Selection and Use of Wild Lachancea thermotolerans Strains from Rioja AOC with Bioacidificant Capacity as Strategy to Mitigate Climate Change Effects in Wine Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media Used for Fermentation
2.2. Laboratory Scale Microfermentations
2.3. Fermentation Monitoring
2.4. Oenological Parameters Analysis
2.5. Pilot Scale Microvinification
2.6. Determination of Wine Aroma Compounds
2.7. Organoleptic Evaluation
2.8. Statistical Analysis in XLSTAT
3. Results
3.1. Isolation of L. thermotolerans Strains
3.2. Laboratory Scale Microfermentations with Four Strains
3.3. Laboratory Scale Microfermentations with Different Inoculation Strategies
3.4. Pilot Scale Microvinifications
3.5. Wine Aromatic Compounds on Pilot Scale Microvinification
3.6. Organoleptic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Conditions | Volatile Acidity (g/L) | Alcohol % (v/v) | Total SO2 (mg/L) | Glycerol (g/L) |
|---|---|---|---|---|
| ScF Control | 0.67 ± 0.09 a | 15.80 ± 0.14 a | 7.50 ± 0.71 a | 10.75 ± 0.07 a |
| Lt07 | 0.82 ± 0.06 a | 11.74 ± 0.63 b | 1.50 ± 0.71 b | 11.65 ± 0.64 a |
| Lt29 | 0.86 ± 0.03 a | 11.83 ± 0.55 bc | 4.00 ± 0.00 b | 11.10 ± 0.00 a |
| Lt75 | 0.63 ± 0.07 a | 11.42 ± 0.28 b | 4.00 ± 1.41 b | 10.55 ± 0.78 a |
| Lt97 | 0.78 ± 0.01 a | 11.14 ± 0.06 c | 4.50 ± 0.71 ab | 11.10 ± 0.14 a |
| Conditions | Volatile Acidity (g/L) | Alcohol % (v/v) | Residual Sugar (g/L) |
|---|---|---|---|
| ScF Control | 0.79 ± 0.01 ab | 15.40 ± 0.02 a | 1.00 ± 0.01 a |
| Lt29CO105 | 0.59 ± 0.01 a | 14.94 ± 0.01 b | 1.00 ± 0.01 a |
| Lt97CO105 | 0.63 ± 0.01 a | 14.83 ± 0.03 b | 1.00 ± 0.01 a |
| Lt29CO104 | 0.83 ± 0.01 ab | 12.45 ± 0.28 c | 3.95 ± 1.10 a |
| Lt97CO104 | 1.00 ± 0.23 b | 12.32 ± 0.04 c | 2.47 ± 1.74 a |
| Lt29SEQ24 | 0.87 ± 0.01 ab | 10.89 ± 0.11 d | 23.85 ± 2.47 b |
| Lt97SEQ24 | 0.87 ± 0.02 ab | 11.09 ± 0.09 d | 22.00 ± 1.56 b |
References
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate Change Effects on Grapevine Physiology and Biochemistry: Benefits and Challenges of High Altitude as an Adaptation Strategy. Front. Plant Sci. 2022, 13, 835425. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez De Toda, F. Current Viticultural Techniques to Mitigate the Effects of Global Warming on Grape and Wine Quality: A Comprehensive Review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a Tool to Improve pH in Red Wines from Warm Regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Comuzzo, P.; Del Fresno, J.M.; Voce, S.; Loira, I.; Morata, A. Emerging Biotechnologies and Non-Thermal Technologies for Winemaking in a Context of Global Warming. Front. Microbiol. 2023, 14, 1273940. [Google Scholar] [CrossRef]
- Colibaba, L.C.; Bosoi, I.; Pușcalău, M.; Bodale, I.; Luchian, C.; Rotaru, L.; Cotea, V.V. Climatic Projections vs. Grapevine Phenology: A Regional Case Study. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13381. [Google Scholar] [CrossRef]
- Llanaj, C.; McGregor, G. Climate Change, Grape Phenology, and Frost Risk in Southeast England. Aust. J. Grape Wine Res. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Baltazar, M.; Castro, I.; Gonçalves, B. Adaptation to Climate Change in Viticulture: The Role of Varietal Selection—A Review. Plants 2025, 14, 104. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Yeasts for the Future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Vejarano, R.; Gil-Calderón, A. Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Bourbon-Melo, N.; Palma, M.; Rocha, M.P.; Ferreira, A.; Bronze, M.R.; Elias, H.; Sá-Correia, I. Use of Hanseniaspora guilliermondii and Hanseniaspora opuntiae to Enhance the Aromatic Profile of Beer in Mixed-Culture Fermentation with Saccharomyces cerevisiae. Food Microbiol. 2021, 95, 103678. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as Biocontrol Agent and Wine Aroma Enhancer in Combination with a Native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Nadai, C.; Giacomini, A.; Corich, V. The Addition of Wine Yeast Starmerella bacillaris to Grape Skin Surface Influences Must Fermentation and Glycerol Production. OENO One 2021, 55, 47–55. [Google Scholar] [CrossRef]
- Canonico, L.; Gattucci, S.; Moretti, L.; Agarbati, A.; Comitini, F.; Ciani, M. Ethanol Reduction in Montepulciano Wine: Starmerella bombicola Sequential Fermentation at Pilot Scale Under Aeration Conditions. Foods 2025, 14, 618. [Google Scholar] [CrossRef]
- Rojas, V. Acetate Ester Formation in Wine by Mixed Cultures in Laboratory Fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of Pure and Mixed Cultures of the Main Wine Yeast Species on Grape Must Fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of Mixed Culture Fermentations on Yeast Populations and Aroma Profile. LWT 2012, 49, 8–13. [Google Scholar] [CrossRef]
- Benito, S. Combined Use of Lachancea thermotolerans and Schizosaccharomyces pombe in Winemaking: A Review. Microorganisms 2020, 8, 655. [Google Scholar] [CrossRef]
- Vicente, J.; Baran, Y.; Navascués, E.; Santos, A.; Calderón, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological Management of Acidity in Wine Industry: A Review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. 16 Years Research on Lactic Acid Production with Yeast—Ready for the Market. Biotechnol. Genet. Eng. Rev. 2010, 27, 229–256. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non- Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on Chemical Composition and Sensory Profiles of Viognier Wines. J. Fungi 2022, 8, 474. [Google Scholar] [CrossRef]
