Potential of Different Eighteen Grapevine Genotypes to Produce Wines in a Hot Region: First Insights into Volatile and Sensory Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Vineyard and Wine Experiment
2.2. Reagents
2.3. Physicochemical General Analysis
2.4. Analysis of Wine Volatile Compounds
2.5. Wine Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. General Wine Composition
3.2. Volatile Composition Results
| Identified Compound (Compound Code Figure 2) | Odor Descriptors α | Retention Index | Significance Level from ANOVA |
|---|---|---|---|
| Ethyl butanoate (C1) | apple | 1047 | *** |
| 1-Propanol (C2) | ripe fruit, alcohol | 1050 | *** |
| Isobutanol (C3) | wine, solvent, bitter | 1105 | *** |
| Isoamyl acetate (C4) | banana | 1133 | *** |
| 1-Butanol (C5) | medicinal | 1155 | *** |
| Isoamylic alcohols (C6) | malt, burnt | 1223 | *** |
| Ethyl hexanoate (C7) | apple peel, fruit | 1245 | ** |
| 1-Pentanol (C8) | green, wax | 1263 | ** |
| Hexyl acetate (C9) | fruit, herb | 1282 | *** |
| Acetoin (C10) | butter/cream | 1289 | *** |
| Ethyl lactate (C11) | fruity | 1354 | *** |
| 1-Hexanol (C12) | resin, flower, green | 1362 | *** |
| trans-3-Hexenol (C13) | moss, fresh | 1373 | *** |
| 3-Ethoxy-1-propanol (C14) | ripe pear [55] | 1381 | *** |
| cis-3-hexen-1-ol (C15) | grass | 1391 | *** |
| Ethyl octanoate (C16) | fruity, fat | 1441 | ** |
| Methional (C17) | cooked potato | 1417 | *** |
| Ethyl 3-hydroxybutanoate (C18) | fruity [56] | 1525 | *** |
| Butyrolactone (C19) | caramel, sweet [57] | 1633 | *** |
| Ethyl decanoate (C20) | grape | 1642 | ** |
| Butanoic acid (C21) | rancid, cheese, sweat | 1649 | *** |
| Diethyl succinate (C22) | wine, fruit | 1684 | *** |
| Isovaleric acid (C23) | sweat, acid, rancid | 1687 | *** |
| Ethyl 9-decenoate (C24) | rose [58] | 1695 | *** |
| Methionol (C25) | sweet, potato | 1723 | *** |
| Ethyl 4-hydroxybutanoate (C26) | fruity [59] | 1813 | *** |
| 2-Phenylethyl acetate (C27) | rose, honey, tobacco | 1821 | *** |
| Hexanoic acid (C28) | fat, cheese, barnyard | 1861 | *** |
| Benzyl alcohol (C29) | fruity, walnut, bitter almond [54] | 1884 | *** |
| 2-Phenylethanol (C30) | honey, spice, rose, lilac | 1918 | *** |
| Diethyl malate (C31) | brown sugar, sweet | 2050 | *** |
