, S.A.; Aslam, R.; Makroo, H.A. High pressure extraction and its application in the extraction of bio-active compounds: A review. J. Food Process Eng. 2019, 42, e12896. [Google Scholar] [CrossRef]
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Alcaraz, L.; Pérez-Ferreras, S.; León-González, M.E.; Rosales-Conrado, N.; López, F.A. Extraction of polyphenols and synthesis of new activated carbon from spent coffee grounds. Sci. Rep. 2019, 9, 17706. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
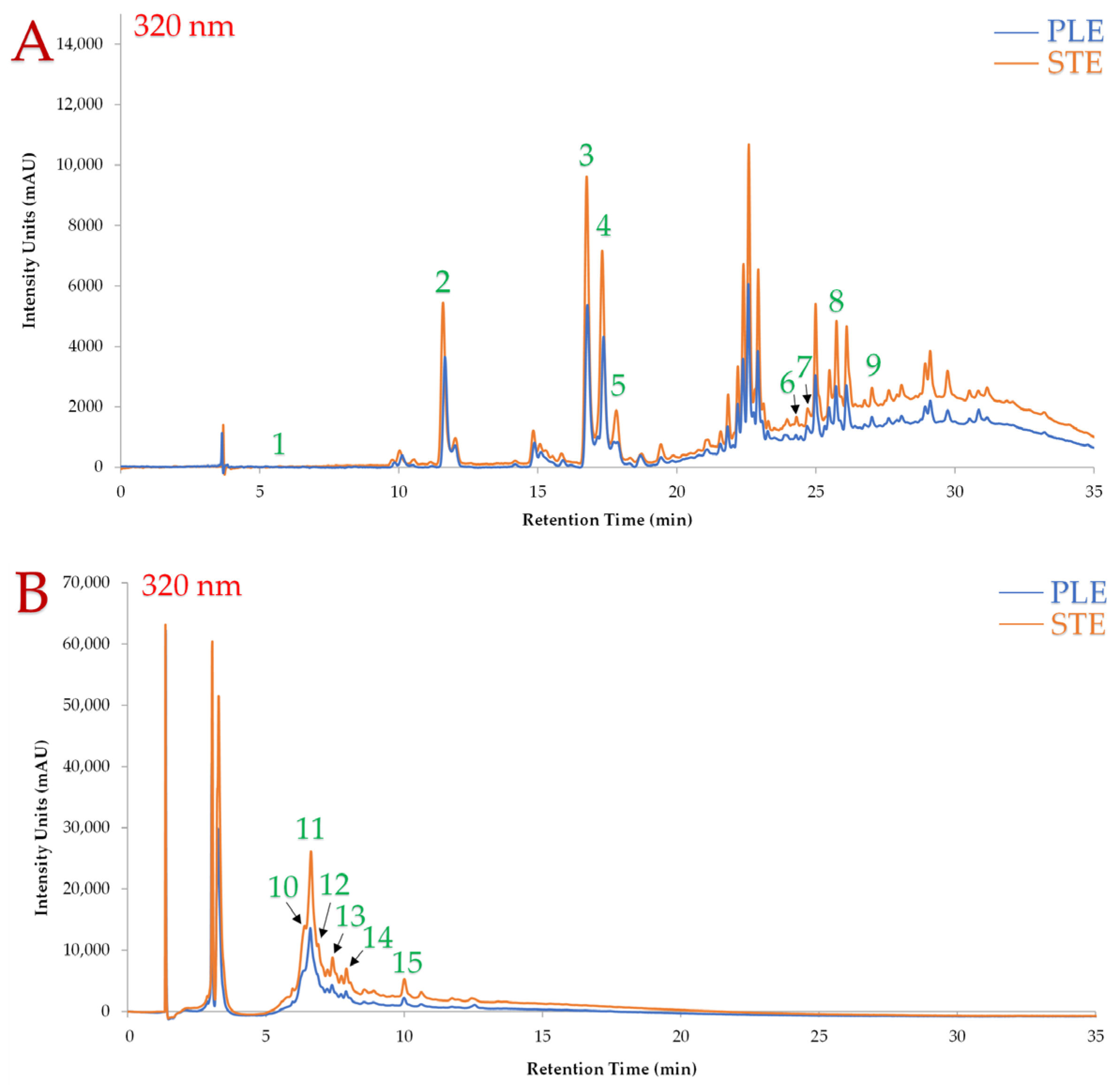
| Design Point | Independent Variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| X1 (C, %) | X2 (T, °C) | X3 (t, min) | TPC (mg GAE/g) | TIP (mg/g) | CAF (mg/g) | FRAP (μmol AAE/g) | |
| 1 | 0 (50) | 0 (100) | 0 (15) | 6.32 | 5.27 | 0.51 | 51.09 |
| 2 | −1 (0) | 0 (100) | 1 (25) | 2.11 | 2.64 | 0.77 | 12.44 |
| 3 | −1 (0) | −1 (40) | 0 (15) | 2.96 | 3.05 | 0.32 | 10.35 |
| 4 | 1 (100) | 0 (100) | 1 (25) | 5.31 | 4.70 | 0.30 | 33.28 |
| 5 | −1 (0) | 0 (100) | −1 (5) | 3.63 | 3.68 | 0.30 | 13.07 |
| 6 | 0 (50) | 1 (160) | −1 (5) | 8.49 | 8.52 | 0.54 | 58.86 |
| 7 | 0 (50) | 0 (100) | 0 (15) | 8.31 | 8.31 | 0.48 | 47.57 |
| 8 | 1 (100) | −1 (40) | 0 (15) | 2.38 | 2.52 | 0.06 | 18.10 |
| 9 | 0 (50) | −1 (40) | 1 (25) | 7.78 | 7.68 | 0.65 | 44.80 |
| 10 | 0 (50) | 1 (160) | 1 (25) | 12.24 | 11.83 | 1.01 | 89.71 |
| 11 | 0 (50) | −1 (40) | −1 (5) | 8.19 | 7.71 | 0.72 | 44.64 |
| 12 | −1 (0) | 1 (160) | 0 (15) | 7.38 | 7.58 | 1.23 | 52.71 |
| 13 | 1 (100) | 0 (100) | −1 (5) | 4.28 | 4.24 | 0.10 | 32.51 |
| 14 | 0 (50) | 0 (100) | 0 (15) | 8.92 | 8.30 | 0.74 | 68.30 |
| 15 | 1 (100) | 1 (160) | 0 (15) | 4.93 | 4.97 | 0.44 | 37.59 |
| Factor | TPC | TIP | CAF | FRAP |
|---|---|---|---|---|
| Least squares regression | ||||