- Vicente, J.; Vladic, L.; Navascués, E.; Brezina, S.; Santos, A.; Calderón, F.; Tesfaye, W.; Marquina, D.; Rauhut, D.; Benito, S. A Comparative Study of Lachancea thermotolerans Fermentative Performance under Standardized Wine Production Conditions. Food Chem. X 2024, 21, 101214. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of Non- Saccharomyces Yeasts on the Volatile Chemical Profile of Shiraz Wine: Shiraz Wines Fermented by Non-Saccharomyces Yeasts. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Banilas, G.; Sgouros, G.; Nisiotou, A. Development of Microsatellite Markers for Lachancea thermotolerans Typing and Population Structure of Wine-Associated Isolates. Microbiol. Res. 2016, 193, 1–10. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Branco, P.; Almeida, M.G.; Caldeira, J.; Albergaria, H.; Arneborg, N. Cell-to-Cell Contact and Antimicrobial Peptides Play a Combined Role in the Death of Lachanchea thermotolerans during Mixed-Culture Alcoholic Fermentation with Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2015, 362, fnv103. [Google Scholar]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological Traits of Lachancea thermotolerans Show Signs of Domestication and Allopatric Differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on Chemical Composition and Sensory Profiles of Merlot Wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans Yeast Strains as an Alternative to theTraditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airen Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Del Fresno, J.M.; Malfeito-Ferreira, M.; Mena-Morales, A.; García-Romero, E.; Heras, J.M.; Loira, I.; González, C.; Morata, A. Wine Bioacidification: Fermenting Airén Grape Juices with Lachancea thermotolerans and Metschnikovia pulcherrima Followed by Sequential Saccharomyces cerevisiae Inoculation. Int. J. Food Microbiol. 2025, 427, 110977. [Google Scholar] [CrossRef]
- Sgouros, G.; Mallouchos, A.; Filippousi, M.-E.; Banilas, G.; Nisiotou, A. Molecular Characterization and Enological Potential of A High Lactic Acid-Producing Lachancea thermotolerans Vineyard Strain. Foods 2020, 9, 595. [Google Scholar] [CrossRef]
- Benito, S. The Impacts of Lachancea thermotolerans Yeast Strains on Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G. Potential Role of Yeast Strains Isolated from Grapes in the Production of Taurasi DOCG. Front. Microbiol. 2016, 7, 809. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Bañuelos, M.A.; Heras, J.M.; Cuerda, R.; Morata, A. Industrial Performance of Several Lachancea thermotolerans Strains for pH Control in White Wines from Warm Areas. Microorganisms 2020, 8, 830. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; López, C.; Song, C.; Vejarano, R.; Loira, I.; Palomero, F.; Suarez Lepe, J.A. Use of Fumaric Acid to Control pH and Inhibit Malolactic Fermentation in Wines. Food Addit. Contam. Part A 2020, 37, 228–238. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Battjes, J.; Melkonian, C.; Mendoza, S.N.; Haver, A.; Al-Nakeeb, K.; Koza, A.; Schrubbers, L.; Wagner, M.; Zeidan, A.A.; Molenaar, D.; et al. Ethanol-Lactate Transition of Lachancea thermotolerans Is Linked to Nitrogen Metabolism. Food Microbiol. 2023, 110, 104167. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; González, C.; Bañuelos, M.A.; Cuerda, R.; Heras, J.M.; Vaquero, C.; Suárez-Lepe, J.A. Biological Acidification by Lachancea thermotolerans. In White Wine Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–142. [Google Scholar]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identificationof Yeasts by RFLP Analysis of the 5.85 rRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Benito, S.; Marquina, D.; Santos, A. Microsatellite Typing of Lachancea thermotolerans for Wine Fermentation Monitoring. Int. J. Food Microbiol. 2023, 394, 110186. [Google Scholar] [CrossRef]
- Legras, J.-L.; Karst, F. Optimisation of Interdelta Analysis for Saccharomyces cerevisiae Strain Characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Morris, E.O.; Eddy, A.A. Method for the measurement of wild yeast infection in pitching yeast. J. Inst. Brew. 1957, 63, 34–35. [Google Scholar] [CrossRef]
- OIV. Compendium of Intenational Methods of Wine and Must Analysis, 2023rd ed.; OIV: Dijon, France, 2023; Volume 1–2. [Google Scholar]
- Mislata, A.M.; Puxeu, M.; Tomás, E.; Nart, E.; Ferrer-Gallego, R. Influence of the Oxidation in the Aromatic Composition and Sensory Profile of Rioja Red Aged Wines. Eur. Food Res. Technol. 2020, 246, 1167–1181. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First Study of Determination of Aromatic Compounds of Red Wine from Vitis vinifera Cv. Castañal Grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Graña, M.; Oliveira, J.M. Determination of Odorants in Varietal Wines from International Grape Cultivars (Vitis vinifera) Grown in NW Spain. S. Afr. J. Enol. Vitic. 2016, 34, 212–222. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors (3rd ed.). International Organization for Standarization (ISO): Geneva, Switzerland, 2023.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms (2nd ed.). International Organization for Standarization (ISO): Brussels, Belgique, 2007.