| Octanoic acid (C32) | sweat, cheese | 2074 | *** |
| Decanoic acid (C33) | rancid, fat | 2288 | ** |
| Ethyl monosuccinate (C34) | - | 2404 | *** |
| Tyrosol (C35) | - | 3025 | *** |
| Varietal White Wine | Esters | Alcohols | C6 Alcohols | Acids | Cetones | Lactones | Others | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Acetoin | Butyrolactone | Methional | Tyrosol | |||||||
| Alvadurão | 24.42 bcd | 222.16 abc | 1.99 bcd | 19.96 c | 0.17 abcd | 2.99 de | 33.23 d | 0.68 abcde | ||
| Bastardo Branco | 16.70 abc | 200.45 ab | 1.31 abc | 8.16 ab | 0.09 abcd | 2.92 de | 13.79 a | 0.54 abc | ||
| Castelão Branco | 14.35 ab | 197.04 ab | 1.88 abcd | 14.45 abc | 0.05 ab | 1.83 abcd | 16.46 ab | 0.48 ab | ||
| Cayetana blanca (Sarigo) | 17.41 abcd | 360.43 c | 2.42 d | 17.63 bc | 0.07 ab | 2.62 bcde | 28.62 bcd | 0.82 bcde | ||
| Cercial | 26.76 cd | 302.97 bc | 1.61 abcd | 14.09 abc | 0.16 abcd | 5.36 fg | 32.21 cd | 0.99 cde | ||
| Folha de Figueira (Dona Branca) | 13.83 ab | 187.18 ab | 0.83 a | 10.68 abc | 0.06 ab | 2.04 abcd | 23.05 abcd | 0.46 ab | ||
| Fernão Pires | 28.58 d | 306.29 bc | 1.74 abcd | 12.12 abc | 0.17 bcd | 6.31 g | 27.71 abcd | 0.84 bcde | ||
| Galego Dourado | 15.53 abc | 207.58 ab | 1.13 abc | 11.97 abc | 0.09 abc | 2.79 cde | 20.45 abcd | 0.63 abcd | ||
| Lameiro | 19.43 abcd | 255.61 abc | 1.02 abc | 11.09 abc | 0.10 abcd | 3.19 de | 18.92 abc | 1.00 de | ||
| Larião | 8.09 a | 132.50 a | 2.08 cd | 14.40 abc | 0.22 d | 0.51 a | 27.06 abcd | 0.25 a | ||
| Palomino fino (Malvasia Rei) | 12.44 a | 246.98 abc | 1.72 abcd | 8.57 ab | 0.07 ab | 1.78 abcd | 20.72 abcd | 0.61 abcd | ||
| Molinha Macia | 9.82 a | 184.30 ab | 1.87 abcd | 15.02 abc | 0.04 a | 0.94 ab | 20.89 abcd | 0.39 ab | ||
| Albillo Mayor (Pardina) | 11.11 a | 232.77 abc | 1.87 abcd | 9.65 ab | 0.08 abc | 1.67 abcd | 25.83 abcd | 0.61 abcd | ||
| Parellada | 9.74 a | 172.69 ab | 1.40 abcd | 6.84 a | 0.04 a | 1.52 abcd | 14.24 a | 0.48 ab | ||
| Pedro Ximenez | 18.78 abcd | 284.75 bc | 1.18 abc | 13.44 abc | 0.07 abc | 2.78 cde | 21.49 abcd | 1.12 e | ||
| Roupeiro Branco | 9.83 a | 188.61 ab | 1.14 abc | 11.80 abc | 0.04 a | 1.16 abc | 21.37 abcd | 0.50 ab | ||
| Trajadura | 25.26 bcd | 223.05 abc | 0.95 ab | 12.49 abc | 0.14 abcd | 4.17 ef | 21.70 abcd | 0.61 abcd | ||
