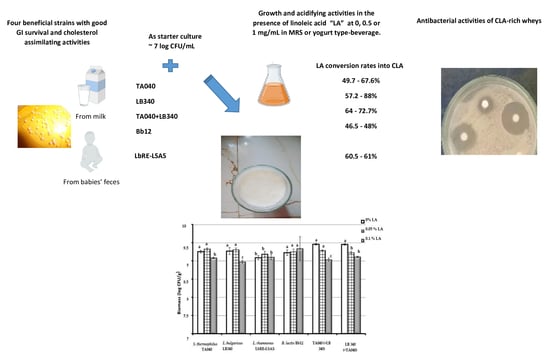
CLA-Producing Probiotics for the Development of a Yogurt-Type Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Linoleic Acid (LA)
2.3. Preparation of Yogurt-Type Beverage
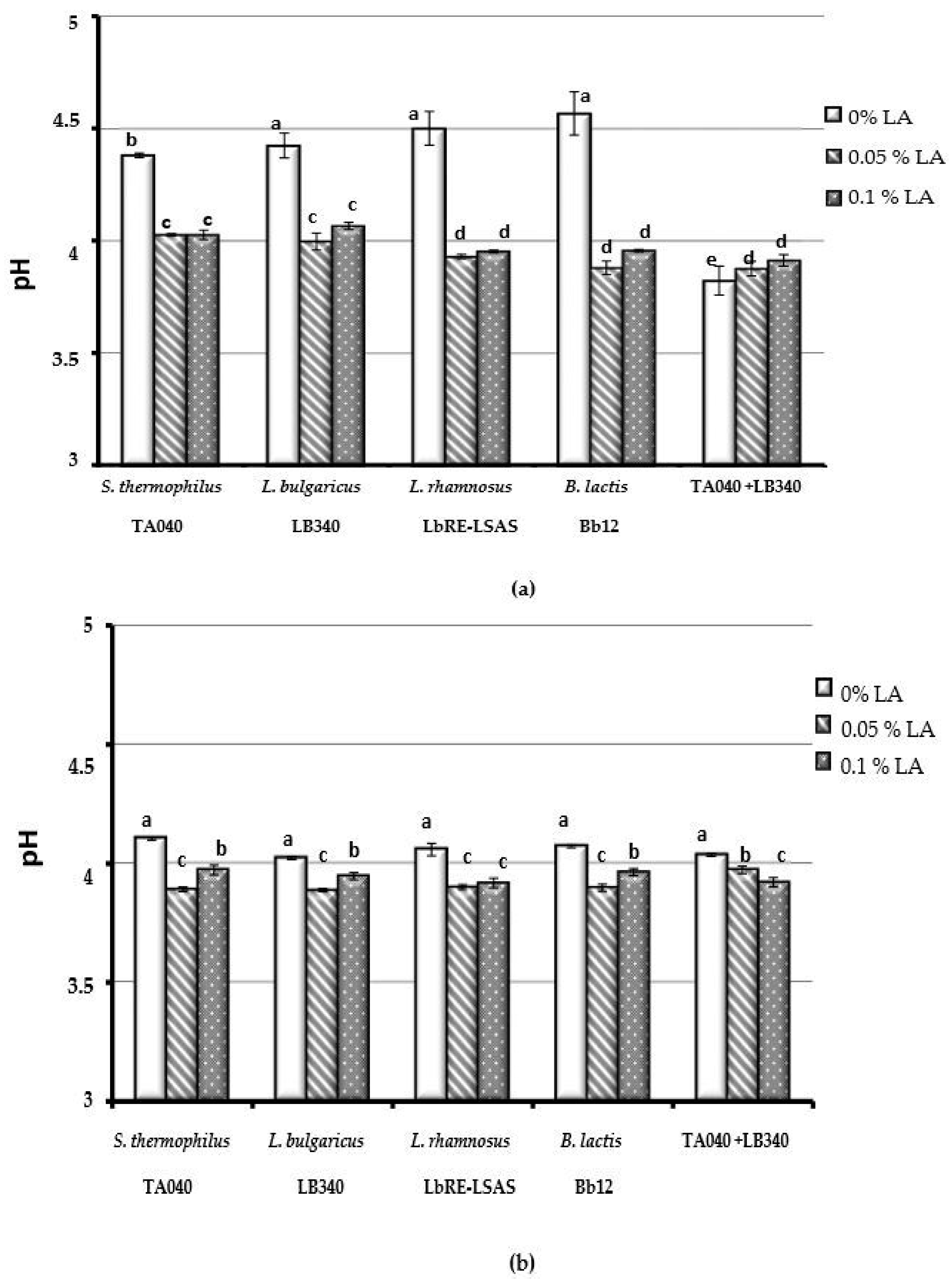
2.4. Biomass and pH
2.5. CLA Contents Released from the Bacterial Conversion of LA
2.5.1. Extraction
2.5.2. Methylation
2.5.3. Gas Chromatography (GC) Analysis
2.5.4. Linoleic Acid’s Bacterial Conversion Ability
2.6. Antibacterial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. All Probiotic Bacteria Were Able to Convert Linoleic Acid into CLA
3.1.1. Bacterial Growth Was High for All Strains and Affected by Linoleic Acid in MRS Medium Only
3.1.2. The Acidifying Activity of Bacterial Strains Was Not Affected by Linoleic Acid
3.2. Bioproduction of CLA Under Yogurt-Type Beverage Conditions Was Good
3.3. Good Antibacterial Effect Against Virulent Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLA | Conjugated linoleic acid isomer |
| LA | Linoleic acid |
| LAB | Lactic acid bacteria |
| RA | Rumenic acid |
| PUFAs | Polyunsaturated fatty acids |
References
- Balthazar, C.F.; Guimarães, J.F.; Coutinho, N.M.; Pimentel, T.C.; Ranadheera, C.S.; Santillo, A.; Albenzio, M.; Cruz, A.G.; Sant’Ana, A.S. The future of functional food: Emerging technologies application on prebiotics, probiotics and postbiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2560–2586. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Keddar, K.; Ziar, H.; Belmadani, N.; Monnoye, M.; Gérard, P.; Riazi, A. Probiotic Bacteria from Human Milk Can Alleviate Oral Bovine Casein Sensitization in Juvenile Wistar Rats. Microorganisms 2023, 11, 1030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemaire, M.; Dou, S.; Cahu, A.; Formal, M.; Le Normand, L.; Romé, V.; Nogret, I.; Ferret-Bernard, S.; Rhimi, M.; Cuinet, I.; et al. Addition of dairy lipids and probiotic Lactobacillus fermentum in infant formula programs gut microbiota and entero-insular axis in adult minipigs. Sci. Rep. 2018, 8, 11656. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.A.-O. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Efsa Panel on Dietetic Products, Nutrition and Allergies (NDA). Statement on the safety of the “conjugated linoleic acid (CLA) rich oils” Clarinol® and Tonalin® TG 80 as Novel Food ingredients. EFSA J. 2012, 10, 2700. [Google Scholar] [CrossRef]
- Ha, Y.L.; Grimm, N.K.; Pariza, M.W. Anticarcinogens from fried ground beef: Heat-altered derivatives of linoleic acid. Carcinogenesis 1987, 8, 1881–1887. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hariri, M. Effect of conjugated linoleic acid supplementation on serum leptin concentration: A systematic review and metaanalysis. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 185–193. [Google Scholar] [CrossRef] [PubMed]
- May, K.S.; den Hartigh, L.J. Modulation of Adipocyte Metabolism by Microbial Short-Chain Fatty Acids. Nutrients 2021, 13, 3666. [Google Scholar] [CrossRef]