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Luyt, N.A.; De Witt, R.N.; Divol, B.; Patterton, H.G.; Setati, M.E.; Taillandier, P.; Bauer, F.F. Physical Cell-Cell Contact Elicits Specific Transcriptomic Responses in Wine Yeast Species. Microbiol. Spectr. 2024, 12, e00572-23. [Google Scholar] [CrossRef]
- Fairbairn, S.; Engelbrecht, L.; Setati, M.E.; Du Toit, M.; Bauer, F.F.; Divol, B.; Rossouw, D. Combinatorial Analysis of Population Dynamics, Metabolite Levels and Malolactic Fermentation in Saccharomyces cerevisiae/Lachancea thermotolerans Mixed Fermentations. Food Microbiol. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An Integrative View of the Role of Lachancea thermotolerans in Wine Technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications, 1st ed.; Wiley: Chichester, UK, 2005. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley: Chichester, UK, 2016. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining Wine Aroma from Compositional Data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative Study of Aromatic Compounds in Two Young White Wines Subjected to Pre-Fermentative Cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortega, L.; Escudero, A.; Cacho, J.F. A Comparative Study of the Ability of Different Solvents and Adsorbents to Extract Aroma Compounds from Alcoholic Beverages. J. Chromatogr. Sci. 2000, 38, 469–476. [Google Scholar] [CrossRef]
- López, R.; Ezpeleta, E.; Sánchez, I.; Cacho, J.; Ferreira, V. Analysis of the Aroma Intensities of Volatile Compounds Released from Mild Acid Hydrolysates of Odourless Precursors Extracted from Tempranillo and Grenache Grapes Using Gas Chromatography-Olfactometry. Food Chem. 2004, 88, 95–103. [Google Scholar] [CrossRef]
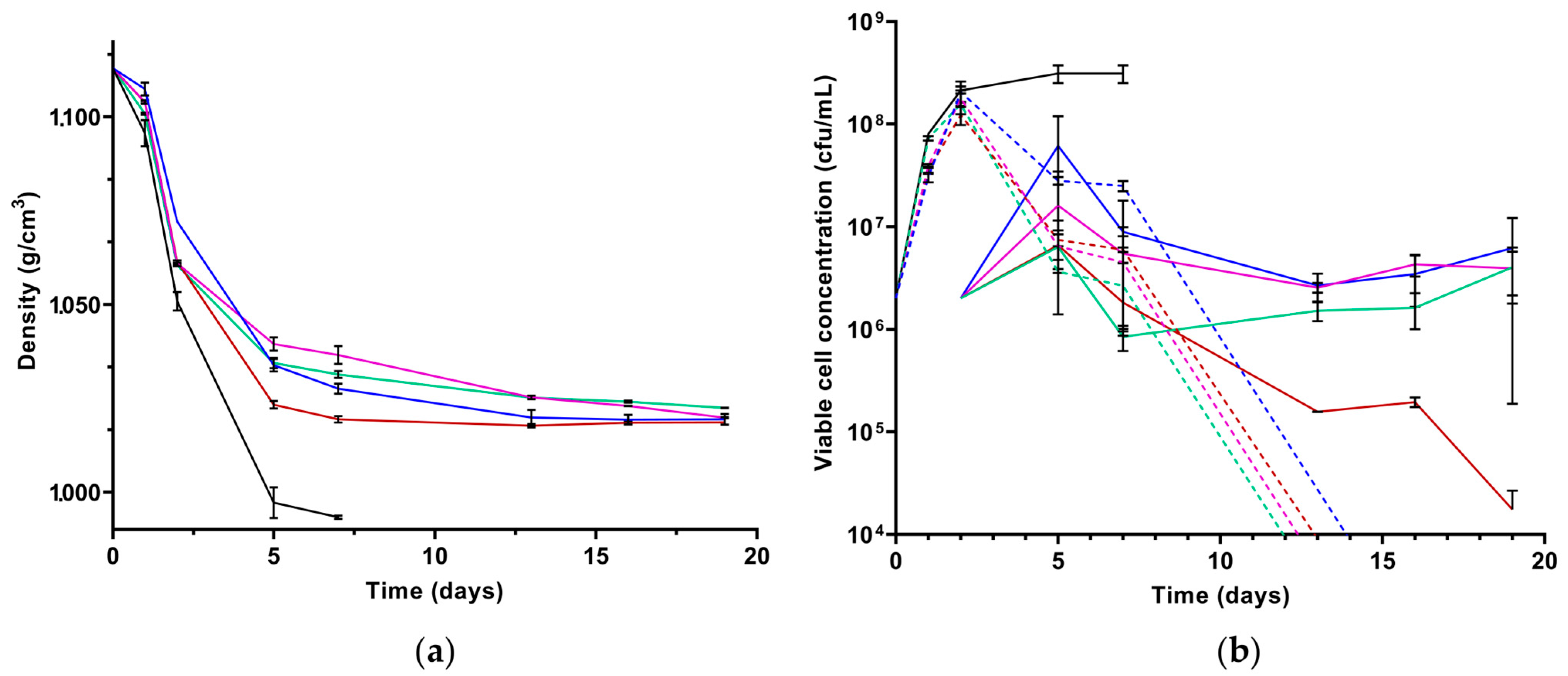
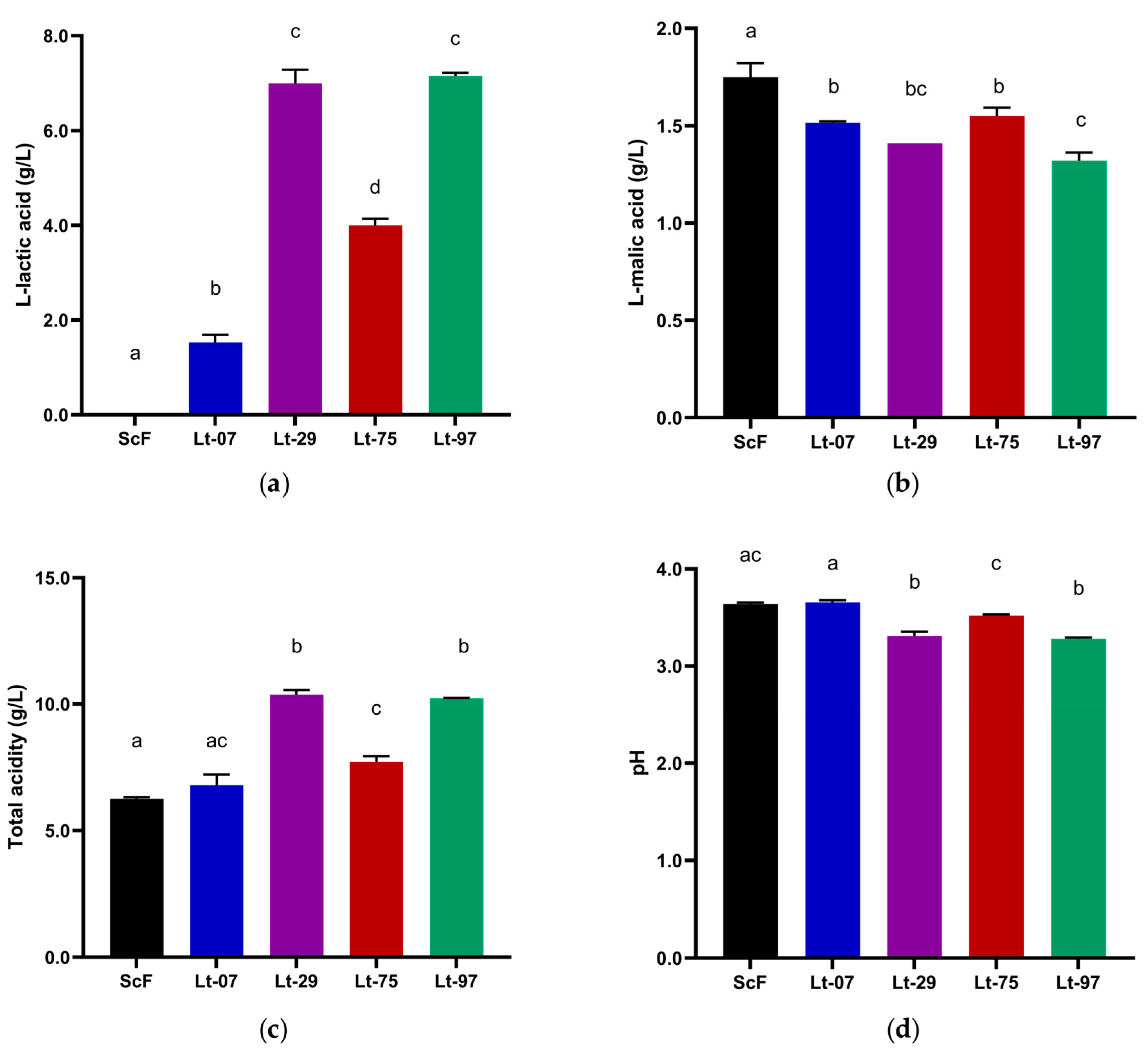
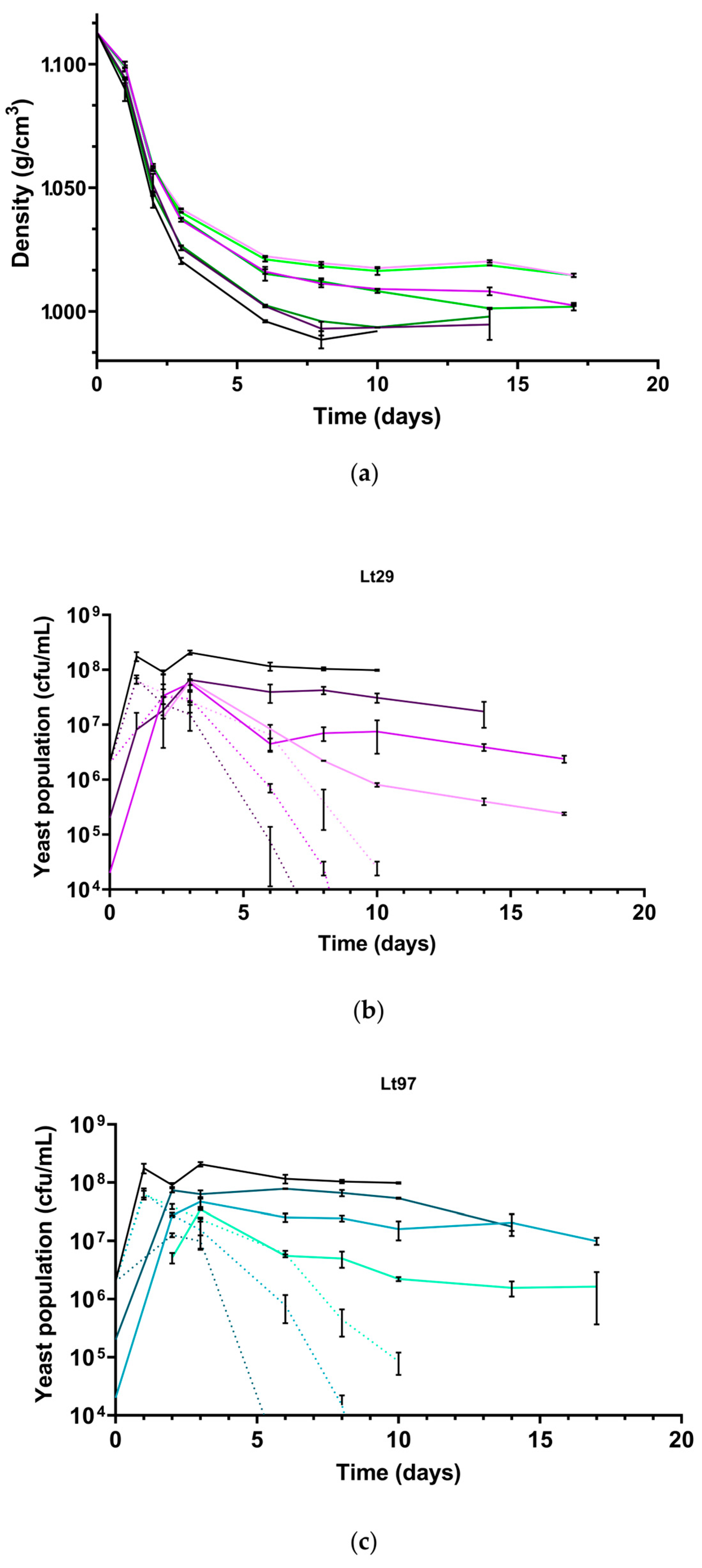
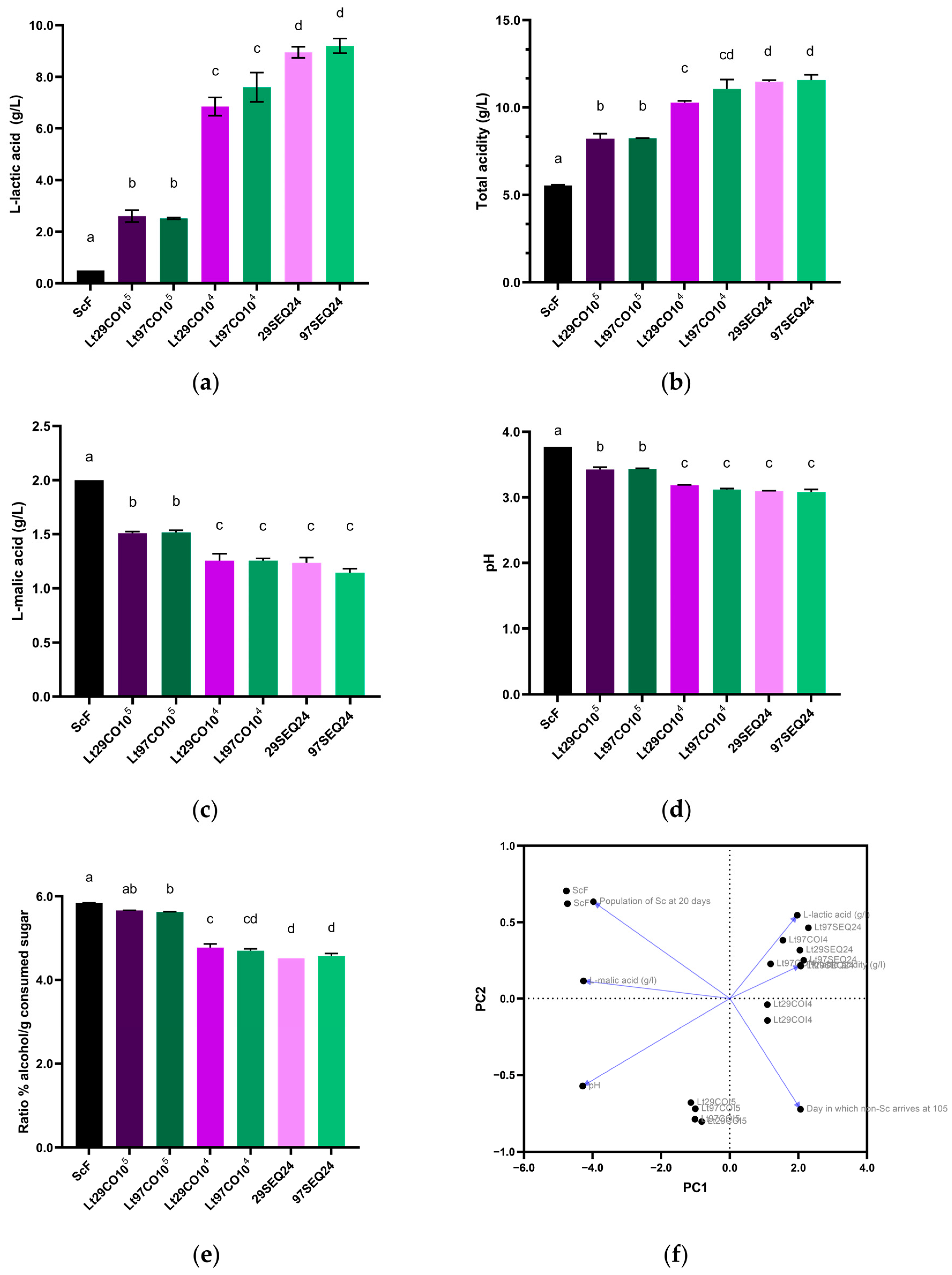
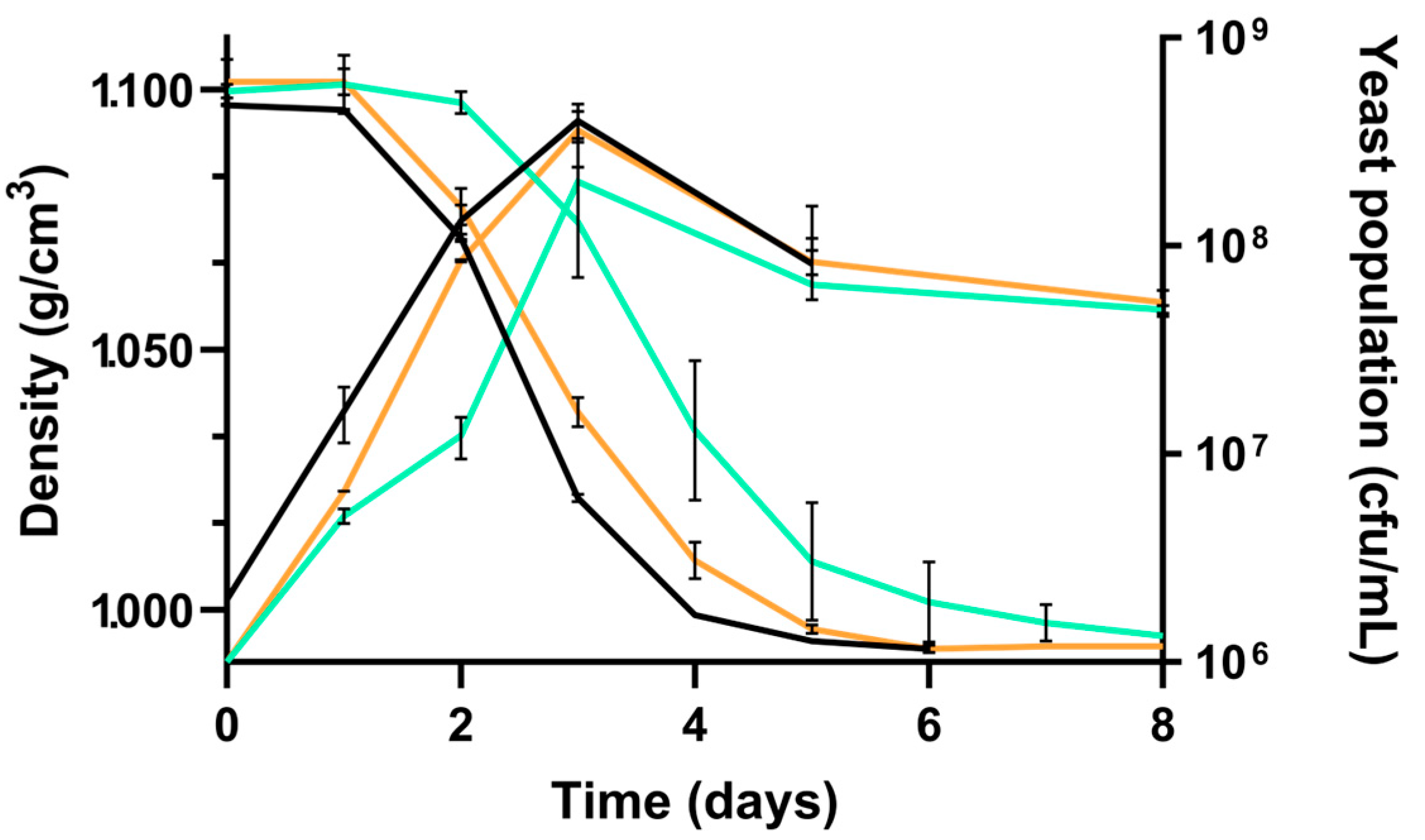