| Chasselas Cioutat (Uva Salsa) | 11.99 a | 211.91 ab | 1.18 abc | 19.36 c | 0.20 cd | 0.84 a | 30.32 bcd | 0.58 abcd | ||
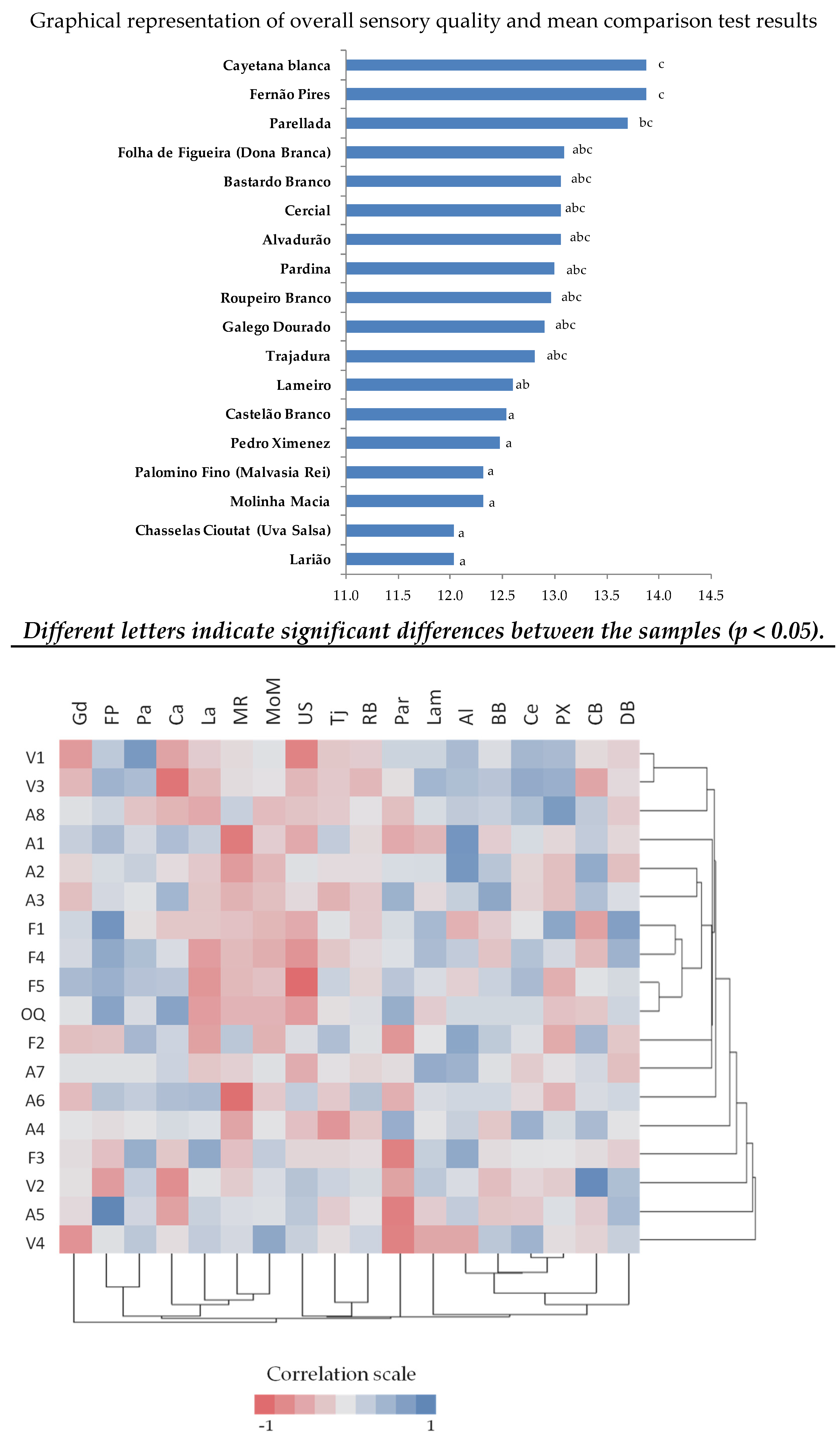
3.3. Sensory Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate changes and food quality: The potential of microbial activities as mitigating strategies in the wine sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2018, 99, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Costea, M.; Lengyel, E.; Stegăruş, D.; Rusan, N.; Tăuşan, I. Assessment of climatic conditions as driving factors of wine aromatic compounds: A case study from Central Romania. Theor. Appl. Climatol. 2019, 137, 239–254. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Chen, W.K.; Wang, Y.; Bai, X.-J.; Cheng, G.; Duan, C.-Q.; Wang, J.; He, F. Effect of the Seasonal Climatic Variations on the Accumulation of Fruit Volatiles in Four Grape Varieties Under the Double Cropping System. Front. Plant Sci. 2022, 12, 809558. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Francis, I.L.; Williamson, P.O. Consumer sensory science in wine research. Aust. J. Grape Wine Res. 2015, 21, 554–567. [Google Scholar] [CrossRef]
- Lucas, C.; Iobbi, A.; Dupas de Matos, A.; Tomasino, E. Understanding the relationship between tropical fruit aroma, acceptance, and emotional response in chardonnay wines. Food Res. Int. 2023, 174, 113496. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. Climate Change Impacts on Vineyards in Warm and Dry Areas: Challenges and Opportunities. Am. J. Enol. Vitic. 2023, 74, 0740033. [Google Scholar] [CrossRef]
- Eiras-Dias, J.E.; Cunha, J.; Brazão, J.; Clímaco, P. Promover e valorizar as castas minoritárias: O exemplo da casta Malvasia de Colares. Vida Rural 2016, 1818, 36–38. [Google Scholar]
- Alifragkis, A.; Cunha, J.; Pereira, J.; Fevereiro, P.; Eiras-Dias, J.E. Identity, synonymies and homonynies of minor grapevine cultivars maintained in the portuguese ampelographic collection. Ciência Téc. Vitiv. 2015, 30, 43–52. [Google Scholar] [CrossRef]
- Wineclimadapt-Seleção e Caracterização das Castas Mais Bem Adaptadas a Cenários de Alterações Climáticas. Available online: https://wineclimadapt.pt/bases-de-dados (accessed on 1 January 2025).