- Ziar, H.; Yahla, I.; Riazi, A. Conjugated isomers of linoleic acid and health: What about those of bacterial origin? Nutr. Santé 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W., Jr.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonanome, A.; Grundy, S.M. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N. Engl. J. Med. 1988, 318, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Cuesta, E.P.; Gilliland, S.E. Production of Free Conjugated Linoleic Acid by Lactobacillus acidophilus and Lactobacillus casei of Human Intestinal Origin. J. Dairy. Sci. 2003, 86, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Coakley, M.; Banni, S.; Johnson, M.C.; Mills, S.; Devery, R.; Fitzgerald, G.; Ross, R.P.; Stanton, C. Inhibitory effect of conjugated alpha-linolenic acid from bifidobacteria of intestinal origin on SW480 cancer cells. Lipids 2009, 44, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ziar, H.; Gérard, P.; Riazi, A. Effect of prebiotic carbohydrates on growth, bile survival and cholesterol uptake abilities of dairy-related bacteria. J. Sci. Food Agric. 2014, 94, 1184–1190. [Google Scholar] [CrossRef]
- Loor, J.J.; Ueda, K.; Ferlay, A.; Chilliard, Y.; Doreau, M. Biohydrogenation, duodenal flow, and intestinal digestibility of trans fatty acids and conjugated linoleic acids in response to dietary forage: Concentrate ratio and linseed oil in dairy cows. J. Dairy Sci. 2004, 87, 2472–2485. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.; Braga, T.; Malcata, X.; Gomes, A.; Fontecha, J. Quantitative and qualitative determination of CLA produced by Bifidobacterium and lactic acid bacteria by combining spectrophotometric and Ag+-HPLC techniques. Food Chem. 2011, 125, 1373–1378. [Google Scholar] [CrossRef]
- Coakley, M.; Ross, R.P.; Nordgren, M.; Fitzgerald, G.; Devery, R.; Stanton, C. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J. Appl. Microbiol. 2003, 94, 138–145. [Google Scholar] [CrossRef]
- Lin, T.Y. Influence of lactic cultures, linoleic acid and fructooligosaccharides on conjugated linoleic acid concentration in non-fat set yoghurt. Aust. J. Dairy Technol. 2003, 58, 11–14. [Google Scholar]
- Gao, H.; Yang, B.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, H. Linoleic acid induces different metabolic modes in two Bifidobacterium breve strains with different conjugated linoleic acid-producing abilities. LWT 2021, 142, 110974. [Google Scholar] [CrossRef]