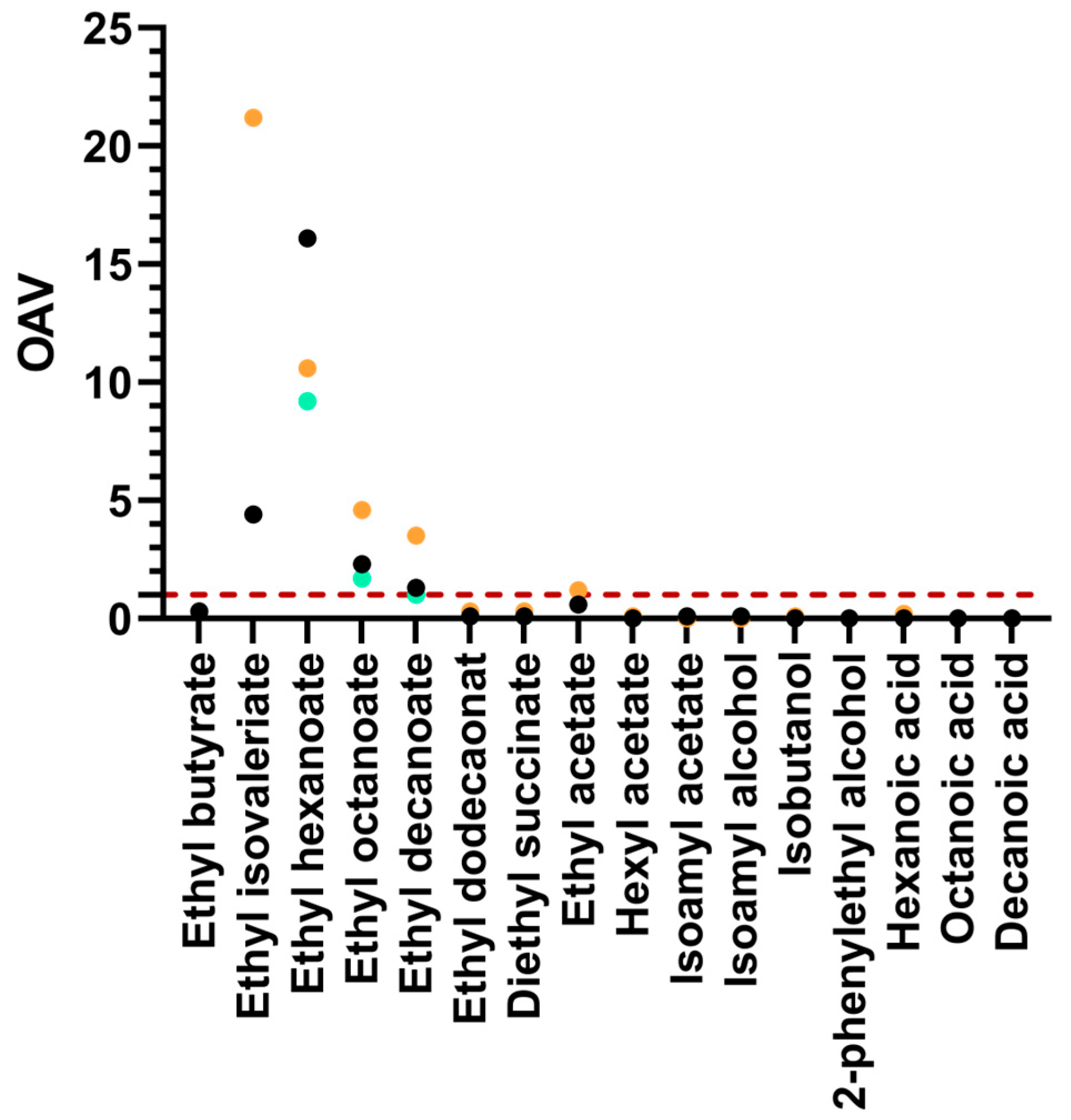
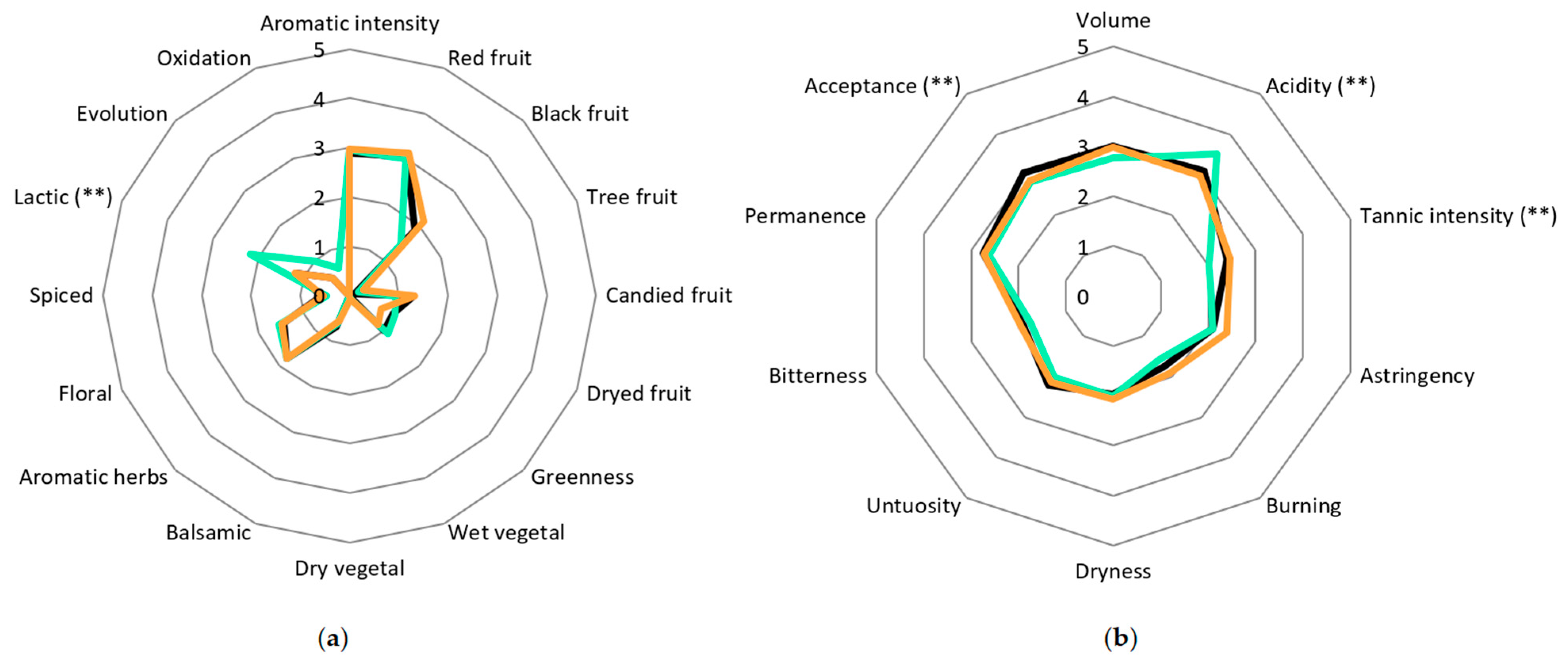
| Must | Density (g/cm3) | Total Sugar (g/L) | NFA (mg/L) | pH | Total Acidity (g/L) |
|---|---|---|---|---|---|
| Laboratory scale | 1.112 ± 0.001 | 264.70 ± 8.30 | 211.76 ± 5.86 | 3.85 ± 0.02 | 3.18 ± 0.20 |
| Pilot scale | 1.098 ± 0.001 | 231.72 ± 5.23 | 185.76 ± 7.09 | 3.46 ± 0.01 | 3.57 ± 0.10 |
| Conditions | L-Malic Acid (g/L) | L-Lactic Acid (g/L) | Total Acidity (g/L) | Volatile Acidity (g/L) | pH | Alcohol % (v/v) |
|---|---|---|---|---|---|---|
| ScM | 0.54 ± 0.11 a | 0.92 ± 0.01 a | 5.49 ± 0.01 a | 0.40 ± 0.01 a | 3.55 ± 0.01 a | 14.13 ± 0.03 ab |
| Lt97 + ScM | 0.63 ± 0.02 a | 4.65 ± 0.98 b | 8.86 ± 1.07 b | 0.53 ± 0.13 a | 3.29 ± 0.07 b | 13.78 ± 0.23 b |
| ScC | 0.73 ± 0.11 a | 0.97 ± 0.03 a | 5.03 ± 0.04 a | 0.33 ± 0.01 a | 3.60 ± 0.01 a | 14.75 ± 0.28 a |
| Concentration (µg/L) | ScM | Lt97 + ScM | ScC |
|---|---|---|---|