- Procedimento Para A Admissão À Certificação, De Parcelas De Multiplicação De Variedades De Videira Minoritárias No Encepamento Nacional. Available online: https://www.dgav.pt/wp-content/uploads/2023/03/Castas-minoritarias_3-3-2023.pdf (accessed on 1 January 2025).
- DGAV-Manual de Procedimentos Certificação de Material de Propagação de Videira. Available online: https://www.dgav.pt/wp-content/uploads/2021/06/DGAV_manualproced_videira.pdf (accessed on 1 January 2025).
- Gutiérrez-Gamboa, G.; Liu, S.-Y.; Pszczólkowski, P. Resurgence of minority and autochthonous grapevine varieties in South America: A review of their oenological potential. J. Sci. Food Agric. 2020, 100, 465–482. [Google Scholar] [CrossRef]
- Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G.; Villalobos-Soublett, E.; Zurita-Silva, A. Effects of Rootstocks on Blade Nutritional Content of Two Minority Grapevine Varieties Cultivated under Hyper-Arid Conditions in Northern Chile. Agronomy 2021, 11, 327. [Google Scholar] [CrossRef]
- Díaz-Fernández, Á.; Díaz-Losada, E.; Cortés-Diéguez, S. Diversity among Traditional Minority Red Grape Varieties According to Their Aromatic Profile. Agronomy 2022, 12, 1799. [Google Scholar] [CrossRef]
- Muñoz-Organero, G.; Espinosa, F.E.; Cabello, F.; Zamorano, J.P.; Urbanos, M.A.; Puertas, B.; Lara, M.; Domingo, C.; Puig-Pujol, A.; Valdés, M.E.; et al. Phenological Study of 53 Spanish Minority Grape Varieties to Search for Adaptation of Vitiviniculture to Climate Change Conditions. Horticulturae 2022, 8, 984. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M.R. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef]
- Puig-Pujol, A.; Domingo, C.; Guerrero, L.; Elorduy, X.; Gomis-Bellmunt, A. Sensory analysis of wines made with mino1rity varieties found in Spain. BIO Web Conf. 2023, 56, 02027. [Google Scholar] [CrossRef]
- Díaz-Fernández, Á.; Cortés-Diéguez, S.; Muñoz-Organero, G.; Cabello, F.; Puertas, B.; Puig-Pujol, A.; Domingo, C.; Valdés-Sánchez, M.E.; Moreno Cardona, D.; Cibriain, J.F.; et al. The Valorization of Spanish Minority Grapevine Varieties—The Volatile Profile of Their Wines as a Characterization Feature. Agronomy 2024, 14, 1033. [Google Scholar] [CrossRef]
- Vázquez-Pateiro, I.; Arias-González, U.; Mirás-Avalos, J.M.; Falqué, E. Evolution of the Aroma of Treixadura Wines during Bottle Aging. Foods 2020, 9, 1419. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fernández, Á.; Díaz-Losada, E.; González, J.M.D.; Cortés-Diéguez, S. Part II—Aroma Profile of Twenty White Grapevine Varieties: A Chemotaxonomic Marker Approach. Agronomy 2023, 13, 1168. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Sánchez, C.; Gonzalez-Hernandez, M.; Bueno, M.; Peña, C.; Fernández-Zurbano, P.; Ballester, J.; Parga-Dans, E.; González, P.A. Natural versus conventional production of Spanish white wines: An exploratory study. J. Sci. Food Agric. 2023, 103, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Coutinho, P.; Coelho, E.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Relationships between the varietal volatile composition of the musts and white wine aroma quality. A four year feasibility study. LWT 2010, 43, 1508–1516. [Google Scholar] [CrossRef]
