Gain of Body Fat and Intake of Energy in Rats with Low Dose of Caloric and Non-Caloric Sweeteners Used in Reformulation Beverage in Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
Sweeteners Selection
2.2. Baseline and Consecutive Measurements
2.3. Weekly Weight and Caloric Intake Records
2.4. Sacrifice and Blood Collection
2.5. Dissection of Adipose Tissue
2.6. Measurement of Biochemical Indicators
2.7. Statistical Analysis
3. Results
3.1. Drink Intake and Weight Gain of Wistar Rats
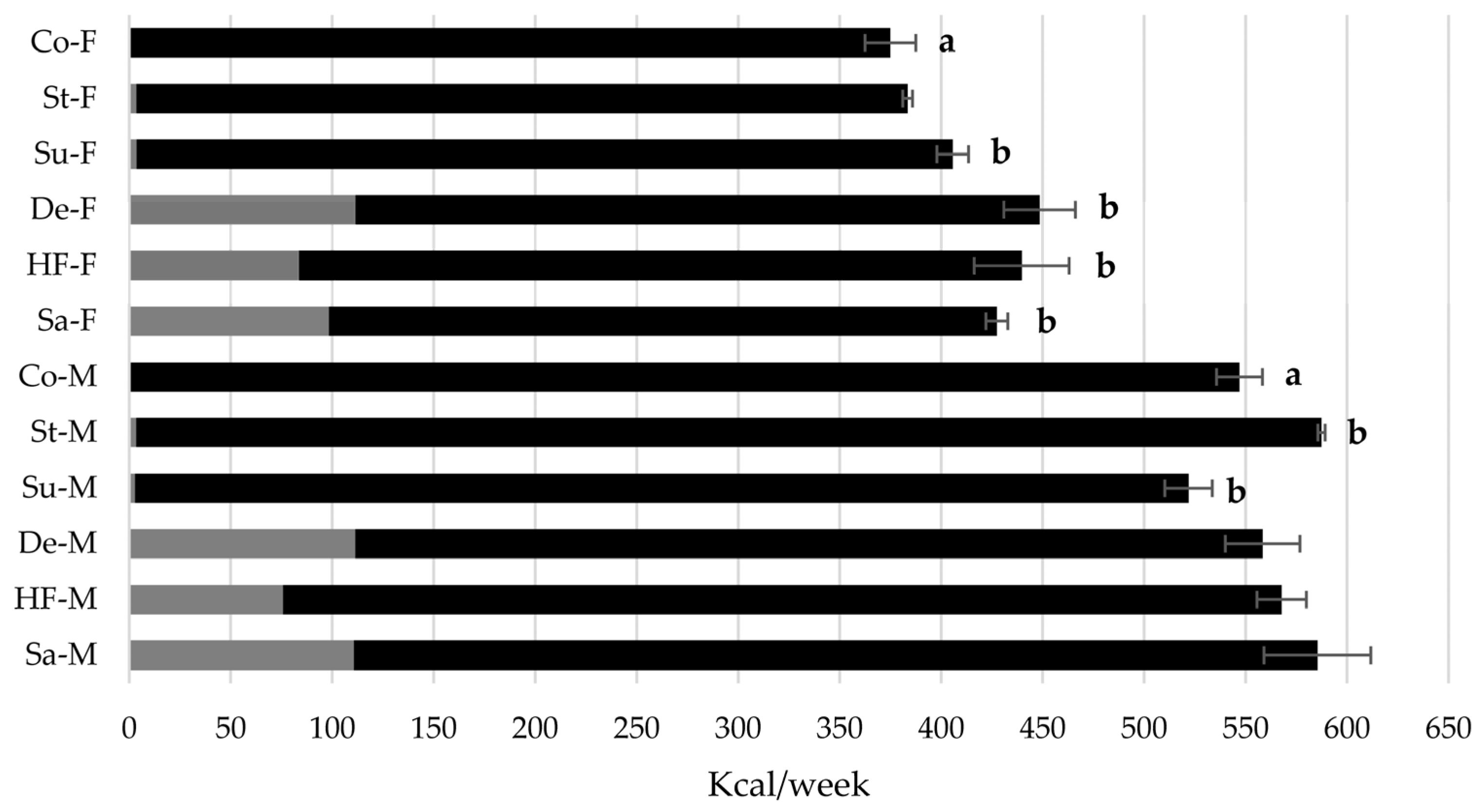
3.2. Energy Intake in Wistar Rats
3.3. Adipose Tissue in Wistar Rats
3.4. Biochemical and Adiposity Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCSs | Non-caloric sweeteners |
| CSs | Caloric sweeteners |
| GAT | Gonadal adipose tissue |
| MAT | Mesenteric adipose tissue |
| HDL | High-density lipoprotein |
| ANOVA | Analysis of variance |
| HFCS | High-fructose corn syrup |
| TC | Total cholesterol |
| TG | Triglycerides |
References
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrera, A.; Cruz-Lopez, M. Childhood obesity: Current situation in Mexico. Nutr. Hosp. 2019, 36, 463–469. [Google Scholar] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Gaona-Pineda, E.B.; Rodriguez-Ramirez, S.; Morales-Ruan, C.; Cuevas-Nasu, L.; Mendez-Gomez-Humaran, I.; Valenzuela-Bravo, D.G.; Avila-Arcos, M.A. Sobrepeso, obesidad y consumo de azúcares en población escolar y adolescente de México. Ensanut 2020–2022. Salud Publica Mex. 2023, 65, s570–s580. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Galvan-Valencia, O.; Hernandez-Barrera, L.; Oviedo-Solis, C.; Barquera, S. Prevalencia de obesidad y factores de riesgo asociados en adultos mexicanos: Resultados de la Ensanut 2022. Salud Publica Mex. 2023, 65, s238–s247. [Google Scholar] [CrossRef]
- Sanchez-Pimienta, T.G.; Batis, C.; Lutter, C.K.; Rivera, J.A. Sugar-Sweetened Beverages Are the Main Sources of Added Sugar Intake in the Mexican Population. J. Nutr. 2016, 146, 1888S–1896S. [Google Scholar] [CrossRef]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Lim, S.; Ezzati, M.; Mozaffarian, D.; Global Burden of Diseases Nutrition; Chronic Diseases Expert Group (NutriCoDE). Estimated Global, Regional, and National Disease Burdens Related to Sugar-Sweetened Beverage Consumption in 2010. Circulation 2015, 132, 639–666. [Google Scholar] [CrossRef]
- Braverman-Bronstein, A.; Camacho-Garcia-Formenti, D.; Zepeda-Tello, R.; Cudhea, F.; Singh, G.M.; Mozaffarian, D.; Barrientos-Gutierrez, T. Mortality attributable to sugar sweetened beverages consumption in Mexico: An update. Int. J. Obes. 2020, 44, 1341–1349. [Google Scholar] [CrossRef]