| Ethyl butyrate | 133.1 ± 9.5 a | 105.2 ± 9.1 a | 135.6 ± 33.9 a |
| Ethyl isovaleriate | 13.1 ± 1.2 a | 13.1 ± 1.8 a | 63.7 ± 55.2 a |
| Ethyl hexanoate | 1285.7 ± 193.7 b | 736.6 ± 117.3 a | 849.2 ± 113.2 b |
| Ethyl octanoate | 1341.3 ± 648.1 a | 1012.7 ± 180.2 a | 2650.4 ± 119.0 b |
| Ethyl decanoate | 674.8 ± 73.6 a | 515.4 ± 120.9 a | 1772.1 ± 119 b |
| Ethyl dodecanoate | 162.6 ± 17.7 a | 116.2 ± 21.2 a | 394.4 ± 47.4 b |
| Diethyl succinate | 112.4 ± 23.7 a | 111.0 ± 34.3 a | 349.2 ± 39.2 b |
| Total ethyl esters | 3723.1 ± 541.2 a | 2610.2 ± 453 b | 6214.6 ± 281.8 c |
| Ethyl acetate | 4249.9 ± 320.5 a | 4799.4 ± 1193.5 a | 8805.4 ± 613.2 b |
| Hexyl acetate | 28.8 ± 0.7 a | 32.8 ± 4.6 a | 54.7 ± 30.6 a |
| Isobutyl acetate | 334.9 ± 17.2 a | 445.6 ± 55.5 a | 591.2 ± 517.4 a |
| Isoamyl acetate | 16.7 ± 3.2 a | 19.9 ± 4.4 a | n.d. |
| Total acetates Isoamyl alcohol | 4630.3 ± 338.2 a 4427.2 ± 338.2 a | 5297.7 ± 1248.8 a 4192.3 ± 578.5 a | 9451.4 ± 953.2 b 1048.2 ± 305.5 b |