| Intercept | 7.850 * | 7.293 * | 0.577 * | 55.653 * |
| X1—solvent concentration | 0.102 | −0.065 | −0.215 * | 4.114 |
| X2—temperature | 1.466 * | 1.493 * | 0.184 | 15.123 * |
| X3—extraction time | 0.356 | 0.338 | 0.134 | 3.894 |
| X1X2 | −0.47 | −0.520 | −0.133 | −5.718 |
| X1X3 | 0.637 | 0.375 | −0.068 | 0.350 |
| X2X3 | 1.04 | 0.835 | 0.135 | 7.673 |
| X12 | −4.390 * | −3.942 * | −0.213 | −31.32 * |
| X22 | 0.953 | 1.178 | 0.149 | 5.356 |
| X32 | 0.373 | 0.463 | 0.004 | −1.507 |
| ANOVA | ||||
| F-value (model) | 4.787 | 3.965 | 3.044 | 5.385 |
| F-value (lack of fit) | 1.508 | 0.708 | 2.985 | 1.094 |
| p-Value (model) | 0.0497 * | 0.0718 ns | 0.1165 ns | 0.0392 * |
| p-Value (lack of fit) | 0.4226 ns | 0.6305 ns | 0.2610 ns | 0.5102 ns |
| R2 | 0.896 | 0.877 | 0.846 | 0.906 |
| Adjusted R2 | 0.709 | 0.656 | 0.568 | 0.738 |
| RMSE | 1.553 | 1.591 | 0.211 | 11.401 |
| PRESS | 142.09 | 118.13 | 2.990 | 7015.5 |
| CV | 46.30 | 44.71 | 58.80 | 54.34 |
| DF (total) | 14 | 14 | 14 | 14 |
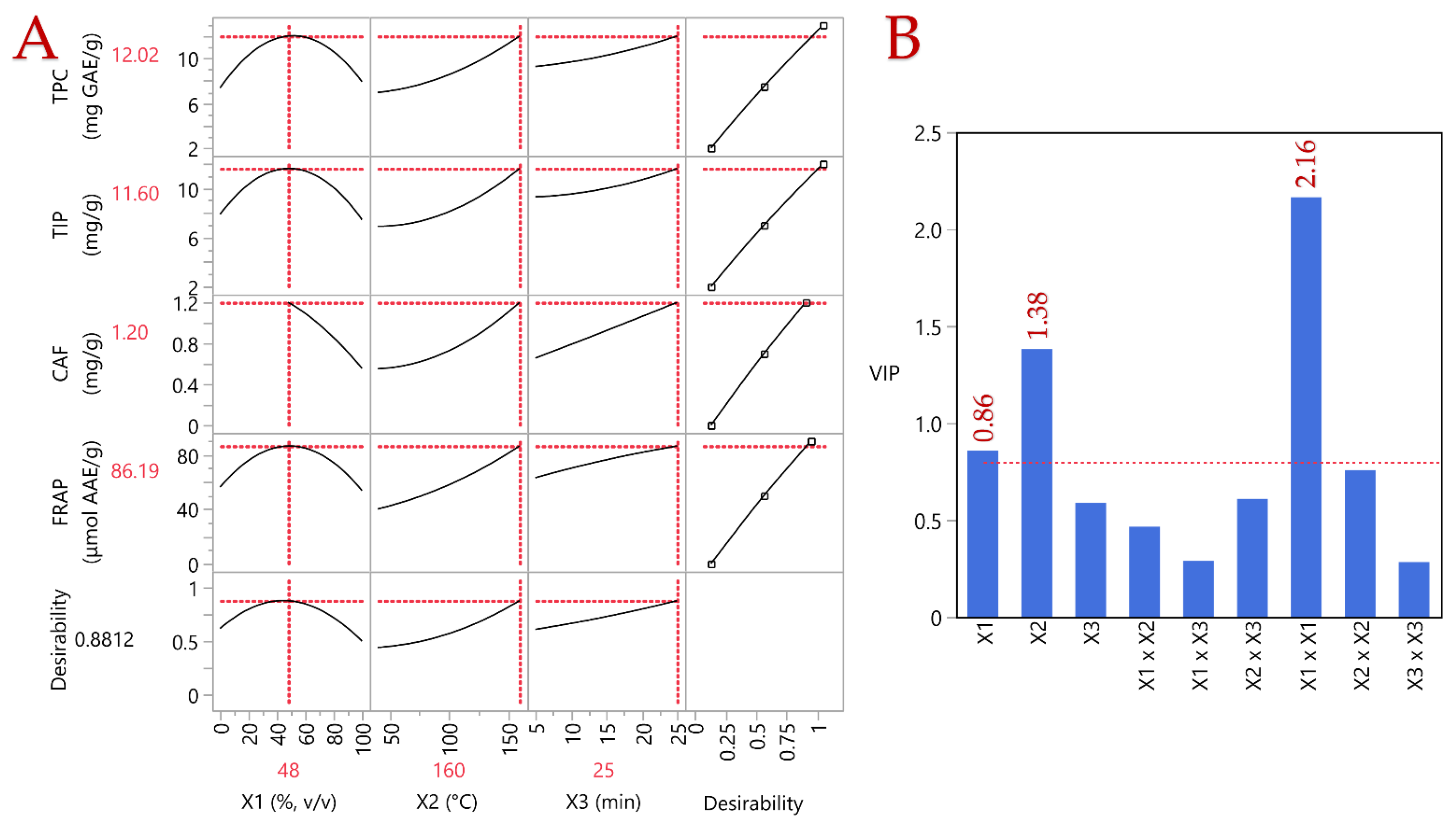
| Parameters | X1 (C, %) | X2 (T, °C) | X3 (t, min) | Desirability | Least Squares Regression |
|---|---|---|---|---|---|
| TPC (mg GAE/g) | 50 | 160 | 25 | 0.8969 | 12.04 ± 3.46 |
| TIP (mg/g) | 50 | 160 | 25 | 0.9432 | 11.6 ± 3.54 |
| CAF (mg/g) | 21 | 160 | 23 | 0.9045 | 1.29 ± 0.45 |
| FRAP (μmol AAE/g) | 50 | 160 | 25 | 0.8474 | 86.19 ± 25.38 |
| Parameter | Polyphenols | Phenolic Acids | Flavonoids | Caffeine | Antioxidant Activity | Centroid |
|---|---|---|---|---|---|---|
| Polyphenols | - | 0.748 | 0.708 | 0.408 | 0.893 | 0.921 |
| Phenolic acids | - | 0.595 | 0.380 | 0.827 | 0.877 | |
| Flavonoids | - | 0.203 | 0.657 | 0.781 | ||
| Caffeine | - | 0.368 | 0.572 | |||
| Antioxidant activity | - | 0.920 | ||||
| Centroid | - |