- Nguyen, M.; Jarvis, S.E.; Tinajero, M.G.; Yu, J.; Chiavaroli, L.; Mejia, S.B.; Khan, T.A.; Tobias, D.K.; Willett, W.C.; Hu, F.B.; et al. Sugar-sweetened beverage consumption and weight gain in children and adults: A systematic review and meta-analysis of prospective cohort studies and randomized controlled trials. Am. J. Clin. Nutr. 2023, 117, 160–174. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Golabek, K.D.; Regulska-Ilow, B. Dietary support in insulin resistance: An overview of current scientific reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Shearrer, G.E.; Daniels, M.J.; Toledo-Corral, C.M.; Weigensberg, M.J.; Spruijt-Metz, D.; Davis, J.N. Associations among sugar sweetened beverage intake, visceral fat, and cortisol awakening response in minority youth. Physiol. Behav. 2016, 167, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef] [PubMed]
- Laviada-Molina, H.; Molina-Segui, F.; Perez-Gaxiola, G.; Cuello-Garcia, C.; Arjona-Villicana, R.; Espinosa-Marron, A.; Martinez-Portilla, R.J. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: Systematic review and meta-analysis. Obes. Rev. 2020, 21, e13020. [Google Scholar] [CrossRef]
- Tobiassen, P.A.; Koster-Rasmussen, R. Substitution of sugar-sweetened beverages with non-caloric alternatives and weight change: A systematic review of randomized trials and meta-analysis. Obes. Rev. 2024, 25, e13652. [Google Scholar] [CrossRef]
- Ahmad, S.Y.; Friel, J.K.; MacKay, D.S. The effect of the artificial sweeteners on glucose metabolism in healthy adults: A randomized, double-blinded, crossover clinical trial. Appl. Physiol. Nutr. Metab. 2020, 45, 606–612. [Google Scholar] [CrossRef]
- Tovar, A.P.; Navalta, J.W.; Kruskall, L.J.; Young, J.C. The effect of moderate consumption of non-nutritive sweeteners on glucose tolerance and body composition in rats. Appl. Physiol. Nutr. Metab. 2017, 42, 1225–1227. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, C.; Mendoza-Garcia, G.; Lopez-Rodriguez, G.; Hernandez-Cabrera, J.; Olivo-Ramirez, D.; Galvan, M. Association of sweetened beverage consumption with abdominal obesity and fasting glycemia in Mexican. Ann. Nutr. Metab. 2023, 78 (Suppl. S3), 134. [Google Scholar]
- Galván, M.; (Universidad Autónoma del Estado de Hidalgo, Pachuca de Soto Hidalgo, México); Ramírez-Ramírez, C.; (Universidad Autónoma del estado de Hidalgo, Pachuca de Soto Hidalgo, México). Analysis of the type and content of caloric and non-caloric sweeteners in 621 non-alcoholic beverages available on the Mexican market before and after front-of-package labeling: 2020–2022. 2025; Unpublished work. [Google Scholar]
- PROFECO. Jugos y bebidas saborizadas en presentaciones pequeñas dirigidos a niñas y niños. Rev. Del Consum. 2025, 578, 22–38. [Google Scholar]