| Isobutanol | 397.8 ± 58.4 a | 425.2 ± 53.3 a | 6372.1 ± 828.2 b |
| 2-phenylethyl alcohol Total alcohols | 1148.0 ± 283 a 5972.9 ± 819.9 a | 898.4 ± 234.3 a 5516.9 ± 863.6 a | 59.1 ± 55.2 b 7479.5 ± 982.7 a |
| Hexanoic acid | 12.8 ± 2.4 a | 10.3 ± 1.7 a | 592.6 ± 49.0 b |
| Octanoic acid | 106.0 ± 8.4 a | 61.6 ± 9.6 b | n.d. |
| Decanoic acid | 57.2 ± 3.3 a | 48.3 ± 9.5 a | n.d. |
| Total fatty acids | 176.0 ± 12.3 a | 120.3 ± 20.7 a | 592.6 ± 49.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Vázquez, D.; Sunyer-Figueres, M.; Vázquez, J.; Puxeu, M.; Nart, E.; de Lamo, S.; Andorrà, I. Selection and Use of Wild Lachancea thermotolerans Strains from Rioja AOC with Bioacidificant Capacity as Strategy to Mitigate Climate Change Effects in Wine Industry. Beverages 2025, 11, 70. https://doi.org/10.3390/beverages11030070