- Piras, S.; Brazão, J.; Ricardo-Da-Silva, J.M.; Anjos, O.; Caldeira, I. Volatile and sensory characterization of white wines from three minority Portuguese grapevine varieties. Ciência Téc. Vitiv. 2020, 35, 49–62. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. The Physicochemical Characterization of the Aroma of Dessert and Sparkling White Wines (Pedro Ximénez, Fino, Sauternes, and Cava) by Gas Chromatography−Olfactometry and Physicochemical Quantitative Analysis. J. Agric. Food Chem. 2008, 56, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Volatile Composition and Sensory Characterization of Dry White Wines Made with Overripe Grapes by Means of Two Different Techniques. Foods 2022, 11, 509. [Google Scholar] [CrossRef]
- del Fresno, J.M.; Escott, C.; Carrau, F.; Herbert-Pucheta, J.E.; Vaquero, C.; González, C.; Morata, A. Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal. Fermentation 2022, 8, 654. [Google Scholar] [CrossRef]
- Vitis International Variety Catalogue. Available online: https://www.vivc.de/index.php?r=cultivarname%2Findex (accessed on 7 May 2024).
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2022. [Google Scholar]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Philips, R.J. Qualitative and quantitative analysis. In High Resolution Gas Chromatography, 3rd ed.; Hyver, K.J., Sandra, P., Eds.; Hewlett-Packard, Co.: Palo Alto, CA, USA, 1989; pp. 1–11. [Google Scholar]
- ISO 8589; Sensory Analysis—General Guidance for the Design of test rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 3591; Sensory Analysis-Wine-Tasting Glass. Standard Reviewed and Confirmed in 2022. International Organization for Standardization: Geneva, Switzerland, 1977.
- ISO 8586; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Fandiño, M.; Vilanova, M.; Caldeira, I.; Silvestre, J.M.; Rey, B.J.; Mirás-Avalos, J.M.; Cancela, J.J. Physicochemical composition and sensory properties of Albariño wine: Fertigation effects. Food Res. Int. 2020, 137, 109533. [Google Scholar] [CrossRef]
- Caldeira, I.; Belchior, A.P.; Clímaco, M.C.; Bruno de Sousa, R. Aroma profile of portuguese brandies aged in chestnut and oak woods. Anal. Chim. Acta 2002, 458, 55–62. [Google Scholar] [CrossRef]
- Macfie, H.J.M.; Bratchell, N.; Greenhoff, H.; Vallis, L.V. Designs to Balance the Effect of Order of Presentation and First-Order Carry-over Effects in Hall Tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Herbert, P.; Cabrita, M.J.; Ratola, N.; Laureano, O.; Alves, A. Free amino acids and biogenic amines in wines and musts from the Alentejo region. Evolution of amines during alcoholic fermentation and relationship with variety, sub-region and vintage. J. Food Eng. 2005, 66, 315–322. [Google Scholar] [CrossRef]