| Parameters | Partial Least Squares (PLS) Regression for PLE | PLE Experimental Values | STE |
|---|---|---|---|
| TPC (mg GAE/g) | 12.02 | 15.99 ± 0.72 | 16.69 ± 0.52 |
| TIP (mg/g) | 11.60 | 10.46 ± 0.56 | 12.75 ± 0.60 * |
| CAF (mg/g) | 1.20 | 1.15 ± 0.07 | 1.60 ± 0.06 * |
| FRAP (μmol AAE/g) | 86.19 | 101.87 ± 3.16 | 102.70 ± 3.49 |
| Polyphenolic Compounds (mg/g) | PLE | STE |
|---|---|---|
| Phenolic acids | ||
| Gallic acid | 0.12 ± 0.01 * | 0.09 ± 0 |
| Neochlorogenic acid | 0.25 ± 0.01 * | 0.14 ± 0.01 |
| Dihydrocaffeic acid | 0.68 ± 0.05 | 0.97 ± 0.02 * |
| Chlorogenic acid | 1.53 ± 0.10 | 1.37 ± 0.09 |
| Caffeic acid | nd | 0.62 ± 0.02 * |
| Syringic acid | 0.79 ± 0.04 | 0.84 ± 0.02 |
| p-Coumaric acid | 0.40 ± 0.03 | 0.44 ± 0.02 |
| Ferulic acid | 0.16 ± 0.01 | 0.20 ± 0.01 * |
| ∑ Phenolic acids | 3.92 ± 0.16 | 4.66 ± 0.28 * |
| Flavonoids | ||
| (+)-Catechin (hydrate) | 0.82 ± 0.05 | 1.06 ± 0.04 * |
| (−)-Epicatechin | 2.27 ± 0.06 | 2.94 ± 0.11 * |
| Rutin | 1.32 ± 0.05 | 1.33 ± 0.08 |
| Apigenin-7-O-glucoside | 0.40 ± 0.01 | 0.39 ± 0.03 |
| Naringin dihydrochalcone | 0.90 ± 0.05 | 0.94 ± 0.03 |
| Quercetin | 0.83 ± 0.04 | 1.43 ± 0.05 * |
| ∑ Flavonoids | 6.54 ± 0.40 | 8.09 ± 0.32 * |
| Total Identified Polyphenols | 10.46 ± 0.56 | 12.75 ± 0.60 * |
| Polyphenolic Compounds (Standards) | Equation | R2 | Retention Time (min) | UVmax |
|---|---|---|---|---|
| Gallic acid | y = 406,13.93x + 241,534.74 | 0.993 | 5.627 | 270 |
| Neochlorogenic acid | y = 33,340.37x − 35,090.94 | 0.997 | 11.405 | 324 |
| Dihydrocaffeic acid | y = 145,111.50x + 68,055.57 | 0.998 | 16.937 | 280 |
| Chlorogenic acid | y = 47,940.59x + 729,821.30 | 0.992 | 18.679 | 323 |
| Caffeic acid | y = 836,011.57x + 606,104.54 | 0.994 | 24.281 | 327 |