Fernández-Vázquez D, Sunyer-Figueres M, Vázquez J, Puxeu M, Nart E, de Lamo S, Andorrà I. Selection and Use of Wild Lachancea thermotolerans Strains from Rioja AOC with Bioacidificant Capacity as Strategy to Mitigate Climate Change Effects in Wine Industry. Beverages. 2025; 11(3):70. https://doi.org/10.3390/beverages11030070
Chicago/Turabian StyleFernández-Vázquez, Daniel, Mercè Sunyer-Figueres, Jennifer Vázquez, Miquel Puxeu, Enric Nart, Sergi de Lamo, and Imma Andorrà. 2025. "Selection and Use of Wild Lachancea thermotolerans Strains from Rioja AOC with Bioacidificant Capacity as Strategy to Mitigate Climate Change Effects in Wine Industry" Beverages 11, no. 3: 70. https://doi.org/10.3390/beverages11030070
APA StyleFernández-Vázquez, D., Sunyer-Figueres, M., Vázquez, J., Puxeu, M., Nart, E., de Lamo, S., & Andorrà, I. (2025). Selection and Use of Wild Lachancea thermotolerans Strains from Rioja AOC with Bioacidificant Capacity as Strategy to Mitigate Climate Change Effects in Wine Industry. Beverages, 11(3), 70. https://doi.org/10.3390/beverages11030070