- Pereira, C.; Mendes, D.; Martins, N.; Gomes da Silva, M.; Garcia, R.; Cabrita, M.J. A Sustainable Approach Based on the Use of Unripe Grape Frozen Musts to Modulate Wine Characteristics as a Proof of Concept. Beverages 2022, 8, 79. [Google Scholar] [CrossRef]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The Interplay between Atmospheric Conditions and Grape Berry Quality Parameters in Portugal. Appl. Sci. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Oliveira, J.; Marta, F.; Filomena, S.; Filipa, B.; Isabel, A. C6 -alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Gonzalez, R.; Guindal, A.M.; Tronchoni, J.; Morales, P. Biotechnological approaches to lowering the ethanol yield during wine fermentation. Biomolecules 2021, 11, 1569. [Google Scholar] [CrossRef]
- Bai, H.; Gambetta, G.A.; Wang, Y.; Kong, J.; Long, Q.; Fan, P.; Duan, W.; Liang, Z.; Dai, Z. Historical long-term cultivar×climate suitability data to inform viticultural adaptation to climate change. Sci. Data 2022, 9, 271. [Google Scholar] [CrossRef]
- Etiévant, P. Wine. In Volatile Compounds in Food and Beverages; Maarse, H., Ed.; Marcell Dekker Inc.: New York, NY, USA, 1991; pp. 483–545. [Google Scholar]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef]
- Clarke, R.J.; Bakker, J. Volatile components. In Wine Flavour Chemistry; Clarke, R., Bakker, J., Eds.; J. Blackwell Publishing Ltd.: London, UK, 2004; pp. 120–188. [Google Scholar]
- Selli, S.; Canbas, A.; Cabaroglu, T.; Erten, H.; Lepoutre, J.-P.; Gunata, Z. Effect of skin contact on the free and bound aroma compounds of the white wine of Vitis vinifera L. cv Narince. Food Control 2006, 17, 75–82. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The Mouthfeel of White Wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956. [Google Scholar] [CrossRef]
- Pons, A.; Nikolantonaki, M.; Lavigne, V.; Shinoda, K.; Dubourdieu, D.; Darriet, P. New Insights into Intrinsic and Extrinsic Factors Triggering Premature Aging in White Wines. In Advances in Wine Research; ACS: Washington, DC, USA, 2015; pp. 229–251. [Google Scholar] [CrossRef]
- Hatanaka, A.; Kajiwara, T.; Horino, H.; Inokuchi, K. Odor-structure relationships in n-hexenols and n-hexenals. Z. Naturforsch. C 1992, 47, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Clues about the Role of Methional as Character Impact Odorant of Some Oxidized Wines. J. Agric. Food Chem. 2000, 48, 4268–4272. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Gurbuz, O.; Rouseff, J.M.; Rouseff, R.L. Comparison of aroma volatiles in commercial Merlot and Cabernet Sauvignon wines using gas chromatography olfactometry and gas chromatography mass spectrometry. J. Agric. Food Chem. 2006, 54, 3990–3996. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: https://www.flavornet.org/ (accessed on 10 May 2024).
- Rocha, S.M.; Coutinho, P.; Delgadillo, I.; Cardoso, A.D.; Coimbra, M.A. Effect of enzymatic aroma release on the volatile compounds of white wines presenting different aroma potentials. J. Sci. Food Agric. 2005, 85, 199–205. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Physicochemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef]