- WHO. Reformulation of Food and Beverage Products for Healthier Diets: Policy Brief; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Jensen, J.D.; Mielby, L.A.; Kidmose, U. Consumer preferences for attributes in sweet beverages and market impacts of beverage innovation. Appetite 2024, 197, 107329. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Gatlin, C.A. Sweeteners: State of knowledge review. Neurosci. Biobehav. Rev. 1993, 17, 313–345. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite 1991, 16, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Delogu, F.; Huddas, C.; Steven, K.; Hachem, S.; Lodhia, L.; Fernandez, R.; Logerstedt, M. A Dissociation Between Recognition and Hedonic Value in Caloric and Non-caloric Carbonated Soft Drinks. Front. Psychol. 2016, 7, 36. [Google Scholar] [CrossRef][Green Version]
- Chen, H.H.; Chu, C.H.; Wen, S.W.; Lai, C.C.; Cheng, P.W.; Tseng, C.J. Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats. Nutrients 2019, 11, 2851. [Google Scholar] [CrossRef]
- Gentry, R.T.; Wade, G.N. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J. Comp. Physiol. Psychol. 1976, 90, 747–754. [Google Scholar] [CrossRef]
- Sclafani, A.; Hertwig, H.; Vigorito, M.; Feigin, M.B. Sex differences in polysaccharide and sugar preferences in rats. Neurosci. Biobehav. Rev. 1987, 11, 241–251. [Google Scholar] [CrossRef]
- Michon, C.; O’sullivan, M.; Delahunty, C.; Kerry, J. The investigation of gender-related sensitivity differences in food perception. J. Sens. Stud. 2009, 24, 922–937. [Google Scholar] [CrossRef]
- Martínez, C.; González, E.; Garc, R.S.; Salas, G.; Constantino-Casas, F.; Macías, L.; Gracia, I.; Tovar, C.; Durán-de-Bazúa, C. Effects on body mass of laboratory rats after ingestion of drinking water with sucrose, fructose, aspartame, and sucralose additives. Open Obes. J. 2010, 2, 116–124. [Google Scholar] [CrossRef]
- Burgeiro, A.; Cerqueira, M.G.; Varela-Rodriguez, B.M.; Nunes, S.; Neto, P.; Pereira, F.C.; Reis, F.; Carvalho, E. Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet. Nutrients 2017, 9, 638. [Google Scholar] [CrossRef]
- Barrios-Correa, A.A.; Estrada, J.A.; Martel, C.; Olivier, M.; Lopez-Santiago, R.; Contreras, I. Chronic Intake of Commercial Sweeteners Induces Changes in Feeding Behavior and Signaling Pathways Related to the Control of Appetite in BALB/c Mice. Biomed. Res. Int. 2018, 2018, 3628121. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tapia, M.; Martínez-Medina, J.; Tovar, A.R.; Torres, N. Natural and artificial sweeteners and high fat diet modify differential taste receptors, insulin, and TLR4-mediated inflammatory pathways in adipose tissues of rats. Nutrients 2019, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.W.; Timmons, C.R. Sex differences in response to taste and postingestive consequences of sugar solutions. Physiol. Behav. 1976, 17, 221–225. [Google Scholar] [CrossRef]