- Kankaanpää, P.; Yang, B.; Kallio, H.; Isolauri, E.; Salminen, S. Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of lactobacilli. Appl. Environ. Microbiol. 2004, 70, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.A.; Barclay, D.; Marshall, H.; Moulin, J.; Maire, J.C.; Makrides, M. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: A randomised controlled trial. Br. J. Nutr. 2009, 101, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Puniya, A.K. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J. Food Sci. Technol. 2017, 54, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; Cabral, C.C.; da Costa Lima, B.R.C.; Paschoalin, V.M.F.; Leandro, K.C. Lactococcus lactis ssp. cremoris MRS47, a potential probiotic strain isolated from kefir grains, increases cis-9, trans-11-CLA and PUFA contents in fermented milk. J. Funct. Foods 2017, 31, 172–178. [Google Scholar]
- Derakhshande-Rishehri, S.M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of foods enriched in conjugated linoleic acid (CLA) and CLA supplements with lipid profile in human studies: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef]
- Salamon, R.; Vargáné-Visi, É.; András, C.D.; Csapóné Kiss, Z.; Csapó, J. Synthetic methods to obtain conjugated linoleic acids (CLAs) by catalysis—A review. Acta Aliment. 2015, 44, 229–234. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Meng, X.; Tong, P.; Liu, X. Biosynthesis of c9, t11-conjugated linoleic acid and the effect on characteristics in fermented soy milk. Food Chem. 2022, 368, 130866. [Google Scholar] [CrossRef]
- Asbaghi, O.; Shimi, G.; Hosseini Oskouie, F.; Naseri, K.; Bagheri, R.; Ashtary-Larky, D.; Nordvall, M.; Rastgoo, S.; Zamani, M.; Wong, A. The effects of conjugated linoleic acid supplementation on anthropometrics and body composition indices in adults: A systematic review and dose-response meta-analysis. Br. J. Nutr. 2023, 131, 406–428. [Google Scholar] [CrossRef]
- Putera, H.D.; Doewes, R.I.; Shalaby, M.N.; Ramírez-Coronel, A.A.; Clayton, Z.S.; Abdelbasset, W.K.; Murtazaev, S.S.; Jalil, A.T.; Rahimi, P.; Nattagh-Eshtivani, E.; et al. The effect of conjugated linoleic acids on inflammation, oxidative stress, body composition and physical performance: A comprehensive review of putative molecular mechanisms. Nutr. Metab. 2023, 20, 35. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lin, C.W.; Lee, C.H. Conjugated linoleic acid concentration as affected by lactic cultures and added linoleic acid. Food Chem. 1999, 67, 1–5. [Google Scholar] [CrossRef]
- Gorissen, L.; Leroy, F.; De Vuyst, L.; De Smet, S.; Raes, K. Bacterial production of conjugated linoleic and linolenic acid in foods: A technological challenge. Crit. Rev. Food Sci. Nutr. 2015, 55, 1561–1574. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Mei, Y.; Yang, B.; Zhao, J.; Stanton, C.; Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res. 2024, 93, 101257. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Nadeem, M.; Al-Asmari, F.; Imran, M.; Ambreen, S.; Rahim, M.A.; Oranab, S.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Lactiplantibacillus plantarum on the Conversion of Linoleic Acid of Vegetable Oil to Conjugated Linoleic Acid, Lipolysis, and Sensory Properties of Cheddar Cheese. Microorganisms 2023, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Yahla, I.; Ziar, H.; Benali, M.; Riazi, A. Bacterial Conjugated Linoleic Acid Effect on Hepatic and Adipose Tissues of High-Fat Diet-Induced Obese Rat. S. Asian J. Exp. Biol. 2016, 6, 143–149. [Google Scholar] [CrossRef]