- Jordão, A.M.; Gonçalves, F.J.; Correia, A.C.; Cantão, J.; Rivero-Pérez, M.D.; González Sanjosé, M.L. Proanthocyanidin content, antioxidant capacity and scavenger activity of Portuguese sparkling wines (Bairrada Appellation of Origin). J. Sci. Food Agric. 2010, 90, 2144–2152. [Google Scholar] [CrossRef]


| Prime Name of the Variety | Variety Number VIVC | Grape Variety Name in the Vineyard | Wine Code |
|---|---|---|---|
| Alvadurao | 20806 | Alvadurão | Al |
| Bastardo Branco | 1026 | Bastardo Branco | BB |
| Castelao Branco | 2321 | Castelão Branco | CB |
| Cayetana Blanca | 5648 | Cayetana | Ca |
| Cercial | 16437 | Cercial | Ce |
| Folha de Figueira | 14142 | Dona Branca | DB |
| Fernão Pires | 4100 | Fernão Pires | FP |
| Galego Dourado | 4325 | Galego Dourado | GD |
| Lameiro | 6706 | Lameiro | Lam |
| Lariao | 6757 | Larião | La |
| Palomino Fino | 8888 | Malvasia Rei | MR |
| Molinha Macia | 40728 | Molinha Macia | MoM |
| Albillo Mayor | 12581 | Pardina | Pa |
| Parellada | 8938 | Parellada | Par |
| Pedro Ximenez | 9080 | Pedro Ximenez | PX |
| Roupeiro Branco | 17716 | Roupeiro Branco | RB |
| Trajadura | 12629 | Trajadura | Tj |
| Chasselas Cioutat | 2476 | Uva Salsa | US |
| Significance Level from ANOVA | *** | *** | *** | *** | *** | *** | *** |
|---|---|---|---|---|---|---|---|
| Varietal Wine | Density (g/cm3) | Alcoholic Strength (% vol) | Total Acidity (g Tartaric Acid/L) | Volatile Acidity (g Acetic Acid/L) | Fixed Acidity (g Tartaric Acid/L) | Reducing Substances (g Glucose/L) | pH |
| Parellada | 0.9887 ab | 12.8 g | 4.67 cde | 0.41 de | 4.16 bcd | 2.31 abcd | 3.61 e |
| ±0.0002 | ±0.1 | ±0.04 | ±0.02 | ±0.01 | ±1.90 | ±0.02 | |
| Cayetana Blanca | 0.9901 abcd | 11.6 d | 5.10 fgh | 0.28 bc | 4.76 gh | 1.38 ab | 3.68 fg |
| ±0.0000 | ±0.0 | ±0.09 | ±0.03 | ±0.05 | ±0.01 | ±0.00 | |
| Albillo Mayor | 0.9902 bcde | 11.7 de | 5.13 fgh | 0.27 bc | 4.80 h | 1.44 ab | 3.67 efg |
| ±0.0000 | ±0.1 | ±0.17 2 | ±0.01 | ±0.18 | ±0.27 | ±0.02 | |
| Trajadura | 0.9882 a | 15.5 k | 5.50 h | 0.55 fg | 4.81 h | 3.53 abcd | 3.78 hi |
| ±0.0001 | ±0.0 | ±0.05 | ±0.03 | ±0.10 | ±0.18 | ±0.02 | |
| Fernão Pires | 0.9888 ab | 14.9 j | 5.41 fgh | 0.61 g | 4.65 fgh | 5.16 cd | 3.63 ef |
| ±0.0003 | ±0.0 | ±0.01 | ±0.03 | ±0.03 | ±1.12 | ±0.02 | |
| Galego Dourado | 0.9896 abc | 13.9 h | 5.00 def | 0.52 fg | 4.35 def | 5.45 d | 3.66 ef |
| ±0.0002 | ±0.0 | ±0.02 | ±0.02 | ±0.05 | ±0.86 | ±0.01 | |
| Cercial | 0.9889 ab | 14.1 hi | 6.57 i | 0.52 fg | 5.92 i | 2.88 abcd | 3.36 b |
| ±0.0005 | ±0.0 | ±0.00 | ±0.02 | ±0.02 | ±0.68 | ±0.01 | |
| Lameiro | 0.9895 abc | 14.3 i | 5.44 gh | 0.31 cd | 5.05 h | 4.11 abcd | 3.72 gh |
| ±0.0004 | ±0.0 | ±0.02 | ±0.01 | ±0.03 | ±0.23 | ±0.03 | |
| Folha de Figueira | 0.9900 abcd | 14.6 j | 4.24 ab | 0.47 ef | 3.64 a | 4.49 bcd | 4.15 j |
| ±0.0007 | ±0.1 | ±0.30 | ±0.01 | ±0.28 | ±1.50 | ±0.02 | |
| Palomino Fino | 0.9899 abcd | 11.9 de | 4.58 bcd | 0.28 bc | 4.23 cde | 2.1 abcd | 3.62 e |
| ±0.0001 | ±0.1 | ±0.02 | ±0.01 | ±0.02 | ±0.21 | ±0.01 | |