- Mendoza-Pérez, S.; García-Gómez, R.S.; Ordaz-Nava, G.; Gracia-Mora, M.I.; Macías-Rosales, L.; Morales-Rico, H.; Salas-Garrido, G.; Pérez-Armendáriz, E.M.; Bustamante-García, R.; Durán-Domínguez-de-Bazúa, M.D.C. Consumption of sweeteners at different stages of life: Effects on body mass, food and drink intake in male and female Wistar rats. Int. J. Food Sci. Nutr. 2021, 72, 935–946. [Google Scholar] [CrossRef]
- Ranawana, D.; Henry, C. Are caloric beverages compensated for in the short-term by young adults? An investigation with particular focus on gender differences. Appetite 2010, 55, 137–146. [Google Scholar] [CrossRef]
- Davy, B.M.; Van Walleghen, E.L.; Orr, J.S. Sex differences in acute energy intake regulation. Appetite 2007, 49, 141–147. [Google Scholar] [CrossRef]
- Mattes, R.D.; Popkin, B.M. Nonnutritive sweetener consumption in humans: Effects on appetite and food intake and their putative mechanisms. Am. J. Clin. Nutr. 2009, 89, 1–14. [Google Scholar] [CrossRef]
- Yang, Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101. [Google Scholar]
- Smeets, P.A.; Weijzen, P.; de Graaf, C.; Viergever, M.A. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage 2011, 54, 1367–1374. [Google Scholar] [CrossRef]
- Jacquillet, G.; Debnam, E.S.; Unwin, R.J.; Marks, J. Acute saccharin infusion has no effect on renal glucose handling in normal rats in vivo. Physiol. Rep. 2018, 6, e13804. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Gómez, C.A.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Ramírez-Durán, N.; Valdés-Ramos, R.; Mondragón-Velásquez, T.; Escoto-Herrera, J.A. Chronic consumption of sweeteners and its effect on glycaemia, cytokines, hormones, and lymphocytes of GALT in CD1 mice. BioMed Res. Int. 2018, 2018, 1345282. [Google Scholar] [CrossRef]
- Kim, E.; Lim, S.M.; Kim, M.S.; Yoo, S.H.; Kim, Y. Phyllodulcin, a Natural Sweetener, Regulates Obesity-Related Metabolic Changes and Fat Browning-Related Genes of Subcutaneous White Adipose Tissue in High-Fat Diet-Induced Obese Mice. Nutrients 2017, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Ahmad, R.S.; Arshad, M.S.; Mushtaq, Z.; Hussain, S.M.; Hameed, A. Antihyperlipidemic efficacy of aqueous extract of Stevia rebaudiana Bertoni in albino rats. Lipids Health Dis. 2018, 17, 175. [Google Scholar] [CrossRef]
- Elnaga, N.A.; Massoud, M.I.; Yousef, M.; Mohamed, H.H. Effect of stevia sweetener consumption as non-caloric sweetening on body weight gain and biochemical’s parameters in overweight female rats. Ann. Agric. Sci. 2016, 61, 155–163. [Google Scholar] [CrossRef]