| Syringic acid | y = 172,124.17x + 1,804,823.19 | 0.988 | 24.578 | 320 |
| p-Coumaric acid | y = 54,706.25x + 346,333.79 | 0.993 | 25.235 | 312 |
| Ferulic acid | y = 233,188.64x + 1,666,648.36 | 0.991 | 27.120 | 310 |
| (+)-Catechin (hydrate) | y = 81,185.19x + 811,110.11 | 0.996 | 5.934 | 310 |
| (−)-Epicatechin | y = 15,754.99x + 177,996.05 | 0.992 | 6.317 | 278 |
| Rutin | y = 35,025.67x + 191,393.19 | 0.993 | 6.381 | 352 |
| Apigenin-7-O-glucoside | y = 52,641.52x − 43,026.57 | 0.996 | 7.271 | 336 |
| Naringin dihydrochalcone | y = 35,117.47x + 362,530.33 | 0.994 | 7.556 | 281 |
| Quercetin | y = 85,950.46x + 943,732.12 | 0.993 | 10.989 | 360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christoforidis, A.; Mantiniotou, M.; Athanasiadis, V.; Lalas, S.I. Caffeine and Polyphenolic Compound Recovery Optimization from Spent Coffee Grounds Utilizing Pressurized Liquid Extraction. Beverages 2025, 11, 74. https://doi.org/10.3390/beverages11030074
Christoforidis A, Mantiniotou M, Athanasiadis V, Lalas SI. Caffeine and Polyphenolic Compound Recovery Optimization from Spent Coffee Grounds Utilizing Pressurized Liquid Extraction. Beverages. 2025; 11(3):74. https://doi.org/10.3390/beverages11030074
Chicago/Turabian StyleChristoforidis, Athanasios, Martha Mantiniotou, Vassilis Athanasiadis, and Stavros I. Lalas. 2025. "Caffeine and Polyphenolic Compound Recovery Optimization from Spent Coffee Grounds Utilizing Pressurized Liquid Extraction" Beverages 11, no. 3: 74. https://doi.org/10.3390/beverages11030074
APA StyleChristoforidis, A., Mantiniotou, M., Athanasiadis, V., & Lalas, S. I. (2025). Caffeine and Polyphenolic Compound Recovery Optimization from Spent Coffee Grounds Utilizing Pressurized Liquid Extraction. Beverages, 11(3), 74. https://doi.org/10.3390/beverages11030074