| Roupeiro Branco | 0.9896 abc | 12.0 e | 4.10 a | 0.28 bc | 3.75 ab | 1.97 abc | 3.83 ij |
| ±0.0006 | ±0.2 | ±0.08 | ±0.01 | ±0.01 | ±1.05 | ±0.01 | |
| Bastardo Branco | 0.9902 bcde | 12.5 f | 6.77 i | 0.54 fg | 6.10 i | 2.65 abcd | 3.22 a |
| ±0.0000 | ±0.0 | ±0.04 | ±0.02 | ±0.06 | ±0.34 | ±0.00 | |
| Pedro Ximenez | 0.9899 abcd | 14.0 h | 4.33 abc | 0.37 cde | 3.86 abc | 5.53 d | 3.85 i |
| ±0.0004 | ±0.1 | ±0.01 | ±0.01 | ±0.03 | ±1.05 | ±0.01 | |
| Alvadurão | 0.9908 cde | 13.1 g | 6.60 i | 0.28 bc | 6.25 i | 2.15 abcd | 3.53 d |
| ±0.0000 | ±0.1 | ±0.07 | ±0.05 | ±0.13 | ±0.06 | ±0.01 | |
| Castelão Branco | 0.9897 abc | 12.0 e | 5.02 efg | 0.30 bc | 4.65 fgh | 1.91 abc | 3.44 c |
| ±0.0000 | ±0.1 | ±0.04 | ±0.01 | ±0.05 | ±0.12 | ±0.01 | |
| Chasselas Cioutat | 0.9915 def | 9.5 b | 4.43 abc | 0.20 ab | 4.18 bcd | 1.30 ab | 3.34 b |
| ±0.0002 | ±0.1 | ±0.10 | ±0.0012 | ±0.03 | ±0.11 | ±0.03 | |
| Larião | 0.9930 f | 8.5 a | 4.44 abc | 0.14 a | 4.26 cde | 0.95 a | 3.44 c |
| ±0.0012 | ±0.0 | ±0.01 | ±0.01 | ±0.00 | ±0.03 | ±0.01 | |
| Molinha Macia | 0.9919 ef | 9.9 c | 4.56 bc | 0.31 bcd | 4.17 bcd | 0.80 a | 3.68 efg |
| ±0.0000 | ±0.0 | ±0.11 | ±0.00 | ±0.11 | ±0.00 | ±0.00 |
| Descriptor | Effect of Variety Factor (Significance Level) |
|---|---|
| Color intensity (V1) | ** |
| Green hue (V2) | * |
| Yellow hue (V3) | *** |
| Limpidity (V4) | * |
| Aroma positive intensity (A1) | ** |
| Floral (A2) | ns |
| Terpenic/muscat (A3) | ns |
| White fruit (A4) | ns |
| Tropical fruit (A5) | * |
| Citrus fruit (A6) | ns |
| Dried fruits (A7) | ns |
| Herbaceous/green (A8) | ns |
| Sweetness (F1) | *** |
| Sourness (F2) | * |
| Bitterness (F3) | ns |
| Body (F4) | * |
| Harmonious persistency (F5) | ** |
| Overall quality (OQ) | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldeira, I.; Roque, R.; Anjos, O.; Lourenço, S.; de Deus, J.; Damásio, M.; Silvestre, J. Potential of Different Eighteen Grapevine Genotypes to Produce Wines in a Hot Region: First Insights into Volatile and Sensory Profiles. Beverages 2025, 11, 68. https://doi.org/10.3390/beverages11030068
Caldeira I, Roque R, Anjos O, Lourenço S, de Deus J, Damásio M, Silvestre J. Potential of Different Eighteen Grapevine Genotypes to Produce Wines in a Hot Region: First Insights into Volatile and Sensory Profiles. Beverages. 2025; 11(3):68. https://doi.org/10.3390/beverages11030068
Chicago/Turabian StyleCaldeira, Ilda, Rita Roque, Ofélia Anjos, Sílvia Lourenço, João de Deus, Miguel Damásio, and José Silvestre. 2025. "Potential of Different Eighteen Grapevine Genotypes to Produce Wines in a Hot Region: First Insights into Volatile and Sensory Profiles" Beverages 11, no. 3: 68. https://doi.org/10.3390/beverages11030068
APA StyleCaldeira, I., Roque, R., Anjos, O., Lourenço, S., de Deus, J., Damásio, M., & Silvestre, J. (2025). Potential of Different Eighteen Grapevine Genotypes to Produce Wines in a Hot Region: First Insights into Volatile and Sensory Profiles. Beverages, 11(3), 68. https://doi.org/10.3390/beverages11030068