- Shukla, R.; Gupta, S.; Gambhir, J.K.; Prabhu, K.M.; Murthy, P.S. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis in hypercholesterolaemic rabbits. J. Ethnopharmacol. 2004, 92, 47–51. [Google Scholar] [CrossRef]
| Males | ||||||
|---|---|---|---|---|---|---|
| Week | Sucrose | HFCS | Dextrose | Sucralose | Stevia | Control |
| 1 | 307.3 ± 9.5 bc | 290.8 ± 2.4 b | 331.3 ± 10.2 bd | 203.2 ± 10.0 bd | 272.0 ± 32.9 b | 170.7 ± 6.9 ad |
| 4 | 423.9 ± 25.6 bc | 432.5 ± 14.8 b | 416.2 ± 21.4 b | 286.3 ± 2.5 bd | 338.3 ± 18.3 bd | 238.8 ± 13.7 ad |
| 8 | 391.3 ± 37.9 bc | 433.0 ± 7.7 b | 432.5 ± 35.6 b | 216.3 ± 26.6 d | 304.3 ± 52.2 b | 188.3 ± 3.6 ad |
| 12 | 417.7 ± 58.8 bc | 447.2 ± 11.5 b | 434.3 ± 2.5 b | 256.3 ± 38.7 bd | 242.4 ± 30.8 bd | 172.2 ± 4.9 a |
| 16 | 408.3 ± 27.4 bc | 391.7 ± 9.1 b | 400.0 ± 3.3 b | 259.9 ± 58.4 d | 255.0 ± 68.5 d | 158.3 ± 10.9 a |
| Total | 386.2 ± 18.2 bc | 372.6 ± 10.8 b | 383.3 ± 3.7 b | 238.2 ± 27.6 bd | 281.3 ± 33.8 bd | 182.6 ± 3.2 a |
| Female | ||||||
| 1 | 306.5 ± 11.9 bc | 302.7 ± 46.4 b | 314.9 ± 14.6 b | 284.8 ± 13.7 b | 260.9 ± 12.4 bd | 180.5 ± 5.3 a |
| 4 | 414.7 ± 25.2 b | 406.5 ± 18.8 b | 397.5 ± 2.7 b | 344.5 ± 32.3 b | 347.2 ± 37.4 b | 180.8 ± 10.0 a |
| 8 | 333.8 ± 20.6 bc | 419.9 ± 18.2 bd | 408.7 ± 12.4 bd | 364.2 ± 39.2 b | 334.3 ± 21.2 b | 139.9 ± 25.6 a |
| 12 | 403.0 ± 30.7 bc | 407.5 ± 28.9 b | 448.6 ± 35.6 b | 278.0 ± 28.9 bd | 272.8 ± 20.1 bd | 154.9 ± 34.1 a |
| 16 | 367.5 ± 11.9 bc | 390.8 ± 2.8 bd | 461.5 ± 37.3 bd | 308.3 ± 3.6 bd | 315.8 ± 33.7 b | 140.8 ± 13.7 a |
| Total | 350.1 ± 22.9 bc | 364.2 ± 9.1 b | 379.6 ± 30.8 b | 296.8 ± 19.0 bd | 281.2 ± 5.2 bd | 154.6 ± 18.5 a |
| Males | ||||
|---|---|---|---|---|
| Group | Gonadal | Mesenteric | Retroperitoneal | Total |
| Sucrose | 2.8 ± 0.38 | 2.4 ± 0.31 | 3.5 ± 0.91 | 8.7 ± 1.2 |
| HFCS | 2.3 ± 0.29 | 2.2 ± 0.52 | 3.2 ± 0.34 | 7.7 ± 0.62 |
| Dextrose | 3.7 ± 0.61 b | 2.8 ± 0.80 b | 3.9 ± 1.2 | 10.4 ± 2.5 b |
| Sucralose | 2.9 ± 0.26 | 1.9 ± 0.38 | 3.3 ± 0.49 | 8.0 ± 0.91 |
| Stevia | 3.1 ± 0.53 | 2.2 ± 0.21 | 4.6 ± 0.64 b | 10 ± 1.16 b |
| Control | 2.3 ± 0.33 a | 1.7 ± 0.32 a | 2.7 ± 0.19 a | 6.7 ± 0.60 a |
| Females | ||||
| Sucrose | 3.3 ± 0.94 | 1.5 ± 0.56 | 1.6 ± 0.50 | 6.4 ± 1.9 |
| HFCS | 4.7 ± 0.81 b | 1.9 ± 0.48 | 2.2 ± 0.56 | 8.9 ± 0.43 b |
| Dextrose | 4.1 ± 0.24 b | 1.7 ± 0.45 | 2.2 ± 0.56 | 7.8 ± 0.45 b |
| Sucralose | 3.4 ± 0.49 | 1.8 ± 0.30 | 2.1 ± 0.56 | 7.3 ± 0.72 |
| Stevia | 1.7 ± 0.55 | 1.4 ± 0.14 | 1.6 ± 0.08 | 4.7 ± 0.64 |
| Control | 2.2 ± 0.75 a | 1.2 ± 0.44 | 1.7 ± 0.66 | 5.2 ± 0.46 a |
| Males | |||||||