- McDonald, M.I.; Graham, I.; Harvey, K.J.; Sinclair, A. Antibacterial activity of hydrolysed linseed oil and linolenic acid against methicillin-resistant staphylococcus aureus. Lancet 1981, 2, 1056. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Do unsaturated fatty acids function as endogenous antibacterial and antiviral molecules? Am. J. Clin. Nutr. 2006, 83, 390–391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, J.; Wang, X.; Chen, K.; Shen, H. Screening of Lactobacillus with high conjugated linoleic acid production capacity. China Oils Fats 2021, 46, 102–106. [Google Scholar]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241, Erratum in Front. Microbiol. 2014, 5, 683. https://doi.org/10.3389/fmicb.2014.00683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Tong, F.; Sun, R.; Zhang, Y.; Pang, Z.; Liu, X. Screening and Identification of High-Yielding Strains of Conjugated Linoleic Acid and Optimization of Conditions for the Conversion of CLA. Foods 2024, 13, 1830. [Google Scholar] [CrossRef]

| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | % Bioconversion | 9t C18:1 | C18:3 n-6 | C18:3 n-3 | Total CLAs | 9c, 11t CLA | 10t, 12c CLA | tt CLA Mixture | |
| S. thermophilus 1 TA 040 | 42.49 ± 2.02 | ND | ND | 0.1652 b | 0.1502 a | 0.0825 a | 0.0677 a | ND | |
| L. bulgaricus LB 340 | 45.34 ± 4.72 | 0.0013 b,* | 0.0029 c | 0.2512 a | 0.1225 a | 0.0512 b | 0.0423 b | 0.0289 a | |
| B. lactis Bb12 | 40.62 ± 3.11 | 0.0018 b,* | 0.0064 b | 0.0874 c | 0.1106 b | 0.0523 b | 0.0607 a | ND | |
| L. rhamnosus LbRE-LSAS | 50.32 ± 5.44 | 0.0031 a | 0.0143 a | 0.0788 c | 0.0728 c | 0.0364 c | 0.0364 b | ND | |
| TA 040 + LB 340 | 35.23 ± 3.33 | 0.0018 b,* | 0.0126 a | 0.0522 d | 0.0822 bc | 0.0424 b | 0.0321 b | 0.0064 b | |
| (b) | |||||||||
| Strain | % Bioconversion | 9t C18:1 | 11t C18:1 | C18:3 n-6 | C18:3 n-3 | Total CLAs | 9c, 11t CLA | 10t, 12c CLA | ttCLA Mixture |
| S. thermophilus 1 TA 040 | 64.74 ± 2.66 | 0.0190 b | ND | 0.0240 c | 0.2150 c | 0.0300 c | 0.0182 d | 0.0118 b | ND |
| L. bulgaricus LB 340 | 87.92 ± 4.55 | 0.0400 a | 0.0380 a | 0.0460 b | 0.3190 b | 0.0881 b | 0.0545 b | ND | 0.0336 |
| B. lactis Bb12 | 46.65 ± 4.87 | 0.0320 a | 0.0320 a | 0.0450 b | 0.4160 a | 0. 1893 a | 0.1035 a | 0.0454 a | 0.0404 |
| L. rhamnosus LbRE-LSAS | 60.58 ± 5.22 | 0.0080 c | 0.0230 b | 0.0270 c | 0.2000 c | 0.0496 c | 0.0354 c | 0.0142 b | ND |
| TA 040 + LB 340 | 72.76 ± 3.33 | 0.0140 b | 0.0260 b | 0.1350 a | 0.2400 c | ND | ND | ND | ND |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | % Bioconversion | 9t C18:1 | C18:3 n-6 | C18:3 n-3 | Total CLAs | 9c, 11t CLA | 10t, 12c CLA | tt CLA Mixture | |
| S. thermophilus 1 TA 040 | 37.7 ± 3.33 | 0.2917 a | 0.0534 | ND | 0.0623 ‡,b | ND | ND | ND | |