|---|---|---|---|---|---|---|---|
| Group | Glucose (mg/dL) | Triglycerides (mg/dL) | ColT (mg/dL) | ColHDL (mg/dL) | Insulin (ng/mL) | Leptin (ng/mL) | Adiponectin (ng/mL) |
| Sucrose | 94.0 ± 9.8 | 257.0 ± 15.6 b | 73.1 ± 12.9 | 27.5 ± 3.6 | 1.2 ± 1.2 | 17.7 ±8.3 | 36.5 ± 7.2 |
| HFCS | 94.6 ± 11.8 | 273.0 ± 44.7 b | 80.3 ± 15.4 | 32.7 ± 5.4 | 0.4 ± 0.2 b | 19.1 ± 8.8 | 37.6 ± 6.1 |
| Dextrose | 72.0 ± 8.0 | 299.7 ± 62.3 b | 75.9 ± 9.5 | 27.0 ± 4.8 | 1.9 ± 1.3 a | 21.3 ± 9.1 | 36.2 ± 5.6 |
| Sucralose | 85.8 ± 16.7 | 352.4 ± 36.2 b | 94.3 ± 7.0 | 26.6 ± 3.1 | 1.8 ± 1.4 a | 17.8 ± 9.8 | 27.7 ± 7.0 |
| Stevia | 87.0 ± 7.2 | 242.3 ± 36.4 b | 89.4 ± 6.3 | 39.3 ± 8.0 b | 0.9 ± 0.2 | 18.8 ± 6.7 | 28.6 ± 6.9 |
| Control | 78.8 ± 7.0 | 122.3 ± 51.2 a | 73.7 ± 7.4 | 28.7 ± 3.9 a | 1.7 ± 1.7 | 14.7 ± 5.8 | 28.8 ± 12.1 |
| Females | |||||||
| Sucrose | 63.8 ± 12.0 | 105.8 ± 32.1 b | 77.7 ± 11.2 | 33.6 ± 2.7 | 0.4 ± 0.1 | 5.2 ± 2.9 | 29.6 ± 4.2 |
| HFCS | 90.4 ± 11.8 b | 61.2 ± 21.1 | 83.7 ± 18.3 | 34.4 ± 2.5 | 0.4 ± 0.1 | 4.9 ± 4.6 | 28.6 ± 8.4 |
| Dextrose | 60.6 ± 19.2 | 88.4 ± 22.8 b | 100.1 ± 18.4 | 39.9 ± 4.7 | 0.8 ± 0.4 b | 6.8 ± 3.1 | 36.5 ± 5.0 |
| Sucralose | 67.5 ± 9.9 | 108.9 ± 15.7 b | 76.1 ± 12.9 | 34.3 ± 3.1 | 0.3 ± 0.1 a | 3.0 ± 1.1 | 27.5 ± 8.5 |
| Stevia | 70.9 ± 10.0 | 63.4 ± 18.9 | 82.6 ± 12.3 | 36.6 ± 2.7 | 0.5 ± 0.1 | 2.4 ± 1.7 | 24.8 ± 4.6 |
| Control | 65.1 ± 10.5 a | 56.0 ± 6.1 a | 72.0 ± 9.1 | 36.8 ± 5.2 | 0.7 ± 0.1 | 2.8 ± 1.8 | 25.1 ± 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rodríguez, G.; Galván, M.; Galván-Valencia, O.; Gómez-Castillo, J. Gain of Body Fat and Intake of Energy in Rats with Low Dose of Caloric and Non-Caloric Sweeteners Used in Reformulation Beverage in Mexico. Beverages 2025, 11, 69. https://doi.org/10.3390/beverages11030069
López-Rodríguez G, Galván M, Galván-Valencia O, Gómez-Castillo J. Gain of Body Fat and Intake of Energy in Rats with Low Dose of Caloric and Non-Caloric Sweeteners Used in Reformulation Beverage in Mexico. Beverages. 2025; 11(3):69. https://doi.org/10.3390/beverages11030069
Chicago/Turabian StyleLópez-Rodríguez, Guadalupe, Marcos Galván, Oscar Galván-Valencia, and Jocelyn Gómez-Castillo. 2025. "Gain of Body Fat and Intake of Energy in Rats with Low Dose of Caloric and Non-Caloric Sweeteners Used in Reformulation Beverage in Mexico" Beverages 11, no. 3: 69. https://doi.org/10.3390/beverages11030069
APA StyleLópez-Rodríguez, G., Galván, M., Galván-Valencia, O., & Gómez-Castillo, J. (2025). Gain of Body Fat and Intake of Energy in Rats with Low Dose of Caloric and Non-Caloric Sweeteners Used in Reformulation Beverage in Mexico. Beverages, 11(3), 69. https://doi.org/10.3390/beverages11030069