| L. bulgaricus LB 340 | 61.74 ± 1.66 | 0.0140 c | ND | ND | 0.0628 b | 0.0304 bc | 0.0302 a | ND | |
| B. lactis Bb12 | 39.74 ± 2.99 | 0.0336 b | ND | ND | 0.0619 b | 0.0619 a | ND | ND | |
| L. rhamnosus LbRE-LSAS | 24.92 ± 1.66 | 0.0068 d | ND | ND | 0.0855 a | 0.0461 ab | 0.0393 a | ND | |
| TA 040 + LB 340 | 46.74 ± 3.33 | 0.0065 d | ND | ND | 0.0432 c | 0.0233 c | 0.0199 b | ND | |
| (b) | |||||||||
| Strain | % Bioconversion | 9t C18:1 | 11t C18:1 | C18:3 n-6 | C18:3 n-3 | Total CLAs | 9c, 11t CLA | 10t, 12c CLA | ttCLA Mixture |
| S. thermophilus 1 TA 040 | 49.69 ± 2.66 | 0.0220 a | 0.0340 a | 0.0390 b | 0.2590 b | 0.0723 a | 0.0493 a | 0.0230 b | ND |
| L. bulgaricus LB 340 | 57.27 ± 0.33 | 0.0180 a | 0.0240 b | 0.0470 b | 0.2170 c | 0.0539 b | 0.0330 b | 0.0209 b | ND |
| B. lactis Bb12 | 48.22 ± 0.66 | 0.0031 b | 0.0380 a | 0.0420 b | 0.1050 d | 0. 0837 a | 0.0536 a | 0.0301 a | ND |
| L. rhamnosus LbRE-LSAS | 61.33 ± 1.75 | 0.0030 b | 0.0390 a | 0.0380 b | 0.3910 a | 0.0572 b | 0.0429 a | 0.0143 c | ND |
| TA 040 + LB 340 | 63.90 ± 0.66 | 0.0020 b | 0.0320 a | 0.0620 a | 0.2080 bc | 0.0751 a | 0.0361 b | 0.0390 a | ND |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Zone of Inhibition (mm) | ||||||
| Supernatants from LA Probiotic Cultures | S. typhimurium | E. coli | S. aureus | S. typhimurium | E. coli | S. aureus |
| MRS | WHEY | |||||
| TA 040 | 25 ± 0.66 | - | - | - | - | - |
| LB 340 | - | - | - | 20 ± 0.11 | - | 10 ± 0.66 |
| TA040 + LB340 | 20 ± 0.05 | - | - | 18 ± 0.05 | - | 18 ± 0.02 |
| LbRE-LSAS | - | 40 ± 0.33 | - | - | 30 ± 0.66 | 8 ± 0.11 |
| Bb12 | - | - | 30 ± 0.67 | 8 ± 0.08 | 18 ± 0.11 | 18 ± 0.02 |
| (b) | ||||||
| Zone of Inhibition (mm) | ||||||
| Supernatants from LA Probiotic Cultures | S. typhimurium | E. coli | S. aureus | S. typhimurium | E. coli | S. aureus |
| MRS | WHEY | |||||
| TA 040 | 35 ± 0.12 | - | - | - | - | - |
| LB 340 | - | - | - | 25 ± 0.05 | - | 12 ± 0.15 |
| TA040 + LB340 | 25 ± 0.33 | - | - | 20 ± 0.01 | - | 18 ± 0.87 |
| LbRE-LSAS | - | 45 ± 0.66 | - | - | 40 ± 0.11 | 10 ± 0.22 |
| Bb12 | - | - | 40 ± 0.33 | 10 ± 0.66 | 20 ± 0.33 | 25 ± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziar, H.; Gérard, P.; Riazi, A. CLA-Producing Probiotics for the Development of a Yogurt-Type Beverage. Beverages 2025, 11, 50. https://doi.org/10.3390/beverages11020050
Ziar H, Gérard P, Riazi A. CLA-Producing Probiotics for the Development of a Yogurt-Type Beverage. Beverages. 2025; 11(2):50. https://doi.org/10.3390/beverages11020050
Chicago/Turabian StyleZiar, Hasnia, Philippe Gérard, and Ali Riazi. 2025. "CLA-Producing Probiotics for the Development of a Yogurt-Type Beverage" Beverages 11, no. 2: 50. https://doi.org/10.3390/beverages11020050
APA StyleZiar, H., Gérard, P., & Riazi, A. (2025). CLA-Producing Probiotics for the Development of a Yogurt-Type Beverage. Beverages, 11(2), 50. https://doi.org/10.3390/beverages11020050